Abstract
We studied the in vitro protective activities of human immunoglobulin G1 (IgG1), IgG3, and IgM antibodies against group B meningococci by constructing sets of chimeric mouse-human antibodies (chIgG1, chIgG3, and chIgM, respectively) with identical binding regions against the P1.7 and P1.16 epitopes on PorA. This was done by cloning the V genes of three mouse hybridoma antibodies and subsequently transfecting vectors containing the homologous heavy- and light-chain genes into NSO cells. Cell clones secreting intact human chIgG1, chIgG3, or chIgM antibodies originating from three parent mouse antibodies were isolated. The functional affinities appeared to be similar for all human isotypes and surprisingly also for the pentameric chIgM antibody. chIgG1 exhibited greater serum bactericidal activity (SBA) than chIgG3, while chIgG3 was more efficient in inducing a respiratory burst (RB) associated with opsonophagocytosis than chIgG1 was. On the other hand, chIgM exhibited SBA similar to that of chIgG1, but it exhibited much higher RB activity than chIgG3 and chIgG1 exhibited. The antibodies against the P1.16 epitope were more efficient in terms of SBA than the antibodies against the P1.7 epitope were; thus, 10- to 40-fold-lower concentrations of antibodies against P1.16 than of antibodies against P1.7 were needed to induce SBA. On the other hand, antibodies against these epitopes were equally effective in inducing RB. Our results revealed differences in the functional activities of human chIgG1, chIgG3, and chIgM antibodies against meningococci, which might influence their protective effects against meningococcal disease.
Immune protection against systemic meningococcal disease depends on recognition of bacterial surface antigens by antibodies, followed by activation of complement leading to bacteriolysis, also called serum bactericidal activity (SBA), and/or opsonophagocytosis (OP). The class 1 outer membrane porin protein, PorA, is expressed by almost all meningococcal strains (9, 45, 46), and antigenic variation among PorA proteins is the basis of serosubtyping (9). PorA can induce bactericidal antibodies in humans and mice when they are immunized with meningococcal outer membrane vesicles (OMVs) (7, 28, 35, 38, 42), and monoclonal antibodies (MAbs) against PorA can be protective in an infant rat model (38). Thus, the PorA protein is considered to be an important vaccine antigen and is therefore the main component in a Dutch candidate vaccine (43).
We have previously shown that human chimeric immunoglobulin G1 (chIgG1) and chIgG3 are very efficient in inducing complement activation and complement-mediated cell lysis (2, 10) and induce OP through Fc receptors and complement receptors on effector cells (1, 2). The human IgM antibody isotype is considered to be an efficient activator of the complement cascade, although there has been no real direct comparison with IgG by using antibodies with identical antigen binding regions.
In this paper, we describe cloning of the VL and VH genes of three anti-PorA MAbs, one against the P1.7 epitope (208,D-5) and two against the P1.16 epitope (151,F-9 and 184,F-12), located on loops 1 (VR1) and 4 (VR2) (42), respectively. The V genes were subcloned into expression vectors containing the constant part of human immunoglobulin G1 (IgG1), IgG3, and IgM and transfected into NSO cells. Transfected cells producing chimeric antibodies were cloned, and the chimeric antibodies were purified and tested for functional affinity, SBA, and respiratory burst (RB) activity. The results showed that there were differences in in vitro models of immune protection that were related to both the antibody isotype and antibody specificity.
(Some of the results were presented at the 12th International Pathogenic Neisseria Conference, Galveston, Tex., November 2000, and at the 13th International Pathogenic Neisseria Conference, Oslo, Norway, September 2002.)
MATERIALS AND METHODS
Mouse MAbs and meningococcal strains.
P1.7-specific MAb 208,D-5 was generated from the same fusion that was described previously for P1.7 MAb 207,B-4 by using LiCl-lithium acetate-extracted OMVs from group B meningococcal strain 188/87 (serogroup B, serotype 15, serosubtype P1.7,16d) as the immunogen (26). P1.16-specific MAbs 151,F-9 and 184,F-12 were produced by two different fusions by using deoxycholate-extracted OMVs from strain 44/76 (serogroup B, serotype 15, serosubtype P1.7,16) as the immunogen (8). Fusion with NSO myeloma cells was performed by standard methods (21). The specificity of the antibodies was tested by an enzyme-linked immunosorbent assay (ELISA) by using microtiter plates coated with OMVs or whole bacteria of various group B meningococcal strains in addition to strain 44/76 and by immunoblotting with and without a renaturing detergent (49). The specificity was verified by testing against synthetic peptides (47). The sequences of the two P1.16 MAbs were very similar, although not identical (18). When they were tested against 10-mer overlapping peptides (8), they both reacted with the core sequence DTNNN (E. Rosenquist, unpublished data); this specificity is identical to the specificity of the previously described P1.16 MAb, MN12H2 (41).
Construction of vectors and transfectants producing chIgG1, chIgG3, and chIgM antibodies against the P1.7 and P1.16 epitopes.
The V-region genes of the anti-P1.7 and anti-P1.16 MAbs were isolated by using reverse transcription-PCR, and subcloned into expression vectors containing human Cκ and γ1, γ3, and μ genes, respectively (Fig. 1), by using a recently described method (17). The vectors were sequenced to verify the presence of the correct genes. Transient transfectants were constructed by employing COS cells and testing the resulting supernatants for secreted antibodies. These antibodies showed positive binding in ELISA with wells coated with OMVs of meningococcal strain 44/76. The same vectors were subsequently used to transfect NSO cells. Clones were screened for the production of antibodies reacting with OMVs of strain 44/76 in an ELISA. Two or more clones that exhibited the highest antibody production were selected for each antibody isotype.
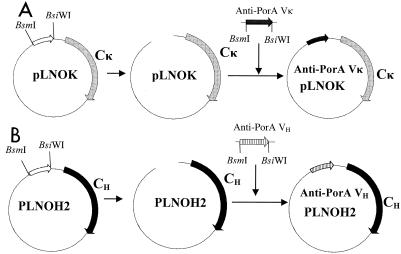
FIG. 1.
Construction of expression vectors. (A) The Vκ gene was subcloned into the pLNOK vector (30). The vector was digested with the BsmI and BsiWI restriction enzymes to remove the foreign Vκ gene, and the anti-P1.16 Vκ gene fragments were ligated into the pLNOK vector at these sites, creating pLNOK/anti-PorA P1.16 and P1.7, respectively. (B) The VH gene was subcloned into the pLNOH2 vector (30). The anti-PorA P1.16 and P1.7 VH genes were digested with the BsiWI and BsmI restriction enzymes before ligation into the linearized pLNOH2 vector containing either γ1, γ3, or the μ chain to create pLNOH2/anti-PorA P1.16 and P1.7.
