Abstract
Nontypeable Haemophilus influenzae (NTHI) is an important etiological agent of otitis media (OM) and of exacerbated chronic obstructive pulmonary diseases (COPD). Inflammation is a hallmark of both diseases. Interleukin-8 (IL-8), one of the important inflammatory mediators, is induced by NTHI and may play a significant role in the pathogenesis of these diseases. Our studies demonstrated that a soluble cytoplasmic fraction (SCF) from NTHI induced much greater IL-8 expression by human epithelial cells than did NTHI lipooligosaccharides and envelope proteins. The IL-8-inducing activity was associated with molecules of ≤3 kDa from SCF and was peptidase and lipase sensitive, suggesting that small lipopeptides are responsible for the strong IL-8 induction. Moreover, multiple intracellular signaling pathways were activated in response to cytoplasmic molecules. The results indicated that the p38 mitogen-activated protein kinase (MAPK) and Src-dependent Raf-1-Mek1/2-extracellular signal-regulated kinase mitogen-activated protein kinase (ERK MAPK) pathways are required for NTHI-induced IL-8 production. In contrast, the phosphatidylinositol 3-kinase (PI3K)-Akt pathway did not affect IL-8 expression, although this pathway was concomitantly activated upon exposure to NTHI SCF. The PI3K-Akt pathway was also directly activated by IL-8 and significantly inhibited by an antagonist of IL-8 receptors during NTHI stimulation. These results indicated that the PI3K-Akt pathway is activated in response to IL-8 that is induced by NTHI and may lead to other important epithelial cell responses. This work provides insight into essential molecular and cellular events that may impact on the pathogenesis of OM and COPD and identifies rational targets for anti-inflammatory intervention.
Nontypeable Haemophilus influenzae (NTHI) is a gram-negative common commensal of the human respiratory tract. It continues to be an important pathogen of the majority of mucosal diseases including otitis media (OM) (32) in children and lower respiratory infections in adults with chronic obstructive pulmonary diseases (COPD) (19). Both diseases are characterized by inflammatory responses with neutrophil infiltration in the infected tissue (30), which mainly depends on the secretion of chemokines such as interleukin-8 (IL-8). The resulting inflammation response leads to release of neutrophil-generated mediators such as elastase and reactive oxygen species to eradicate the bacteria. However, uncontrolled inflammation due to overproduction of proinflammatory cytokines and chemokines can cause indirect host-mediated tissue damage. Emerging studies revealed that higher-than-normal levels of IL-8 were detected in most middle ear effusions of OM (17, 23) and sputum of COPD (27), suggesting that IL-8 contributes to this pathology. This is further supported by an animal model in which direct injection of IL-8 into the murine middle ear induced leukocyte accumulation at that site (13). Many in vitro and in vivo studies have demonstrated that NTHI significantly induces IL-8 production by epithelial cells (7, 14) and in the middle ear effusions of the animal (25). There are also indications that NTHI causes a greater inflammatory response in the middle ear than does another OM pathogen, Streptococcus pneumoniae (18). However, the mechanisms underlying IL-8 up-regulation by NTHI have not been elucidated, and the responsible NTHI molecules remain largely undefined.
It was recently demonstrated that a soluble cytoplasmic fraction (SCF) of NTHI, rather than lipooligosaccharides (LOS) and outer membrane proteins of this bacterium, maximally induced activation of the mucin gene MUC5AC (33). Other investigators also reported that LOS and other surface molecules of NTHI appear not to play a significant role in production of inflammatory mediators, including IL-8 (5, 7). These observations strongly suggested that something other than surface molecules are important and led us to hypothesize that NTHI SCF might be responsible for the overproduction of IL-8 and subsequent tissue damage in NTHI infection. Our results indicated that SCF released from broken NTHI cells markedly activates IL-8 expression to a significantly greater degree than do other NTHI surface molecules. In addition, we demonstrated that the small molecules of NTHI SCF sensitive to peptidase and lipase are responsible for the overproduction of IL-8 in NTHI infection, suggesting that they might be a new category of bacterial inflammation stimulators.
Insight into cellular signaling pathways used by NTHI to induce inflammatory cytokines might help generate novel approaches for the treatment of NTHI-mediated inflammatory disorders. Therefore, we investigated the signaling pathways that lead to the NTHI-induced IL-8 response. Our results indicated that p38 is required for NTHI-induced IL-8 expression. Moreover, additional mitogen-activated protein (MAP) kinase-extracellular signal-regulated kinase (ERK) was activated by NTHI SCF and required for the expression of IL-8. Interestingly, the phosphatidylinositol 3-kinase (PI3K)-Akt pathway, unlike its role in MUC5AC activation, was not involved in IL-8 induction, whereas it was indirectly activated by NTHI through IL-8. These findings connect MUC5AC and IL-8 responses during NTHI infection and provide a broader picture of NTHI pathogenesis. The involved signaling pathways are possible targets for therapeutic intervention in NTHI-induced pathology.
MATERIALS AND METHODS
Reagents.
SB 203580, PD 98059, UO 126, MG 132, wortmannin, caffeic acid phenethylester (CAPE), and SB 225002 were purchased from Calbiochem-Novabiochem Corporation (La Jolla, Calif.). NTHI LOS were a gift from X. X. Gu (Laboratory of Immunology, National Institute on Deafness and Other Communication Disorders). Polymyxin B, peptidase, lipase, and DNase were purchased from Sigma. RNase is a product from Promega (Madison, Wis.). Recombinant human IL-8 was purchased from R&D Systems Inc.
Bacterial strains and culture conditions.
NTHI strain 12 used in this study is a clinical isolate that was described previously (33). The strain was grown in liquid brain heart infusion supplemented with NAD (3.5 μg/ml) and hemin (5 μg/ml) at 37°C with 5% CO2.
Preparation of NTHI cytoplasmic components.
