Abstract
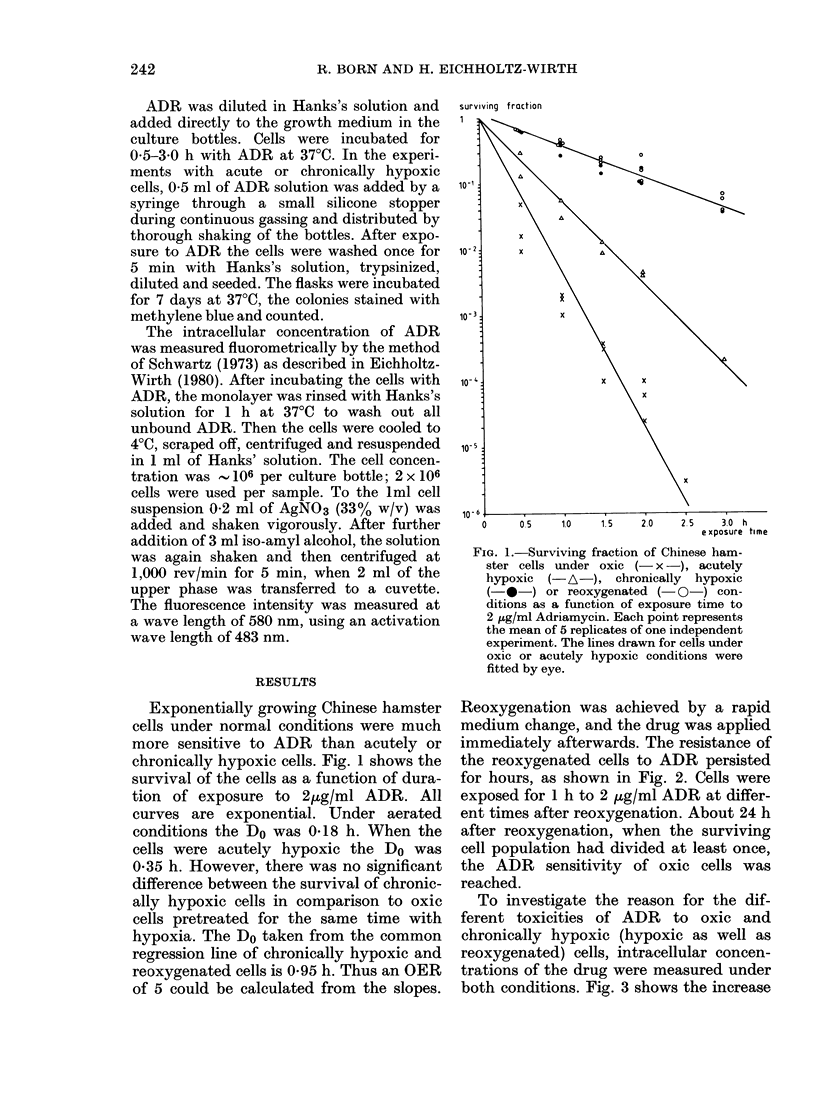
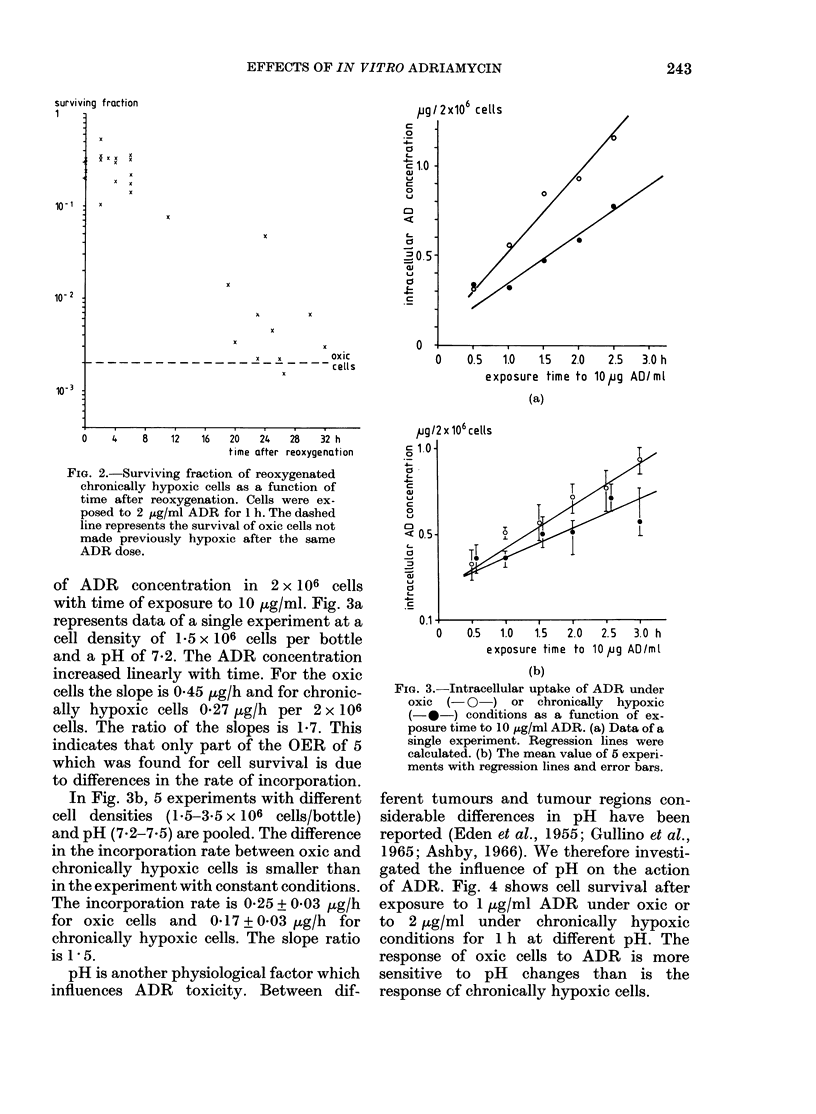
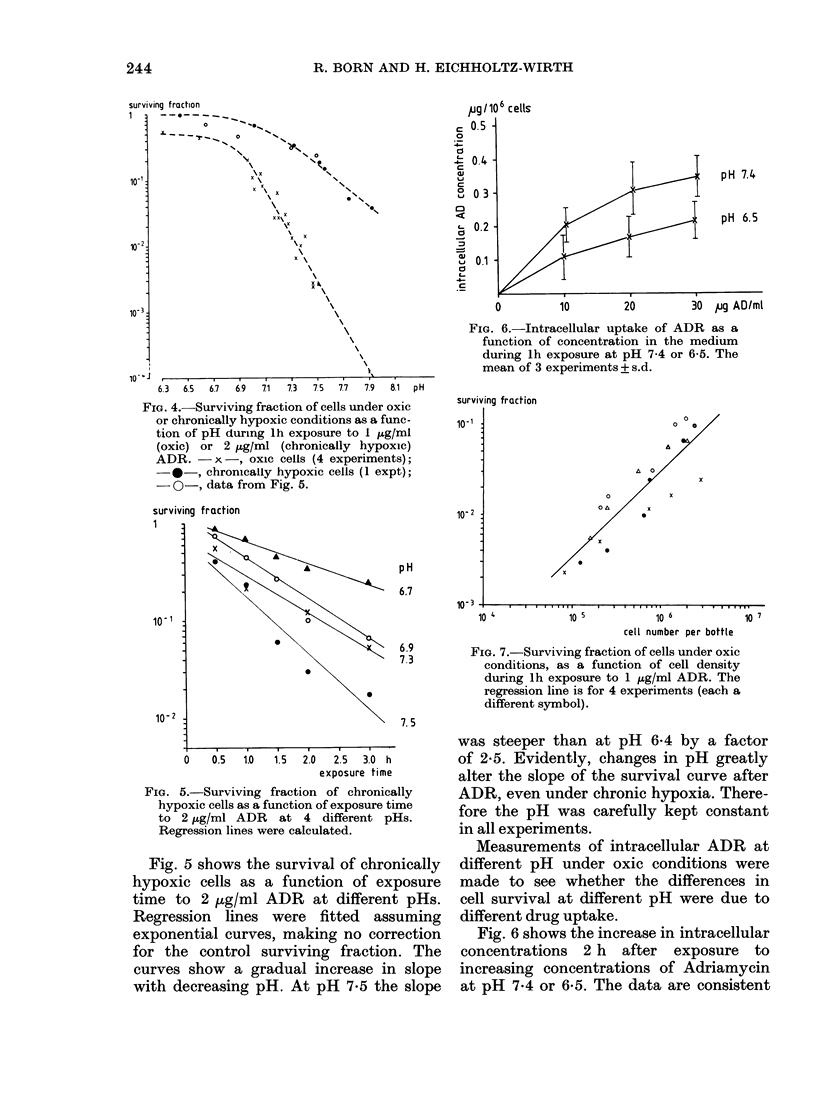
Chronically hypoxic cells were 5 times more resistant to Adriamycin (ADR) than exponentially growing oxic cells. On reoxygenation, resistance decreased slowly to reach the ADR sensitivity of oxic cells after 24 h. With increasing pH, ADR efficiency increased more in oxic than in chronically hypoxic cells. With increasing cell density, ADR efficiency decreased linearly. The differences in ADR efficiency under the various conditions were accompanied by differences in intracellular ADR uptake. Chronically hypoxic cells incorporated 1.6 times less than oxic cells; the incorporation rate at pH 6.5 was half that at pH 7.4; and at a cell density of 5 X 10(5)/bottle the intracellular uptake was 6 times that at 5 X 10(6)/bottle. The observed differences in uptake of ADR were not, however, sufficient to explain the differences in cytotoxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby B. S. pH studies in human malignant tumours. Lancet. 1966 Aug 6;2(7458):312–315. doi: 10.1016/s0140-6736(66)92598-0. [DOI] [PubMed] [Google Scholar]