Verifying the vector and cell constructs.
The V-region genes of the P1.7 and P1.16 MAbs subcloned into expression vectors containing human Cκ and Cγ1, Cγ3, or μ genes were sequenced and were found to be the correct genes (18).
The gene maps of the vectors are shown in Fig. 1. Transient transfectants in COS cells were shown to produce antibodies with binding activity with strain 44/76 and were therefore used subsequently to transfect NSO cells. Positive clones were identified and were shown to produce 6 to 115 μg of the corresponding chimeric MAbs per ml. The chimeric antibodies were purified on a protein A (chIgG1) or protein G (chIgG3) affinity column (Amersham-Bioscience, Uppsala, Sweden). chIgM antibodies were partially purified on a Kaptiv M column (Tecnogen, Piana di Monte Verna, Italy) by using the manufacturer's instructions. IgM was alternatively purified by growing the transfectoma cell clone in UltraCulture medium (BioWhittaker, Verviers, Belgium) containing no calf serum. The cell supernatant was precipitated with 50% saturated (NH4)2SO4, and this was followed by gel filtration on Superdex 200 (Amersham-Bioscience). Gel filtration analysis and agarose gel electrophoresis showed that the protein preparation was highly homogeneous. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis showed that the antibodies were assembled as H2L2 monomers (chIgG1 and chIgG3) and pentamers (chIgM) (data not shown). When tested by an ELISA against wells coated with various strains of meningococci, the chimeric antibodies showed the same reaction pattern as the original mouse MAbs (data not shown).
RB used as a measurement of OP activity.
OP activity was measured by determining the RB in human peripheral blood polymorphonuclear cells (PMNs) (1, 2, 25). RB is a late event in the phagocytic process (4) and is probably related more to meningococcal killing than to mere internalization. In addition, we used untreated viable meningococci in this analysis, which closely resembled the meningococci used for SBA analysis (see below). Briefly, on microplates, twofold serial dilutions of the various antibody preparations (50 μl) in Hanks' balanced salt solution containing 2 mg of bovine serum albumin per ml (HBSS/BSA) were mixed with 5 μl of live, log-phase strain 44/76 meningococci (109 cells/ml) and incubated for 30 min at 37°C with agitation. Then 5 μl of human serum without any antibody activity against meningococci was added as a complement source and incubated for 10 min. Finally, dihydrorhodamine 123-primed PMNs (50 μl, 4 × 106 cells/ml) heterozygous for the FcγRIIa allotype (5) were added, and incubation was continued for another 10 min. The effector/target cell ratio was approximately 1:25. The results were measured by flow cytometry by using an EPICS XL flow cytometer (Coulter, Hialeah, Fla.), gating on the PMN population, and the percentages of RB-positive PMNs were recorded. The RB activity was calculated with the Prism program (GraphPad, San Diego, Calif.) and was expressed as the lowest concentration of antibodies that gave positive fluorescence in 50% of the PMNs. Internal standards were used to compensate for day-to-day variations in bacteria, in effector cells, and in antibody inputs.
SBA.
The SBA assay was performed with the vaccine strain, strain 44/76 (14), by examining a twofold dilution series of the antibody preparations and adding external human plasma without any bactericidal activity (final concentration, 25%) as the complement source (13, 31). Briefly, the assay was done on microplates by using an agar overlay method and applying a bacterial inoculum consisting of 70 to 100 CFU per well. First, the antibody and bacteria were mixed, and then complement was added and the mixture was incubated for 30 min at 35°C in an ordinary atmosphere. Next, brain heart infusion agar was added, and the plates were incubated overnight at 35°C in the presence of 5% CO2. The number of CFU was determined by using a magnifying glass, and the results were recorded as the percentage of CFU at each antibody dilution. The level of SBA of an antibody was defined as the lowest antibody concentration that resulted in a 50% reduction in CFU, as calculated by the Prism program. Internal standards were used to compensate for day-to-day variations in antibody inputs. Positive polyclonal control sera were also included in all experiments (13).
Methods used to measure avidity. (i) Thiocyanate elution method.
The avidity of the antibodies was studied by using the ammonium thiocyanate elution method, as described previously (22). Briefly, microplates coated with OMVs (4 μg/ml and, for some experiments, 0.5 μg/ml) were incubated for 2 h at 37°C with a dilution of each antibody predetermined to give an absorbance of approximately 1.0. To the samples different concentrations of ammonium thiocyanate ranging from 0.1 to 4.0 M were added, and the preparations were incubated for 30 min at room temperature; this was followed by a washing step. Bound antibody was detected by adding a mixture of biotinylated sheep anti-human IgG or anti-human IgM, streptavidin, and biotinylated alkaline phosphatase at optimal dilutions. Finally, the plates were developed by using p-nitrophenyl phosphate before the absorbance at 405 nm was read. The ammonium thiocyanate concentration required to reduce the absorbance by 50% was recorded as the avidity index. This value was calculated by using the Prism program from GraphPad.
(ii) Flow cytometry.
The functional affinities of chIgG and chIgM were also tested by direct binding and inhibition during flow cytometry.
To measure direct binding, we used twofold serial dilutions of chIgG or chIgM in 96-well U-bottom microtiter plates (50 μl/well). Five microliters of a bacterial suspension (109 cells of ethanol-killed strain 44/76 per ml) was added to each well, and the plates were incubated for 45 min at 37°C with agitation. The bacteria were washed by centrifuging the plates at 1,300 × g for 3 min. This procedure was then repeated two times by adding 150 μl of HBSS/BSA. After the final wash, 3 μl of locally produced fluorescein isothiocyanate (FITC)-labeled sheep anti-human IgM or 3 μl of FITC-labeled goat anti-human IgG (ICN Pharmaceuticals, Aurora, Ohio) was added to each well. After incubation with agitation for 45 min at 37°C, the bacteria were washed three times as described above, suspended in 100 μl of HBSS/BSA, and finally analyzed with a flow cytometer, and the median fluorescence intensity was recorded.
For inhibition experiments, twofold serial dilutions of biotin-labeled chIgG or chIgM were added to microtiter plates (50 μl/well); then 5 μl of a bacterial suspension (109 cells/ml) was added to each well, and the plates were incubated for 60 min at 37°C with agitation. The bacteria were washed as described above. After the final wash, 3 μl of FITC-labeled streptavidin (Molecular Probes, Eugene, Oreg.) was added to each well. After incubation with agitation for 30 min at 37°C, the bacteria were washed three times as described above, suspended in 100 μl of HBSS/BSA, and then analyzed with a flow cytometer. Half-saturating dilutions of biotin-labeled chIgG and chIgM were determined from the analysis. For inhibition studies the dilution of the biotin-labeled chIgG or chIgM antibodies was kept constant, and increasing amounts of unlabeled chIgG or chIgM antibodies were added 30 min before biotin-labeled antibodies were added. Inhibition was detected by measuring the decrease in fluorescence due to the decreased amounts of biotin-labeled antibodies bound to the bacteria.