NTHI bacterial cells were harvested when they reached middle to late log phase and resuspended in phosphate-buffered saline (PBS) or double-distilled H2O at the same (1×) or one-third of the original volume (3×). This bacterial cell suspension was sonicated on ice three times at 150 W for 3 min with 5-min intervals between each sonication (Branson Sonifier 250). After residual cells and cell debris were removed by centrifugation (10,000 × g, 4°C for 10 min, Beckman Avanti J-25I), envelope proteins and SCF were separated by ultracentrifugation (100,000 × g, 4°C for 1 h, Beckman Optima XL-100K) and stored at −80°C for later analyses.
Cell culture.
The HeLa cells (human cervix epithelial cells) were cultured in minimal essential medium. HM3 (human colon epithelial) cells were maintained in Dulbecco's modified Eagle's medium. A549 cells (human lung epithelial cells) were maintained in F-12K nutrient mixture medium. All media contained 10% fetal bovine serum (Invitrogen), penicillin (100 U/ml), and streptomycin (0.1 mg/ml).
Reverse transcriptase PCR (RT-PCR) and enzyme-linked immunosorbent assay analyses of IL-8.
Tissue culture dishes (6 cm in diameter) were seeded with 1.5 × 105 HeLa cells in a 5-ml volume of complete Dulbecco's modified Eagle's medium and incubated for 20 h. The cells were starved in serum-free medium for 18 h and treated with or without NTHI SCF in duplicate for 3 and 5 h. Total RNA was extracted from the lysed cells with an RNeasy minikit (Qiagen Inc., Valencia, Calif.) according to the manufacturer's instructions and treated with RNase-free DNase I. cDNAs were synthesized with Moloney murine leukemia virus RT (Superscript II; Life Sciences, Gaithersburg, Md.) using random hexadeoxynucleotides as primers (Promega). After DNA synthesis, the RT was inactivated by heating the sample at 95°C for 10 min. IL-8 cDNA was amplified with primers 5′-AAC ATG ACT TCC AAG CTG GCC-3′ and 5′-TTA TGA ATT CTC AGC CCT CTT C-3′, and cyclophilin was amplified with 5′-CCG TGT TCT TCG ACA TTG CC-3′ and 5′-ACA CCA CAT GCT TGC CAT CC-3′. PCR was performed for 15 min at 95°C, 1 min at 94°C, 1 min at 59°C (50°C for cyclophilin), and 1 min at 72°C for each cycle and 7 min at 72°C after all the cycles. A cycle number that was in the linear range of amplification was selected for PCR analysis: 30 cycles for IL-8 and 26 for cyclophilin. IL-8 protein in the supernatants of HeLa cells treated with NTHI SCF was measured with an IL-8 kit (Biosource International, Inc.) according to the manufacturer's instructions.
Plasmids, transfection, and luciferase assays.
The reporter construct, IL-8 (IL-8-Luc), contains the −135 to +46 bp promoter region of the human IL-8 gene in a luciferase reporter vector, pGL2 (35). Transient transfections and cotransfections of cells were performed in triplicate with Trans IT-LT1 (Panvera, Madison, Wis.) according to the manufacturer's instructions. Forty-two hours after the transfection, the cells were treated with NTHI samples for 4 h and harvested with lysis buffer (250 mM Tris-HCl [pH 7.5], 0.1% Triton X-100, and 1 mM dithiothreitol). Luciferase assays were performed by adding luciferase assay substrate (Promega), 50 μl in 10 μl of cell lysate, on a Monolight 3010 luminometer for 15 s (Analytical Luminescence, San Diego, Calif.). The NTHI-dependent fold induction was calculated relative to the luciferase light units obtained in the absence of NTHI SCF treatment. The normalized luciferase activity was thus expressed as relative luciferase activity (fold activity). For experiments with inhibitors, epithelial cells transfected with IL-8-Luc were pretreated with inhibitors for 1 to 2 h, then treated with NTHI SCF for 4 h, and harvested for luciferase assays.
Western blot analysis.
Epithelial cells were treated with or without NTHI SCF. Twenty-five micrograms of total cell lysate protein in each sample was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and antibodies to phospho-p38 (Thr-180/182), p38, phospho-Akt (Ser-473), Akt, phospho-ERK, and ERK (New England Biolabs, Beverly, Mass.) as described in the manufacturer's instructions. For experiments with inhibitors, epithelial cells were pretreated with the inhibitors for 2 h and then treated with NTHI samples.
Statistical test.
A two-tailed Student t test was used to analyze the statistical differences between samples.
RESULTS
NTHI SCF induces strong IL-8 expression in epithelial cells.
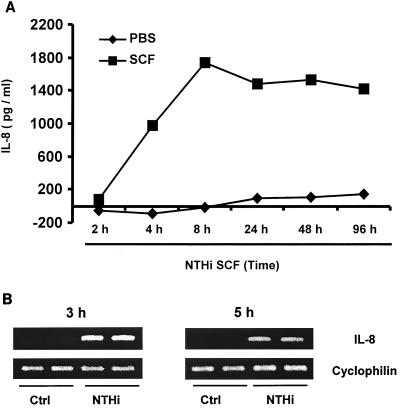
IL-8 protein levels in the supernatant of human epithelial cells were measured with an enzyme-linked immunoassay based on specific anti-IL-8 polyclonal antibody. Incubation of epithelial cells with PBS for 2 to 8 h did not induce detectable levels of IL-8. When the cells were treated with NTHI SCF, strong IL-8 induction was detected at 2 h, significantly increased by 4 h, and peaked at 8 h (Fig. 1A). The highly elevated levels of IL-8 were retained for up to 96 h. Induction of IL-8 at the mRNA level in cells was tested by RT-PCR. IL-8 mRNA expression was dramatically increased in NTHI SCF-stimulated epithelial cells, whereas no IL-8 mRNA was detected in PBS-stimulated cells at the indicated times (Fig. 1B). Thus, quantitation of both IL-8 protein and mRNA levels indicates that NTHI SCF is a strong IL-8 inducer. To further study NTHI-regulated IL-8 expression at the transcriptional level, an IL-8 reporter (IL-8-Luc) was constructed and used to transfect human epithelial cells. The luciferase activity was significantly increased in A549 (human airway epithelial cells) (Fig. 2A) and HeLa (Fig. 2B) cells treated with NTHI SCF, confirming that NTHI SCF induces IL-8 expression at the transcriptional level and that the IL-8 response to NTHI is similar in the two cell lines.