- Born R., Hug O., Trott K. R. The effect of prolonged hypoxia on growth and viability of Chinese hamster cells. Int J Radiat Oncol Biol Phys. 1976 Jul-Aug;1(7-8):687–697. doi: 10.1016/0360-3016(76)90151-6. [DOI] [PubMed] [Google Scholar]
- EDEN M., HAINES B., KAHLER H. The pH of rat tumors measured in vivo. J Natl Cancer Inst. 1955 Oct;16(2):541–556. [PubMed] [Google Scholar]
- Eichholtz-Wirth H. Dependence of the cytostatic effect of adriamycin on drug concenration and exposure time in vitro. Br J Cancer. 1980 Jun;41(6):886–891. doi: 10.1038/bjc.1980.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Smith S. H., Haggerty A. C. Modifications of the acid-base status of the internal milieu of tumors. J Natl Cancer Inst. 1965 Jun;34(6):857–869. [PubMed] [Google Scholar]
- Harris J. W., Shrieve D. C. Effects of adriamycin and X-rays on euoxic and hypoxic EMT-6 cells in vitro. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1245–1248. doi: 10.1016/0360-3016(79)90647-3. [DOI] [PubMed] [Google Scholar]
- Martin W. M., McNally N. J. Cytotoxicity of adriamycin to tumour cells in vivo and in vitro. Br J Cancer. 1980 Dec;42(6):881–889. doi: 10.1038/bjc.1980.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. M., McNally N. J. The cytotoxic action of adriamycin and cyclophosphamide on tumor cells in vitro and in vivo. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1309–1312. doi: 10.1016/0360-3016(79)90660-6. [DOI] [PubMed] [Google Scholar]
- Schwartz H. S. A fluorometric assay for daunomycin and adriamycin in animal tissues. Biochem Med. 1973 Jun;7(3):396–404. doi: 10.1016/0006-2944(73)90060-4. [DOI] [PubMed] [Google Scholar]
- Smith E., Stratford I. J., Adams G. E. Cytotoxicity of adriamycin on aerobic and hypoxic chinese hamster V79 cells in vitro. Br J Cancer. 1980 Oct;42(4):568–573. doi: 10.1038/bjc.1980.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. M., Eddy H. A., Bareham B., Reich K., Vanantwerp D. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1225–1230. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]