Test for cross-inhibition of P1.7 and P1.16 antibodies.
Antibody cross-inhibition was tested by flow cytometry by first titrating chIgG1 and chIgM binding to heat-inactivated meningococcal strain 44/76. On microtiter plates, 100-μl portions of twofold serial dilutions in HBSS/BSA (starting at 10 μg/ml) of chIgG1 and chIgM isotypes of the 184,F-12 (anti-P1.16) and 208,D-5 (anti-P1.7) antibodies, respectively, were added to round-bottom microtiter plates. Then 10 μl of a bacterial culture was added to each well as described above, and the plates were incubated for 30 min at 37°C with agitation. Then the bacteria were washed three times as described above. After the final wash, 2 μl of FITC-labeled goat anti-human IgG was added to each well to detect chIgG or 3 μl of locally produced FITC-labeled sheep anti-human IgM was added to each well to detect chIgM. After incubation with agitation for 30 min at 37°C, the bacteria were washed three times as described above, suspended in 100 μl of HBSS/BSA, and analyzed with a flow cytometer. Nonsaturating, optimal dilutions of chIgG anti-P1.7 and anti-P1.16 were determined from this analysis. For inhibition studies the dilution of the chIgG antibodies was kept constant, and increasing amounts of chIgM antibodies were added to measure inhibition. Inhibition was detected by measuring the decrease in fluorescence of chIgG antibodies.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the nucleotide sequences determined in this study are AF191791 (208,D-5 VH), AF191792 (208,D-5 VK), AY195879 (151,F-9 VH), AY195880 (151,F-9 VK), AY 195881(184,F-12 VH), and AY195882 (184,F-12 VK).
RESULTS
Effects of isotypes on SBA.
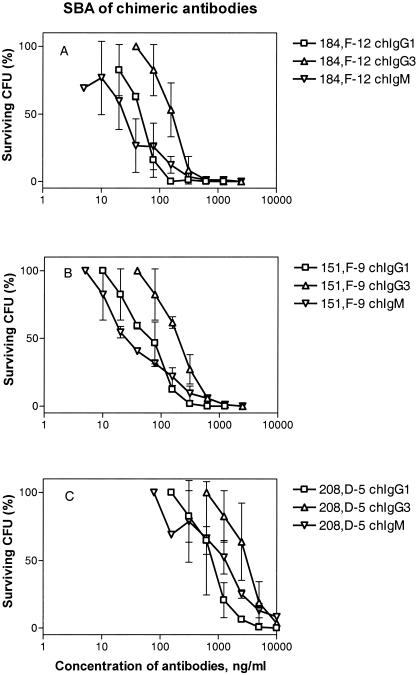
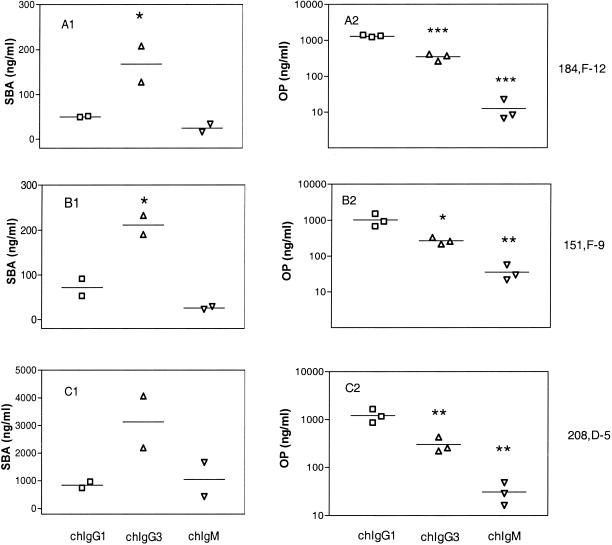
The chIgG1 antibodies exhibited more potent SBA than the corresponding chIgG3 antibodies exhibited, against both the P1.7 and P1.16 epitopes (Fig. 2 and Table 1). For antibodies to the P1.16 epitope, chIgG1 and chIgG3 exhibited SBA (lowest concentration resulting in 50% killing of the inoculum) of 50 to 72 and 168 to 212 ng/ml, respectively (Table 1). On the other hand, the SBA of chIgM was similar to that of chIgG1, and this activity corresponded to 25 to 26 ng/ml against the P1.16 epitope (Table 1). The hierarchy for SBA on a weight basis was consequently chIgM ∼ chIgG1 > chIgG3. The difference in potency of the SBA of chIgG1 and chIgG3 was statistically significant (P < 0.05), as shown in Fig. 3.
FIG. 2.
Dose-response curves for SBA of the IgG1, IgG3, and IgM sets of chimeric antibodies. (A) Antibodies containing the V region of MAb 184,F-12 (anti-P1.16). (B) Antibodies containing the V region of MAb 151,F-9 (anti-P1.16). (C) Antibodies containing the V region of MAb 208,D-5 (anti-P1.7). The results are based on two independent experiments. The standard deviations are indicated by error bars. The SBA was expressed as the lowest antibody concentration (in nanograms per milliliter) able to reduce the CFU by 50%, as shown in Table 1 and Fig. 3.
TABLE 1.
Efficiency of SBA and induction of RB as a measurement of OP of chimeric MAbs against PorA epitopes
| Antibody | Concn (ng/ml)a
|
|||||
|---|---|---|---|---|---|---|
| 208,D-5 (anti-P1.7)
|
151,F-9 (anti-P1.16)
|
184,F-12 (anti-P1.16)
|
||||
| SBA efficiency | RB induction | SBA efficiency | RB induction | SBA efficiency | RB induction | |
| chIgG1 | 839 ± 160 | 1,220 ± 395 | 72 ± 27 | 1,029 ± 423 | 50 ± 2 | 1,296 ± 81 |
| chIgG3 | 3,130 ± 1,320 | 303 ± 113 | 212 ± 30 | 270 ± 57 | 168 ± 57 | 350 ± 76 |
| chIgM | 1,044 ± 874 | 31 ± 16 | 26 ± 4 | 36 ± 19 | 25 ± 13 | 13 ± 9 |
Lowest concentration of the antibodies able to induce SBA (50% reduction in CFU) or RB (RB in 50% of the PMNs). The data are means ± standard deviations.