FIG. 1.
NTHI SCF induces strong IL-8 expression in epithelial cells. (A) IL-8 in cell culture supernatants was quantified using an IL-8-specific enzyme-linked immunosorbent assay kit according to the manufacturer's instructions. Cells were treated with NTHI SCF when they reached 50% confluence. Cell culture supernatants were collected at various times and stored at −20°C until all samples were ready for the assay. Data are averages of duplicate wells. (B) RT-PCR analysis of IL-8 mRNA was performed using total RNA extracted from HeLa cells stimulated with NTHI SCF or PBS in duplicate for 3 and 5 h. The cyclophilin transcripts served as an internal control for the amount of RNA used in each reaction.
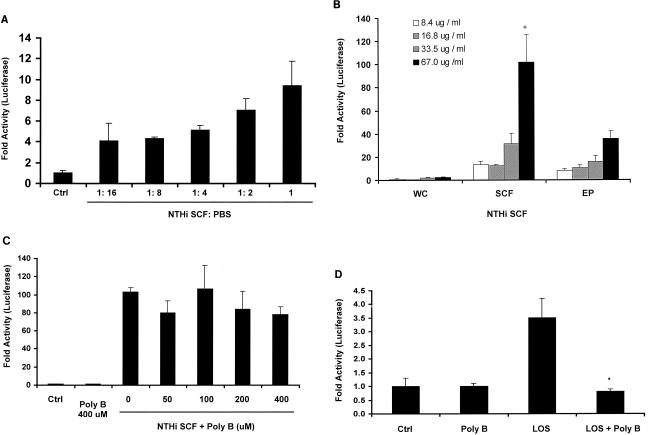
FIG. 2.
NTHI SCF is a more potent IL-8 inducer than LOS and EFs are. (A) A549 (human airway epithelial) cells were transfected with the IL-8-Luc reporter and exposed to NTHI samples in a series of dilutions. (B) SCF and EF were prepared as described in Materials and Methods. IL-8-Luc-transfected HeLa cells were treated with each fraction for 4 h and then lysed for luciferase assays. IL-8-inducing activity in SCF samples is significantly different from that in EF samples at 67.0 μg/ml (*, P ≤ 0.01). (C) NTHI SCF was pretreated with various concentrations of polymyxin B for 10 min at room temperature and then added to IL-8-Luc-transfected HeLa cells. (D) NTHI LOS was sonicated and pretreated with polymyxin B (200 μM) or PBS. The activity in polymyxin B-treated LOS is significantly different from that in nontreated LOS (*, P ≤ 0.05). Data are presented as means ± standard deviations of three individual treatments. Results are representative of two separate experiments.
NTHI SCF is a more potent IL-8 inducer than are NTHI LOS and EFs.
To compare the contribution of NTHI SCF with those of other NTHI fractions in IL-8 expression, NTHI cells were separated into SCF and envelope fraction (EF) following sonication and ultracentrifugation (Materials and Methods). The ability of these fractions to induce IL-8 was examined in IL-8-Luc-transfected epithelial cells. Live whole bacterial cells did not stimulate significant activation of IL-8 promoter after 4 h of incubation, whereas treatment of the cells with SCF induced a strong IL-8 promoter activation. The EF also activated the IL-8 promoter; however, the activity was significantly lower than the activity of SCF at higher concentrations (Fig. 2B). To determine whether IL-8-inducing activity is broadly associated with different NTHI strains, SCFs from nine NTHI clinical isolates were tested. All of them stimulated high but variable levels of IL-8 promoter activation, indicating that the capacity to induce IL-8 is a common property of this species. Considering that LOS of NTHI is an important inducer of proinflammatory cytokines (31), the possibility that SCF was contaminated by LOS was examined. SCF samples were pretreated with polymyxin B to neutralize LOS activity and then incubated with the cells. As shown in Fig. 2C polymyxin B did not prevent the IL-8 induction by SCF. To further examine the contribution of NTHI LOS in IL-8 expression and to ensure the effectiveness of polymyxin B, IL-8-Luc-transfected cells were incubated with LOS and polymyxin B-pretreated LOS. As shown in Fig. 2D NTHI LOS (10 μg/ml) was able to induce IL-8 expression; however, the activity was much lower, less than 4% of SCF activity. Moreover, polymyxin B efficiently abolished LOS activity, indicating that NTHI LOS does not contribute to the strong IL-8-inducing activity of SCF. The culture supernatant of NTHI bacteria was also tested for a secreted IL-8-inducing activity. Only a slightly increased level of IL-8 expression was observed in cells treated with NTHI culture filtrate (data not shown). Taken together, these results clearly demonstrated that the SCF is the most potent fraction responsible for NTHI-induced IL-8.
The IL-8 inducers in NTHI SCF are small and sensitive to peptidase and lipase.
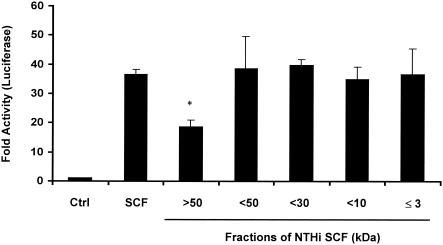
To characterize the IL-8 inducers, NTHI SCF was further fractionated by a series of filters with different pore sizes (Centriplus). Fractions were tested for their IL-8-inducing activity. As shown in Fig. 3, activity was reduced by 50% when molecules smaller than 50 kDa were removed from the SCF. However, the full activity was retained in all fractions containing molecules of ≤3 kDa in size. To determine the biochemical nature of the inducers, the ≤3-kDa fraction was treated with various enzymes at 37°C overnight. The samples were heated to destroy residual activity of enzymes before they were added to epithelial cells. The nontreated ≤3-kDa fraction and enzymes alone in the buffer were also heated and used as controls. The activity in the ≤3-kDa fraction was reduced by 90% when treated with peptidase and lipase but was insensitive to DNase and RNase (data not shown). These results indicated that peptide and ester bonds are required for the IL-8-inducing activity.