FIG. 3.
SBA and RB of the chimeric antibodies: calculated lowest concentrations giving RB in 50% of the PMNs or 50% reduction in the CFU (SBA) in the individual experiments. The lines indicate the means. (A1) SBA of chimeric 184,F-12 antibodies. (B1) SBA of chimeric 151,F-9 antibodies. (C1) SBA of chimeric 208,D-5 antibodies. (A2) RB of chimeric 184,F-12 antibodies. (B2) RB of chimeric 151,F-9 antibodies. (C2) RB of chimeric 208,D-5 antibodies. The Student t test was used to calculate statistically significant differences compared to the values for chIgG1. One asterisk indicates that the P value was <0.05, two asterisks indicate that the P value was <0.01, and three asterisks indicate that the P value was <0.001, as determined by the Prism program.
Antibodies to the P1.16 epitope induced greater SBA than antibodies to the P1.7 epitope induced.
The SBAs induced by the chimeric antibodies against the P1.16 epitope were about 10- to 40-fold greater than the SBA induced by the chimeric antibodies against the P1.7 epitope irrespective of the isotype (Table 1). Thus, for chIgG1, 839 ng of antibodies/ml was needed for P1.7 to cause 50% killing of the inoculum, while only 50 to 72 ng of antibodies per ml was needed for the P1.16 epitope (Table 1) (P < 0.001). For chIgG3, the corresponding concentrations were 3,130 ng/ml for antibodies against P1.7 and 168 to 212 ng/ml for antibodies against P1.16 (P < 0.01). Finally, for chIgM the concentrations that exhibited SBA were 1,044 ng/ml for antibodies to P1.7 and 25 ng/ml for antibodies to the P1.16 epitope (P < 0.05).
Effect of isotype on RB-inducing activity.
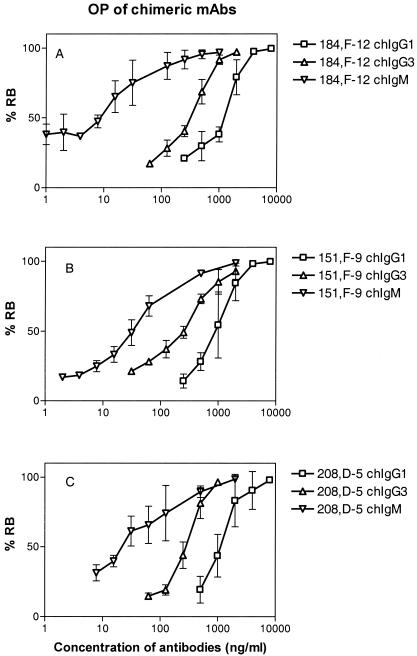
The RB-inducing activities of the chimeric antibodies were tested with live log-phase bacteria (Fig. 4). The chIgG3 molecules showed higher RB activity than the corresponding chIgG1 molecules. Thus, 270 to 350 ng of chIgG3 per ml and 1,029 to 1,296 ng of chIgG1 per ml were needed to induce RB activity against the P1.16 epitope (Table 1). More strikingly, the chIgM isotype was the most active in this respect and showed RB activity at a concentration of 13 to 36 ng/ml (Table 1). The hierarchy for RB activity was thus chIgM ≫ chIgG3 > chIgG1. This hierarchy was statistically significant, as shown in Fig. 3.
FIG. 4.
Dose-response curves for RB of the chIgG1, chIgG3, and chIgM sets of antibodies. (A) Antibodies containing the V region of MAb 184,F-12 (anti-P1.16). (B) Antibodies containing the V region of MAb 151,F-9 (anti-P1.16). (C) Antibodies containing the V region of MAb 208,D-5 (anti-P1.7). OP was measured with a flow cytometer and was expressed as percentage of the effector cells (human PMNs) undergoing RB as described in Materials and Methods. The results are the means of three independent experiments. The error bars indicate standard deviations. The RB activity was expressed as the lowest antibody concentration (in nanograms per milliliter) able to induce RB in 50% of the PMNs, as shown in Table 1 and Fig. 3.
Antibodies to the P1.16 and P1.7 epitopes are equally active for RB.
The RB-inducing activity of the chimeric antibodies against the P1.16 epitope was similar to that of the antibodies against the P1.7 epitope (Table 1). For chIgG1 the RB activity was 1,220 ng/ml against the P1.7 epitope and 1,029 to 1,296 ng/ml against the P1.16 epitope. For chIgG3 the RB activity was 303 ng/ml against P1.7 and 270 to 350 ng/ml against the P1.16 epitope. Similarly, for the most active isotype, IgM, the RB activity was in the same range (13 to 36 ng/ml) for both epitopes.
Antibodies to the P1.7 and P1.16 epitopes bind independently to bacteria.
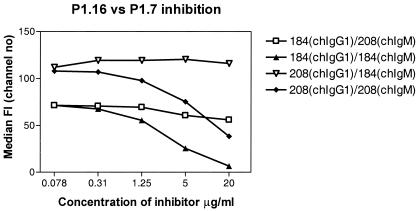
chIgM antibodies to P1.7 were tested for inhibition of chIgG antibodies to P1.16 (cross-inhibition) by flow cytometry. The opposite inhibition reaction (chIgM antibodies to P1.16 inhibiting chIgG antibodies to P1.7) was also tested. Antibodies to P1.16 had no significant inhibitory effect on binding of antibodies to P1.7 antibodies, nor was the opposite type of inhibition observed. As controls, we observed good homologous inhibition when we used chIgM antibodies against P1.16 to inhibit chIgG against P1.16 and chIgM against P1.7 to inhibit chIgG against P1.7 (Fig. 5).
FIG. 5.
Flow cytometer test for inhibition of chIgM antibodies against chIgG antibodies for homologous inhibition (internal positive control) and cross-inhibition between P1.16 and P1.7 epitopes. The results show that there was no cross-inhibition but there was clear homologous inhibition. Abbreviations: 184, 184,F-12; 208, 208,D-5.
chIgG and chIgM have similar functional affinities.
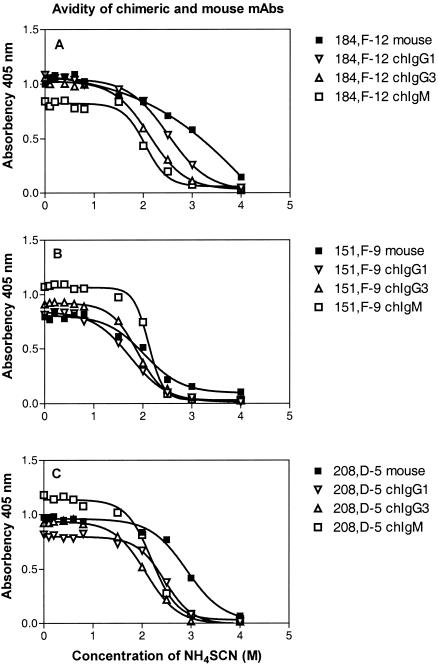
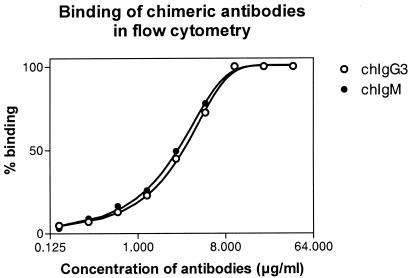
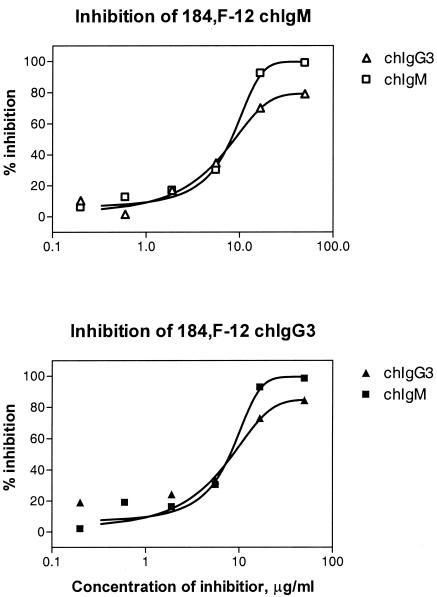
The functional affinities of chIgG and chIgM were tested primarily by the thiocyanate elution method by using ELISA microtiter plates coated with 0.5 to 4 μg of strain 44/76 OMVs per ml. The results showed that the avidity indexes for chIgM and the avidity indexes for chIgG1 and chIgG3 were similar (Fig. 6 and Table 2). The same results were observed when we used wells coated with 0.5 μg/ml (data not shown). In order to verify these somewhat unexpected results, some antibodies were tested further for binding and inhibition by flow cytometry by using whole meningococci and biotin-labeled or unlabeled chIgM and chIgG3. The direct binding activity of chIgM was equal to that of chIgG3 (Fig. 7), and the half-saturation concentrations were 2.8 μg/ml (range, 2.6 to 2.9 μg/ml) for chIgG3 and 2.5 μg/ml (range, 2.3 to 2.6 μg/ml) for chIgM. This indicates that chIgG3 and chIgM have similar functional affinities for whole-cell bacteria in suspension. We next tested the inhibition activity of chIgG3 and chIgM relative to each other by flow cytometry. The flow cytometry inhibition curves of chIgG and chIgM were very similar, indicating that the functional affinities of chIgG and chIgM are similar (Fig. 8).
FIG. 6.
Avidity curves for the chimeric antibodies and the original mouse MAbs. (A) Results obtained with 184,F-12. (B) Results obtained with 151,F-9. (C) Results obtained with 208,D-5. The graphs show the influence of NH4SCN on the binding of the MAbs. The avidity index was the concentration of NH4SCN that resulted in a 50% reduction in absorbance. The curves show the nonlinear regression fit (Boltzman sigmoidal) obtained by using the Prism program supplied by GraphPad.
TABLE 2.
Avidity indices of mouse MAbs and humanized chimeric MAbs
| Antibody | Avidity indices against PorA epitopes (M)a
|
||
|---|---|---|---|
| 208,D-5 (anti-P1.7) | 151,F-9 (anti-P1.16) | 184,F-12 (anti-P1.16) | |
| Original MAb | 2.9 | 2.2 | 3.0 |
| chIgG1 | 2.5 ± 0.1 | 1.7 ± 0.2 | 2.6 ± 0.1 |
| chIgG3 | 2.2 ± 0.2 | 1.7 ± 0.2 | 2.2 ± 0.2 |
| chIgM | 2.1 ± 0.1 | 2.1 ± 0.1 | 1.9 ± 0.2 |
Lowest concentrations of NH4SCN resulting in a 50% reduction in absorbance in ELISA. The data are averages ± standard deviations for three independent measurements.
FIG. 7.
Binding activity of 184,F-12 chIgG3 and 184,F-12 chIgM as measured by flow cytometry. The bacteria were reacted with twofold serial dilutions of chIgG3 and chIgM, and this was followed by development with FITC-labeled anti-IgG and anti-IgM, respectively. The y axis indicates the percentage of binding relative to saturating binding. The data are the data from one of two independent experiments in which essentially the same results were obtained.
FIG. 8.
Inhibition as determined by flow cytometry of biotin-labeled 184,F-12 chIgG3 and 184,F-12 chIgM by the unlabeled 184,F-12 chIgG3 and 184,F-12 chIgM, respectively. The upper curve shows the inhibition of biotin-labeled 184,F-12 chIgM, and the lower curve shows the inhibition of biotin-labeled 184,F-12 chIgG3. The data are the data from one of three independent experiments in which identical results were obtained.
DISCUSSION
Antibodies probably protect against systemic meningococcal disease by complement-mediated bactericidal activity and/or OP destruction of the bacteria (3, 11, 12, 19, 36). The efficiency of protection depends on antibody specificity, avidity, and the isotype of the antibody. The antibody effector functions are assigned to the Fc region of the molecules (6). In the present study, we isolated the V-region genes of three mouse MAbs against a group B meningococcal strain, strain 44/76, which has been prevalent in Norway since the early 1970s. Two of the MAbs (184,F-12 and 151,F-9) are directed against the P1.16 epitope, and the third MAb (208,D-5) is directed against the P1.7 epitope; these epitopes are located in the V2 and V1 extracellular loops of the PorA molecule, respectively (42). Sequence analysis of the V genes encoding the two MAbs against the P1.16 epitope revealed a very high level of similarity, and they probably were derived from the same V germ line genes (18). These germ line genes are the same as those previously described for the P1.16 MAb MN12H2 (41). A similar close relationship among the V genes encoding three P1.7 MAbs (including 208,D-5) has been described previously (47). Thus, the mouse V-region repertoire used against these two different meningococcal protein epitopes seems to be very limited.