FIG. 3.
The IL-8 inducers in NTHI SCF are small and sensitive to peptidase and lipase. NTHI SCF was fractionated with a Centriplus (Millipore) fractionator with different pore sizes according to the manufacturer's instructions. Fractions were tested on IL-8-Luc-transfected epithelial cells for luciferase activity. IL-8-inducing activity in the fraction of >50 kDa is significantly different from that in other fractions (*, P ≤ 0.001). Data are presented as means ± standard deviations of three individual treatments. Results are representative of two separate experiments.
Activation of MAP kinase p38 by NTHI SCF is required for IL-8 up-regulation.
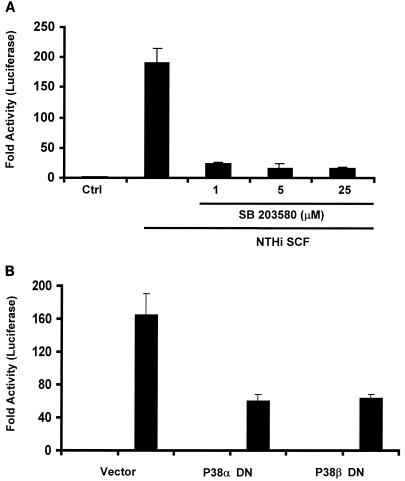
MAP kinase p38 has been found to play an essential role in production of proinflammatory cytokines. We previously found that activation of MAP kinase p38 was induced by NTHI SCF and required for induction of mucin expression (33). To determine the role of p38 activation in NTHI-induced IL-8 expression, we examined the effect of pyridinylimidazole SB 203580, which specifically inhibits phosphorylation of downstream kinases by p38 isoforms α and β. As shown in Fig. 4A, SB 203580 inhibited NTHI SCF-induced IL-8 expression by 90%, indicating that the p38 pathway is required for NTHI-induced IL-8 expression. To confirm the requirement and to determine the contribution of each p38 isoform, we overexpressed dominant-negative forms of p38α and p38β in epithelial cells and examined their effects on NTHI-induced IL-8 expression. The results in Fig. 4B showed that overexpression of either mutant significantly inhibited IL-8 up-regulation. Co-overexpression of both p38 mutants did not significantly further reduce IL-8 expression (data not shown), indicating that inhibition is not additive. These results confirmed that the p38 pathway is also required for NTHI-induced IL-8 expression and suggested that both p38 isoforms, α and β, are involved in IL-8 induction.
FIG. 4.
Activation of MAP kinase p38 by NTHI SCF is required for IL-8 up-regulation. (A) HM3 cells were pretreated with SB 203580 or dimethyl sulfoxide, the solvent for SB 203580, for 1.5 h and were then incubated with NTHI SCF for 4 h before being lysed for luciferase assays. Activities associated with SB 203580-treated cells are significantly different from control (P ≤ 0.005). (B) HM3 cells were cotransfected with IL-8-Luc and a dominant-negative form of either p38α (p38α DN) or p38β (p38β DN). Forty-two hours after transfection, cells were treated with NTHI SCF for 4 h and then assayed for luciferase activity. Cells transfected with the vector alone served as a control. The activities in p38α- and p38β-cotransfected cells are significantly lower than that of the vector control (P ≤ 0.05). Data are presented as means ± standard deviations of three individual treatments. Results are representative of three separate experiments.
MAP kinase ERK is activated by NTHI SCF, and activation is required for induction of IL-8.
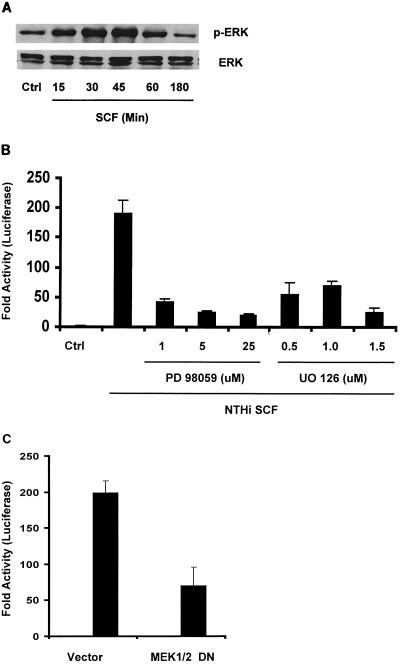
Another MAP kinase ERK has been found to be activated by an increasing number of infectious microbes and involved in microbially induced up-regulation of cytokines (9, 24, 26). The classical ERK pathway is the best-studied protein kinase cascade. Binding of ligands to receptor tyrosine kinases activates the protein kinase Src, which in turn activates Ras, c-Raf, MEK, and the ERK1/2 MAP kinase pathway to link extracellular signals with transcription in the nucleus (22). To determine a possible role for the ERK pathway in IL-8 up-regulation, we first examined ERK phosphorylation. Western blot analysis showed that ERK kinases were strongly activated when epithelial cells were incubated with NTHI SCF (Fig. 5A). Phosphorylated ERK1/2 significantly increased at 15 min, peaked at 45 min, and returned to control levels by 180 min. To identify the biological significance of ERK activation in NTHI-induced IL-8 up-regulation, we tested the effects of PD 98059 and UO 126, which selectively inhibit MEK1/2, a MAP kinase that phosphorylates ERK1/2. As shown in Fig. 5B, both inhibitors significantly prevented NTHI-induced IL-8 up-regulation, indicating that the ERK pathway is another MAP kinase pathway required for the IL-8 induction. To confirm the requirement for the ERK pathway, cells were cotransfected with IL-8-Luc and a dominant-negative form of MEK1/2 (MEK1/2 DN) and then treated with NTHI SCF. Consistent with inhibitor studies, NTHI-induced IL-8 expression was significantly reduced by overexpression of MEK1/2 DN (Fig. 5C). Since MEK1/2 can be activated by different upstream kinases (11), the involvement of Raf, a kinase directly upstream of MEK1/2 in the classical ERK pathway, was evaluated. Overexpression of a dominant-negative form of Raf-1 decreased IL-8-inducing activity by 76% (data not shown). Similarly, overexpression of a dominant form of Src, a more upstream kinase of this pathway, also strongly inhibited IL-8 induction (87%) (data not shown). By using a series of kinase mutants in the pathway, we demonstrated that the classical ERK pathway is activated and involved in NTHI-induced IL-8 expression.
FIG. 5.
MAP kinase ERK is activated by NTHI SCF, and activation is required for IL-8 induction. (A) HeLa cells were treated with NTHI SCF or PBS and then lysed at various times. Equal amounts of proteins from extracts were analyzed by Western blotting with antibodies to phospho-ERK or ERK. (B) IL-8-Luc-transfected HeLa cells were pretreated with selective MEK1/2 inhibitors, PD 98059 and UO 126, for 1.5 h at various doses. Cells were treated with dimethyl sulfoxide alone for a solvent control. Cells were then treated with NTHI SCF for 4 h and lysed for luciferase assay. The activity in cells treated with either inhibitor is significantly different from that in the vector control (P ≤ 0.05). (C) HeLa cells were cotransfected with IL-8-Luc and a dominant-negative form of MEK1/2 (MEK1/2 DN). Forty-two hours after transfection, the cells were treated with or without NTHI SCF for 4 h and assayed for luciferase activity. Vector alone served as the negative control. The activity in cells cotransfected with the dominant-negative form is significantly different from that of the vector control (P ≤ 0.05). Data are presented as means ± standard deviations of three individual treatments. Results are representative of two separate experiments.
The NF-κB is another signaling pathway activated by NTHI SCF and required for IL-8 induction.
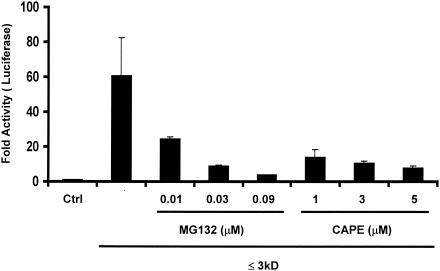
The nuclear transcription factor NF-κB is one of the most important cellular factors involved in the regulation of the host innate antimicrobial response by inducing an array of proinflammatory cytokines and chemokines (21). Many bacterial pathogens induce activation of NF-κB, which in turn binds to the IL-8 promoter to activate IL-8 expression (4). Aberrant activation of the NF-κB pathway appears to drive a number of inflammatory diseases. It was previously found that this pathway is strongly activated by NTHI whole-cell lysates (28) and that NTHI SCF is an even stronger activator (data not shown). To determine whether NF-κB production leads to IL-8 induction, cells were pretreated with two NF-κB inhibitors that act by different mechanisms before they were exposed to NTHI SCF. As shown in Fig. 6, IL-8 responses to NTHI SCF were reduced by up to 94% with MG 132 and up to 87% with CAPE in a dose-dependent manner. Therefore, NF-κB activation is also required for the IL-8 response to NTHI.
FIG. 6.
NF-κB is another signaling pathway activated by NTHI SCF and required for IL-8 induction. IL-8-Luc-transfected HeLa cells were pretreated with NF-κB inhibitors, MG 132 and CAPE, for 1.5 h at various doses. The cells were then treated with the ≤3-kDa fraction for 4 h and lysed for luciferase assay. Control cells were pretreated with dimethyl sulfoxide and treated with a buffer for the ≤3-kDa fraction. The activities in cells treated with inhibitors are significantly different from those for control (P ≤ 0.05). Data are presented as means ± standard deviations of three individual treatments. Results are representative of two separate experiments.
The activation of the PI3K-Akt pathway by NTHI is not required for, but is dependent on, NTHI-induced IL-8 expression.
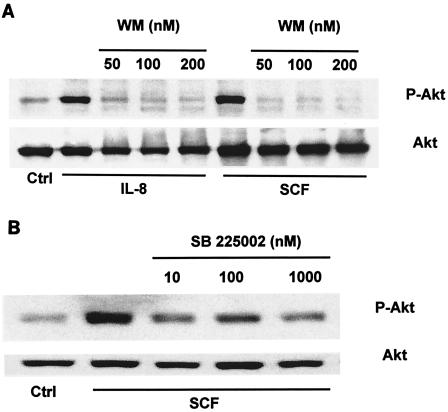
PI3K-Akt is another important signaling pathway activated by most growth factors and is required for IL-8 expression (1, 2, 6, 16). We previously demonstrated that NTHI SCF strongly activated the PI3K-Akt pathway of epithelial cells, which down-regulated NTHI-induced mucin expression (33). A connection between activation of the PI3K-Akt pathway and IL-8 expression induced by NTHI was investigated. Cells were pretreated with wortmannin, a specific inhibitor of PI3K, and then examined for NTHI-induced IL-8 expression. Interestingly, wortmannin did not affect IL-8 expression (data not shown), suggesting that PI3K is not involved in the NTHI-induced IL-8 expression. Recent studies showed that stimulation of neutrophils by chemotactic factors was associated with PI3K activation (20) and that the PI3K of human neutrophils was activated by IL-8 (15). These observation and our results led to the hypothesis that NTHI activates the PI3K-Akt pathway through IL-8 expression. To test this hypothesis, we first examined the effect of IL-8 on Akt activation in epithelial cells. As we expected, direct stimulation of epithelial cells with recombinant IL-8 substantially activated Akt (Fig. 7A) with kinetics similar to those of NTHI SCF-induced Akt activation (33) (data not shown). IL-8-induced Akt activation was abolished by pretreatment of the cells with wortmannin, indicating that Akt activation is under the control of PI3K. These results suggested that the PI3K-Akt pathway responds to IL-8 but did not show that NTHI activated PI3K-Akt through the expression of IL-8. We next pretreated cells with SB 225002 [N-(2-hydroxy-4-nitrophenyl)-N′-(2-bromophenyl)urea, a strong selective antagonist of the IL-8 receptor CXCR2 (34), to block IL-8 from binding to its receptor. As shown in Fig. 7B, SB 225002 significantly prevented NTHI SCF-induced Akt activation, indicating that the PI3K-Akt pathway is activated by NTHI through an IL-8 feedback loop.