The main aim of the present work was to analyze the functional activities of human antibodies against two different epitopes on the PorA protein on group B meningococci. When IgG is considered, the IgG1 and IgG3 isotypes are of particular interest as they are dominant during vaccination with meningococcal OMVs and also during meningococcal infection (29, 40, 48). When we compared chIgG1 with chIgG3 in effector functions against meningococci, chIgG1 performed slightly better than chIgG3 in terms of SBA, when the antibodies were directed against both the P1.16 and P1.7 epitopes (Table 1 and Fig. 3). On the other hand, when RB was used as a measure of OP activity, chIgG3 performed better than chIgG1 (Table 1 and Fig. 3). A similar study of the antibacterial activity of human chIgG1 and chIgG3 based on the P1.16-specific mouse MAb MN12H2 also showed that the SBA of chIgG1 was greater than that of chIgG3, and chIgG3 was better than chIgG1 in terms of OP (44). In this study we extended this IgG isotype pattern of biological activity to include the P1.7 specificity of the MAbs.
The P1.16 and P1.7 epitopes are situated on different loops on the PorA protein, which could behave differently as targets for antibodies to induce SBA and to induce OP. Our comparison showed that antibodies to the P.16 epitope were strikingly more efficient than the corresponding antibodies to the P1.7 epitope in terms of SBA, since 10- to 40-fold-lower antibody concentrations were needed to observe SBA against the P1.16 epitope than to observe SBA against the P1.7 epitope. This greater efficacy of antibodies to P1.16 than of antibodies to P1.7 was observed for all three isotypes tested (Table 1). As far as we know, this is the first detailed analysis of the relative in vitro immunoprotective importance of two independent bacterial epitopes situated on the same bacterial surface protein. The difference in the SBA observed was apparently not due to differences in affinity, as the thiocyanate elution method revealed virtually the same avidity indexes for the two chimeric MAb families (Table 2). A reasonable explanation for the lower SBA of antibodies to P1.7 could be a more distant localization of the P1.7 epitope in relation to the bacterial membrane, since VR1 of P1.7 has a longer loop (40 amino acids) than VR2 of P1.16 (34 amino acids) (42). Thus, antibodies to the P1.7 epitope might be expected to induce the deposition of membrane attack complexes (MACs) farther away from the bacterial membrane, leading to less bacterial lysis than the lysis observed with antibodies to the P1.16 epitope. Interestingly, the long hinge of IgG3 (62 amino acids) (15, 24), compared to the shorter hinge of IgG1 (15 amino acids), does not negatively influence the deposition of MACs on the bacterial membrane, as the differences in SBA between the chIgG1 and chIgG3 antibodies were similar for both the P1.7 and P1.16 epitopes (Table 1). Possibly, the great flexibility of chIgG3 antibodies (37) compensates for any influence of the hinge length on MAC deposition. It is also worth mentioning that chIgG3 is better than chIgG1 in complement-mediated lysis of sheep red blood cells when antigen concentrations on the target cells are low (27). Whether chIgG3 performs better than chIgG1 in terms of SBA against other bacterial antigens less abundant than PorA remains to be seen.
It has been shown previously that the avidity of chIgG3 is greater than that of chIgG1 when they are directed against the NIP-hapten (22). We did not observe any such differences in avidity between chIgG1 and chIgG3 antibodies to the P1.7 and P.16 epitopes on PorA when we used the thiocyanate elution method. A possible explanation for this is the availability and concentration of the NIP-hapten antigens on the red blood cell surface, which might favor stronger binding by the flexible chIgG3 antibodies than by chIgG1 antibodies.
Even more strikingly, we did not observe any enhanced avidity of chIgM compared to the avidity of chIgG. This is surprising, since a polymer bonus effect would be anticipated for the pentameric IgM molecule compared to the monomeric IgG molecule (50). We observed similar avidities both when we used the thiocyanate elution method and when we performed binding and inhibition experiments with flow cytometry. Importantly, flow cytometry measured the relative binding activity to whole bacteria in aqueous suspensions, and the results were therefore expected to reflect the in vivo binding properties of chIgG and chIgM antibodies against PorA epitopes. The flow cytometer analysis showed that the functional affinities of our chIgG and chIgM were similar. These findings indicate that IgM might not always exhibit favorable binding activity with bacteria, at least when the target is a protein epitope and the intrinsic affinity of each binding site is high, as in our experiments. The binding regions in our experiments were taken from an IgG parent molecule and might not be representative of the IgM molecules present in a primary immune response. Interestingly, for mouse antibodies against virus, a lack of an avidity bonus effect upon polymerization for high-affinity antibodies has been reported, parallel to such a bonus effect for low-affinity antibodies (20). As far as we know, the present study is the first study in which the avidities of monoclonal humanized chIgG and chIgM with the same molecular binding regions to protein antigen were compared. One other study dealt with human IgG1 and IgM with identical binding regions, but that study did not include avidity measurements (39). It is conceivable that the greater avidity observed for IgM than for IgG might be valid only for antibodies against abundantly expressed antigens, such as carbohydrate (50), and only for low-avidity antibodies.
The other unexpected observation with the chIgM antibodies was that on a weight basis they had bactericidal activity similar to that of chIgG1, while a study in which switch variants of rat IgM and IgG against O18 lipopolysaccharide of Escherichia coli were used demonstrated that rat IgM was 100- to 1,000-fold more efficient in complement-mediated lysis of lipopolysaccharide-coated sheep erythrocytes than IgG (32). Perhaps IgM requires a high density of epitopes to activate complement more efficiently than IgG, or there might be species differences among IgM molecules. The relative distribution of hexamer and pentamer IgM might also vary and influence complement activation (16, 34). OP was measured by determining the ability of the antibodies to induce RB in human peripheral blood PMN after internalization of opsonized meningococci, as it has been demonstrated that OP and RB activity closely follow each other when meningococci are used as the target (1). For OP, the chIgM antibodies were much more efficient than chIgG3 and chIgG1 in our test system. On a weight basis, our chIgMs were 8- to 30-fold more efficient in terms of OP activity than chIgG3 and 28- to 100-fold more efficient than chIgG1 (Table 1). A similar high level of OP activity of human chIgM compared with the activity of human IgG1 with identical binding regions has been observed for anti-carbohydrate antibodies against the gram-positive group B streptococci (33, 39). Thus, both for abundant cell wall carbohydrate antigens and for less abundant protein antigens, chIgM can be by far the most efficient OP-inducing antibody isotype. However, caution must be used when this notion is generalized, as it has recently been shown that for certain carbohydrate specificities, chIgM can exhibit very low OP activity compared to the activity of chIgG with an identical V region (23). However, a complicating feature in these systems is the striking influence of the isotype on the fine specificity of the antibodies (23). We did not find any change in the fine specificity of chIgM compared to that of chIgG since chIgM could efficiently inhibit chIgG when the antibodies were directed against P1.7 or P1.16 (Fig. 5), and there was equal inhibition of chIgM and chIgG as determined by flow cytometry. However, the possibility that there are subtle differences in fine specificity of the different isotypes, despite identical V-region sequences, cannot be excluded.