FIG. 7.
Activation of the PI3K-Akt pathway by NTHI is not required for, but is dependent on, NTHI-induced IL-8 expression. (A) HM3 cells were pretreated with wortmannin (WM) for 2 h and were then treated with human recombinant IL-8 (25 ng/ml) or NTHI SCF for 30 min. Control cells were pretreated with dimethyl sulfoxide and treated with PBS. Results are representative of two separate experiments. (B) HM3 cells were pretreated with SB 225002 for 2 h and then treated with NTHI SCF for 30 min. Control cells were pretreated with dimethyl sulfoxide and treated with PBS. Equal amounts of proteins from each cell lysate were analyzed by Western blotting with antibodies to phospho-Akt or Akt.
DISCUSSION
IL-8-mediated neutrophil infiltration and activation in response to bacterial infection are a host defense mechanism for eradication of invading bacteria, but unrestricted they can lead to massive leukocyte recruitment and subsequently tissue destruction. NTHI is an important cause of OM and lung infection in COPD. Both are characterized by localized neutrophil infiltration and tissue damage. Local IL-8 accumulation is another hallmark of these conditions. However, direct evidence of IL-8 induction by NTHI at molecular and cellular levels was not available. In this study mechanisms of IL-8 expression in response to NTHI were investigated. We demonstrate that small soluble cytoplasmic molecules from NTHI are responsible for IL-8 up-regulation by epithelial cells and that multiple MAP kinase signaling pathways are required for IL-8 induction. In addition, PI3K-Akt is found to be activated downstream of IL-8 and could be involved in other epithelial cellular responses to NTHI.
Bacteria are able to interact by direct contact with host cells via surface structures. Recently it was reported that the most potent mucin inducers from NTHI are neither surface associated nor secreted (33) but are soluble cytoplasmic molecules (SCF). Here we demonstrate that IL-8 expression was also strongly induced by NTHI SCF. Unlike mucins, which mechanically contribute to the pathogenesis of mucosal infections by blocking airways or middle ear conductance, IL-8 triggers detrimental inflammation. Thus, IL-8 up-regulation by NTHI SCF may initiate procedures which lead to NTHI-induced pathology. The release of SCF from NTHI is not understood. However, autolysis of NTHI occurs in unfavorable growth conditions or is induced by antibiotic treatment. Obviously in vivo studies are needed to assess its clinical relevance to our observations.
The IL-8-inducing activity of NTHI SCF is associated with the ≤3-kDa bacterial fraction and is sensitive to peptidase and lipase, suggesting that the responsible molecules are likely lipopeptides. The fact that IL-8-inducing activity was not impaired by polymyxin B but was destroyed by peptidase supports the conclusion that NTHI LOS is not responsible for significant IL-8 induction. Furthermore, serum, a rich source of CD14 and lipopolysaccharide binding protein, did not enhance ≤3-kDa-fraction-induced IL-8 expression (data not shown), further suggesting that NTHI LOS is not the strong inducer of IL-8 by epithelial cells. Bacterial lipoproteins are another group of cell wall- or membrane-associated molecules known to activate the innate immune system. The lipopeptide-like property of the IL-8 inducers suggests that they may be related to bacterial lipoproteins, which are known to induce cytokines. However, their cytoplasmic location and the small molecular size suggest that they are distinct from all reported natural bacterial lipoproteins. Identification of the active component of SCF is ongoing.
Activation of multiple cellular signaling pathways by NTHI SCF is consistent with NTHI SCF being an important and potent inflammatory stimulator. We demonstrate that the p38 pathway is required for IL-8 expression. The fact that NTHI SCF up-regulated expression of both IL-8 and mucin through activation of p38 shows the relevance of these responses in the host defense against NTHI infection and supports a common pathogenic mechanism in OM and COPD. Therefore, the p38 pathway is a potential target for therapeutic intervention in NTHI inflammation.
NTHI SCF also activates another MAP kinase, ERK1/2. Interestingly, this pathway is not significantly involved in NTHI-induced mucin expression (33) but is clearly required for the expression of IL-8. This pathway could also be a new therapeutic target. The ERK is an important pathway leading to activation of NF-κB, the prominent transcription factor of IL-8 and other cytokines. The Ras-Raf-MEK-MAP-pp90rsk kinase pathway phosphorylates IκBα, which initiates its degradation and subsequent NF-κB translocation to the nucleus (10). The MEK1/2 inhibitor UO 126 significantly reduced both NF-κB and IL-8 secretion (1). This mechanism led to induction of IL-8 expression by a Pseudomonas autoinducer (29). In this study we found that both the ERK and NF-κB pathways were required for NTHI-induced IL-8 induction. Although an NF-κB-independent mechanism has been reported for nonbacterially induced IL-8 expression (12, 35), further studies will very likely show that the ERK pathway leads to IL-8 transcription by inducing activation of NF-κB.
The PI3K-Akt pathway is involved in expression of IL-8 and other proinflammatory cytokines (1, 8). It was previously demonstrated that PI3K-Akt down-regulated NTHI SCF-induced mucin expression through a negative effect on p38 activation (33). Here, however, we demonstrate that IL-8 induction by NTHI is independent of the PI3K-Akt signaling pathway. Moreover, our experiments indicate that PI3K-Akt is activated in response to IL-8 in epithelial cells. The biological significance of PI3K-Akt activation beyond mucin regulation remains to be investigated. Evidently IL-8 is a pivotal regulator of cellular processes through the PI3K-Akt pathway and may also be involved in NTHI pathogenesis in addition to its chemoattractant activity.
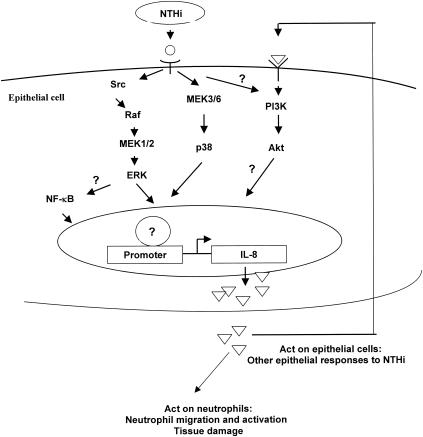
Although IL-8 is important for host defenses, it clearly also has a pathological impact. As depicted in Fig. 8, we speculate that in OM and COPD NTHI cells undergo autolysis due to an unfavorable host environment, such as oxidative stress or nutrient deprivation. A released small cytoplasmic molecule binds to receptors on epithelial cells, which triggers a cascade of multiple cellular signaling pathways, including the p38, ERK, and NF-κB pathways. Activation of these pathways leads to intense IL-8 expression by epithelial cells to defend against NTHI infection. Uncontrolled high levels of IL-8 will cause a massive infiltration of neutrophils into the middle ear or lungs and subsequently result in neutrophil-generated mediators, such as elastase and reactive oxygen species, which can lead to tissue destruction. In addition, IL-8 binds to receptors of epithelial cells and activates the PI3K-Akt pathway that leads to cell proliferation, antiapoptosis, and cytoskeleton rearrangement (3) and other possible epithelial responses to NTHI. Control of neutrophil recruitment and activation in infected tissue by interruption of the signaling cascade would appear to be an attractive strategy for therapeutic intervention. Structural analogs that antagonize the ability of NTHI SCF to induce IL-8 may be a useful means to control inflammation-mediated tissue injury in NTHI infections.
FIG. 8.
Schematic diagram showing the signaling pathways involved in NTHI-induced IL-8 transcription. The small cytoplasmic molecules (○) released from lysed NTHI cells activate p38 and ERK MAP kinase pathways, and probably NF-κB through the ERK pathway, leading to IL-8 expression (▵). High levels of IL-8 secreted by epithelial cells cause massive infiltration and activation of neutrophils in infected tissue, resulting in neutrophil-mediated tissue destruction. The PI3K-Akt pathway of epithelial cells is also activated due to interaction of IL-8 with receptor, which may account for other epithelial responses to NTHI infection.
Acknowledgments
We thank Jiahuai Han for providing p38 expression plasmids.
This work is supported by Public Health Service grants DC005569-01A1 (B.W.) and DC04562 (J.-D.L.) from the National Institutes of Health.
Editor: J. D. Clements
REFERENCES
- 1.Bancroft, C. C., Z. Chen, J. Yeh, J. B. Sunwoo, N. T. Yeh, S. Jackson, C. Jackson, and C. Van Waes. 2002. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-κB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int. J. Cancer 99:538-548. [DOI] [PubMed] [Google Scholar]
- 2.Barchowsky, A., N. V. Soucy, K. A. O'Hara, J. Hwa, T. L. Noreault, and A. S. Andrew. 2002. A novel pathway for nickel-induced interleukin-8 expression. J. Biol. Chem. 277:24225-24231. [DOI] [PubMed] [Google Scholar]
- 3.Booth, J. W., D. Telio, E. H. Liao, S. E. McCaw, T. Matsuo, S. Grinstein, and S. D. Gray-Owen. 2003. Phosphatidylinositol 3-kinases in carcinoembryonic antigen-related cellular adhesion molecule-mediated internalization of Neisseria gonorrhoeae. J. Biol. Chem. 278:14037-14045. [DOI] [PubMed] [Google Scholar]
- 4.Chu, S. H., H. Kim, J. Y. Seo, J. W. Lim, N. Mukaida, and K. H. Kim. 2003. Role of NF-κB and AP-1 on Helicobacter pylori-induced IL-8 expression in AGS cells. Dig. Dis. Sci. 48:257-265. [DOI] [PubMed] [Google Scholar]
- 5.Clemans, D. L., R. J. Bauer, J. A. Hanson, M. V. Hobbs, J. W. St. Geme III, C. F. Marrs, and J. R. Gilsdorf. 2000. Induction of proinflammatory cytokines from human respiratory epithelial cells after stimulation by nontypeable Haemophilus influenzae. Infect. Immun. 68:4430-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong, G., Z. Chen, Z. Y. Li, N. T. Yeh, C. C. Bancroft, and C. Van Waes. 2001. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 61:5911-5918. [PubMed] [Google Scholar]
- 7.Frick, A. G., T. D. Joseph, L. Pang, A. M. Rabe, J. W. St. Geme III, and D. C. Look. 2000. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J. Immunol. 164:4185-4196. [DOI] [PubMed] [Google Scholar]
- 8.Funakoshi, M., Y. Sonoda, K. Tago, S. Tominaga, and T. Kasahara. 2001. Differential involvement of p38 mitogen-activated protein kinase and phosphatidyl inositol 3-kinase in the IL-1-mediated NF-κB and AP-1 activation. Int. Immunopharmacol. 1:595-604. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, J., B. Lemercier, S. Roman-Roman, and G. Rawadi. 1998. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-κB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:34391-34398. [DOI] [PubMed] [Google Scholar]
- 10.Ghoda, L., X. Lin, and W. C. Greene. 1997. The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IκBα and stimulates its degradation in vitro. J. Biol. Chem. 272:21281-21288. [DOI] [PubMed] [Google Scholar]
- 11.Guo, F. F., E. Kumahara, and D. Saffen. 2001. A CalDAG-GEFI/Rap1/B-Raf cassette couples M(1) muscarinic acetylcholine receptors to the activation of ERK1/2. J. Biol. Chem. 276:25568-25581. [DOI] [PubMed] [Google Scholar]
- 12.Hipp, M. S., C. Urbich, P. Mayer, J. Wischhusen, M. Weller, M. Kracht, and I. Spyridopoulos. 2002. Proteasome inhibition leads to NF-κB-independent IL-8 transactivation in human endothelial cells through induction of AP-1. Eur. J. Immunol. 32:2208-2217. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, M., G. Leonard, and D. L. Kreutzer. 1997. Murine model of interleukin-8-induced otitis media. Laryngoscope 107:1405-1408. [DOI] [PubMed] [Google Scholar]