The P1.7 and P1.16 epitopes are located on tips on extracellular loops in the PorA protein. Whether these two epitopes can bind antibodies independently has been a matter of debate. By using chIgM and chIgG against the two epitopes, we tested cross-inhibition by flow cytometry. Our results showed that there was no cross-inhibition, and thus the P1.7 and P1.16 epitopes can bind antibodies independently of each other. Antibodies to the P1.7 epitope should therefore have an additive protective effect with the antibodies against the P1.16 epitope and vice versa.
In summary, we created humanized antibodies against the PorA P1.16 and P1.7 epitopes. In vitro analysis showed that antibodies against the P1.16 epitope were at least 10-fold more effective in inducing bactericidal activity than antibodies against the P1.7 epitope, while antibodies to both epitopes were equally effective in inducing OP, as measured by RB. chIgG1 was more active in SBA than chIgG3, whereas in OP chIgG3 was more active than chIgG1. chIgM was the most active antibody isotype in OP. These properties of the meningococcal antibodies shed more light on their different in vitro functional activities, which might be relevant to their in vivo protective activities and could possibly be used to guide the development of meningococcal vaccines and antibody preparations for therapy and prophylaxis against meningococcal disease.
Acknowledgments
We gratefully acknowledge the technical assistance of Gunnhild Rødal in creating the mouse MAbs, and we thank Lars Norderhaug, Oslo, Norway, for supplying the pLNOK and LNOH2 vectors.
Editor: J. N. Weiser
REFERENCES
- 1.Aase, A., and T. E. Michaelsen. 1994. Opsonophagocytic activity induced by chimeric antibodies of the four IgG subclasses with or without help from complement. Scand. J. Immunol. 39:581-587. [DOI] [PubMed] [Google Scholar]
- 2.Aase, A., E. A. Høiby, and T. E. Michaelsen. 1998. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand. J. Immunol. 47:388-396. [DOI] [PubMed] [Google Scholar]
- 3.Aase, A., G. Bjune, E. A. Høiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggiolini, M., and M. P. Wymann. 1990. Turning on the respiratory burst. Trends Biochem. Sci. 15:69-72. [DOI] [PubMed] [Google Scholar]
- 5.Bredius, R. G., C. A. Fijen, M. De Haas, E. J. Kuijper, R. S. Weening, J. G. van de Winkel, and T. A. Out. 1994. Role of neutrophil FcγRIIa (CD32) and FcγRIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology 83:624-630. [PMC free article] [PubMed] [Google Scholar]
- 6.Burton, D. R., and J. N. Woof. 1992. Human antibody effector function. Adv. Immunol. 51:1-84. [DOI] [PubMed] [Google Scholar]
- 7.Claassen, I., J. Meylis, P. van der Ley, C. Peeters, H. Brons, J. Robert, D. Borsboom, A. van der Ark, I. van Straaten, P. Roholl, B. Kuipers, and J. T. Poolman. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001-1008. [DOI] [PubMed] [Google Scholar]
- 8.Delvig, A. A., E. Wedege, D. A. Caugant, R. Dalseg, J. Kolberg, M. Achtman, and E. Rosenqvist. 1995. A linear B-cell epitope on the class 3 outer-membrane protein of Neisseria meningitidis recognized after vaccination with the Norwegian group B outer-membrane vesicle vaccine. Microbiology 141:1593-1600. [DOI] [PubMed] [Google Scholar]
- 9.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-510. [DOI] [PubMed] [Google Scholar]
- 10.Garred, P., T. E. Michaelsen, and A. Aase. 1989. The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand. J. Immunol. 30:379-382. [DOI] [PubMed] [Google Scholar]
- 11.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halstensen, A., B. Haneberg, L. O. Frøholm, V. Lehmann, C. E. Frasch, and C. O. Solberg. 1984. Human opsonins to meningococci after vaccination. Infect. Immun. 46:673-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Høiby, E. A., E. Rosenqvist, L. O. Frøholm, G. Bjune, B. Feiring, H. Nøkleby, and E. Rønnild. 1991. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH (Natl. Inst. Public Health) Ann. (Oslo) 14:147-155. [PubMed] [Google Scholar]
- 14.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huck, S., P. Fort, D. H. Crawford, M. P. Lefranc, and G. Lefranc. 1986. Sequence of a human immunoglobulin gamma 3 heavy chain constant region gene: comparison with the other human C gamma genes. Nucleic Acids Res. 14:1779-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughey, C. T., J. W. Brewer, A. D. Colosia, W. F. Rosse, and R. B Corley. 1998. Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J. Immunol. 161:4091-4097. [PubMed] [Google Scholar]
- 17.Ihle, O., and T. E. Michaelsen. 2000. Efficient purification of DNA fragments using a protein binding membrane. Nucleic Acids Res. 28:E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ihle, Ø., K. J. Beckstrøm, and T. E. Michaelsen. 2003. Cloning, sequencing and expression of immunoglobulin variable regions of murine monoclonal antibodies specific for the P1.7 and P1.16 PorA protein loops of Neisseria meningitidis. Scand. J. Immunol. 57:453-462. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis, G. A. 1995. Recognition and control of neisserial infection by antibody and complement. Trends Microbiol. 3:198-201. [DOI] [PubMed] [Google Scholar]
- 20.Kalinke, U., A. Oxenius, C. Lopez-Macias, R. M. Zinkernagel, and H. Hengartner. 2000. Virus neutralization by germ-line vs. hypermutated antibodies. Proc. Natl. Acad. Sci. USA 97:10126-10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolberg, J., E. A. Høiby, R. Lopez, and K. Sletten. 1997. Monoclonal antibodies against Streptococcus pneumoniae detect epitopes on eubacterial ribosomal proteins L7/L12 and on streptococcal elongation factor Ts. Microbiology 143:55-61. [DOI] [PubMed] [Google Scholar]
- 22.McCloskey, N., M. W. Turner, and T. D. Goldblatt. 1997. Correlation between the avidity of mouse-human chimeric IgG subclass monoclonal antibodies measured by solid-phase elution ELISA and biospecific interaction analysis (BIA). J. Immunol. Methods 205:67-72. [DOI] [PubMed] [Google Scholar]
- 23.McLean, G. R., M. Torres, N. Elguezabal, A. Nakouzi, and A. Casadevall. 2002. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 169:1379-1386. [DOI] [PubMed] [Google Scholar]