- 14.Khair, O. A., R. J. Davies, and J. L. Devalia. 1996. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur. Respir. J. 9:1913-1922. [DOI] [PubMed] [Google Scholar]
- 15.Knall, C., G. S. Worthen, and G. L. Johnson. 1997. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc. Natl. Acad. Sci. USA 94:3052-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, T. H., H. Avraham, S. H. Lee, and S. Avraham. 2002. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J. Biol. Chem. 277:10445-10451. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell, K. S., J. E. Fitzgerald, J. A. Burleson, G. Leonard, R. Carpenter, and D. L. Kreutzer. 1994. Interleukin-8 expression in otitis media. Laryngoscope 104:989-995. [DOI] [PubMed] [Google Scholar]
- 18.Melhus, A., and A. F. Ryan. 2000. Expression of cytokine genes during pneumococcal and nontypeable Haemophilus influenzae acute otitis media in the rat. Infect. Immun. 68:4024-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy, T. F., and S. Sethi. 1992. Bacterial infection in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 146:1067-1083. [DOI] [PubMed] [Google Scholar]
- 20.Naccache, P. H., S. Levasseur, G. Lachance, S. Chakravarti, S. G. Bourgoin, and S. R. McColl. 2000. Stimulation of human neutrophils by chemotactic factors is associated with the activation of phosphatidylinositol 3-kinase gamma. J. Biol. Chem. 275:23636-23641. [DOI] [PubMed] [Google Scholar]
- 21.Naumann, M. 2000. Nuclear factor-κB activation and innate immune response in microbial pathogen infection. Biochem. Pharmacol. 60:1109-1114. [DOI] [PubMed] [Google Scholar]
- 22.Norum, J. H., K. Hart, and F. O. Levy. 2003. Ras-dependent ERK activation by the human G(s)-coupled serotonin receptors 5-HT4(b) and 5-HT7(a). J. Biol. Chem. 278:3098-3104. [DOI] [PubMed] [Google Scholar]
- 23.Pospiech, L., M. Jaworska, and M. Kubacka. 2000. Soluble l-selectin and interleukin-8 in otitis media with effusion. Auris Nasus Larynx 27:213-217. [DOI] [PubMed] [Google Scholar]
- 24.Rawadi, G., V. Ramez, B. Lemercier, and S. Roman-Roman. 1998. Activation of mitogen-activated protein kinase pathways by Mycoplasma fermentans membrane lipoproteins in murine macrophages: involvement in cytokine synthesis. J. Immunol. 160:1330-1339. [PubMed] [Google Scholar]
- 25.Sato, K., C. L. Liebeler, M. K. Quartey, C. T. Le, and G. S. Giebink. 1999. Middle ear fluid cytokine and inflammatory cell kinetics in the chinchilla otitis media model. Infect. Immun. 67:1943-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroder, N. W., D. Pfeil, B. Opitz, K. S. Michelsen, J. Amberger, U. Zahringer, U. B. Gobel, and R. R. Schumann. 2001. Activation of mitogen-activated protein kinases p42/44, p38, and stress-activated protein kinases in myelo-monocytic cells by Treponema lipoteichoic acid. J. Biol. Chem. 276:9713-9719. [DOI] [PubMed] [Google Scholar]
- 27.Sethi, S., K. Muscarella, N. Evans, K. L. Klingman, B. J. Grant, and T. F. Murphy. 2000. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 118:1557-1565. [DOI] [PubMed] [Google Scholar]
- 28.Shuto, T., H. Xu, B. Wang, J. Han, H. Kai, X. X. Gu, T. F. Murphy, D. J. Lim, and J. D. Li. 2001. Activation of NF-κB by nontypeable Haemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK α/β-IκBα and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc. Natl. Acad. Sci. USA 98:8774-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, R. S., R. Kelly, B. H. Iglewski, and R. P. Phipps. 2002. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169:2636-2642. [DOI] [PubMed] [Google Scholar]
- 30.Tan, C. T., and P. Herman. 1998. Inflammatory mediators and otitis media with effusion. An experimental approach using cell culture. Auris Nasus Larynx 25:25-32. [DOI] [PubMed] [Google Scholar]
- 31.Tong, H. H., Y. Chen, M. James, J. Van Deusen, D. B. Welling, and T. F. DeMaria. 2001. Expression of cytokine and chemokine genes by human middle ear epithelial cells induced by formalin-killed Haemophilus influenzae or its lipooligosaccharide htrB and rfaD mutants. Infect. Immun. 69:3678-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trujillo, H., R. Callejas, G. I. Mejia, and L. Castrillon. 1989. Bacteriology of middle ear fluid specimens obtained by tympanocentesis from 111 Colombian children with acute otitis media. Pediatr. Infect. Dis. J. 8:361-363. [DOI] [PubMed] [Google Scholar]
- 33.Wang, B., D. J. Lim, J. Han, Y. S. Kim, C. B. Basbaum, and J. D. Li. 2002. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J. Biol. Chem. 277:949-957. [DOI] [PubMed] [Google Scholar]
- 34.White, J. R., J. M. Lee, P. R. Young, R. P. Hertzberg, A. J. Jurewicz, M. A. Chaikin, K. Widdowson, J. J. Foley, L. D. Martin, D. E. Griswold, and H. M. Sarau. 1998. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J. Biol. Chem. 273:10095-10098. [DOI] [PubMed] [Google Scholar]
- 35.Wu, G. D., E. J. Lai, N. Huang, and X. Wen. 1997. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J. Biol. Chem. 272:2396-2403. [PubMed] [Google Scholar]