- 24.Michaelsen, T. E., B. Frangione, and E. C. Franklin. 1977. Primary structure of the “hinge” region of human IgG3. Probable quadruplication of a 15-amino acid residue basic unit. J. Biol. Chem. 252:883-889. [PubMed] [Google Scholar]
- 25.Michaelsen, T. E., and A. Aase. 2001. Antibody-induced opsonophagocytosis of serogroup B meningococci measured by flow cytometry, p. 331-337. In A. J. Pollard and M. C. J. Maiden (ed.), Methods in molecular medicine: meningococal vaccines. Methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 26.Michaelsen, T. E., A. Aase, J. Kolberg, E. Wedege, and E. Rosenqvist. 2001. PorB3 outer membrane protein on Neisseria meningitidis is poorly accessible for antibody binding on live bacteria. Vaccine 19:1526-1533. [DOI] [PubMed] [Google Scholar]
- 27.Michaelsen, T. E., P. Garred, and A. Aase. 1991. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur. J. Immunol. 21:11-16. [DOI] [PubMed] [Google Scholar]
- 28.Milagres, L. G., M. C. Gorla, C. T. Sacchi, and M. M. Rodrigues. 1998. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunization with an outer membrane vaccine. Infect. Immun. 66:4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nœss, L. M., T. Aarvak, A. Aase, F. Oftung, E. A. Høiby, R. H. Sandin, and T. E. Michaelsen. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17:754-764. [DOI] [PubMed] [Google Scholar]
- 30.Norderhaug, L., T. Olafsen, T. E. Michaelsen, and I. Sandlie. 1997. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J. Immunol. Methods 204:77-87. [DOI] [PubMed] [Google Scholar]
- 31.Perkins, B., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Høiby, E. Rosenqvist, J. Holst, H. Nøkleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapcynski, E. Tikhomirov, J. D. Wemger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 32.Pluschke, G., G. Bordmann, M. E. Daoudaki, J. D. Lambris, M. Achtman, and M. Neibert. 1989. Isolation of rat IgM to IgG hybridoma isotype switch variants and analysis of the efficiency of rat Ig in complement activation. Eur. J. Immunol. 19:131-135. [DOI] [PubMed] [Google Scholar]
- 33.Raff, H. V., C. Bradley, W. Brady, K. Donaldson, L. Lipsich, G. Maloney, W. Shuford, M. Walls, P. Ward, E. Wolff, et al. 1991. Comparison of functional activities between IgG1 and IgM class-switched human monoclonal antibodies reactive with group B streptococci or Escherichia coli K1. J. Infect. Dis. 163:346-354. [DOI] [PubMed] [Google Scholar]
- 34.Randall, T. D., L. B. King, and R. B. Corley. 1990. The biological effects of IgM hexamer formation. Eur. J. Immunol. 20:1971-1979. [DOI] [PubMed] [Google Scholar]
- 35.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Rønnild, G. Bjune, and H. Nøkleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross, S., P. J. Rosenthal, H. M. Berberich, and P. Densen. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement deficient individuals. J. Infect. Dis. 155:1266-1275. [DOI] [PubMed] [Google Scholar]
- 37.Roux, K. H., L. Strelets, and T. E. Michaelsen. 1997. Flexibility of human IgG subclasses. J. Immunol. 159:3372-3382. [PubMed] [Google Scholar]
- 38.Saukkonen, K., M. Leinonen, H. Abdillahi, and J. T. Poolman. 1989. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine 7:325-328. [DOI] [PubMed] [Google Scholar]
- 39.Shyur, S. D., H. V. Raff, J. F. Bohnsack, D. K. Kelsey, and H. R. Hill. 1992. Comparison of the opsonic and complement triggering activity of human monoclonal IgG1 and IgM antibody against group B streptococci. J. Immunol. 48:1879-1884. [PubMed] [Google Scholar]
- 40.Sjursen, H., E. Wedege, E. Rosenqvist, A. Nœss, A. Halstensen, R. Matre, and C. O. Solberg. 1990. IgG subclass antibodies to serogroup B meningococcal outer membrane antigens following infection and vaccination. APMIS 98:1061-1069. [DOI] [PubMed] [Google Scholar]
- 41.van den Elsen, J., L. Vandeputte-Rutten, J. Kroon, and P. Gros. 1999. Bactericidal antibody recognition of meningococcal PorA by induced fit. Comparison of liganded and unliganded Fab structures. J. Biol. Chem. 274:1495-1501. [DOI] [PubMed] [Google Scholar]
- 42.van der Ley, P., J. E. Heckels, M. Virji, P. Hoogerhout, and J. T. Poolman. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 59:2963-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Voort, E. R., P. van der Ley, J. van der Biezen, S. George, O. Tunnela, H. van Dijken, B. Kuipers, and J. Poolman. 1996. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect. Immun. 64:2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidarsson, G., W. L. van der Pol, J. M. van den Elsen, H. Vile, M. Jansen, J. Duijs, H. C. Morton, E. Boel, M. R. Daha, B. Corthesy, and J. G. van de Winkel. 2001. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J. Immunol. 166:6250-6263. [DOI] [PubMed] [Google Scholar]
- 45.Wang, J. F., D. A. Caugant, G. Morelli, B. Koumarae, and M. Achtman. 1993. Antigenic and epidemiologic properties of the ET-37 complex of Neisseria meningitidis. J. Infect. Dis. 167:1320-1329. [DOI] [PubMed] [Google Scholar]
- 46.Wang, J. F., D. A. Caugant, X. Li, X. Hu, J. T. Poolman, B. A. Crowe, and M. Achtman. 1992. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People's Republic of China. Infect. Immun. 60:5267-5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, J. F., G. A. Jarvis, M. Achtman, E. Rosenqvist, T. E. Michaelsen, A. Aase, and J. M. Griffiss. 2000. Functional activities and immunoglobulin variable regions of human and mouse monoclonal antibodies specific for the P1.7 PorA protein loop of Neisseria meningitidis. Infect. Immun. 68:1871-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wedege, E., and T. E. Michaelsen. 1987. Human immunoglobulin G subclass immune response to outer membrane antigens in meningococcal group B vaccine. J. Clin. Microbiol. 25:1349-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wedege, E., K. Bryn, and L. O. Frøholm. 1988. Restoration of antibody binding to blotted meningococcal outer membrane proteins using various detergents. J. Immunol. Methods 113:51-59. [DOI] [PubMed] [Google Scholar]
- 50.Zebedee, S. L., R. K. Koduri, J. Mukherjee, S. Mukherjee, S. Lee, D. F. Sauer, M. D. Scharff, and A. Casadevall. 1994. Mouse-human immunoglobulin G1 chimeric antibodies with activities against Cryptococcus neoformans. Antimicrob. Agents Chemother. 38:1507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]