Abstract
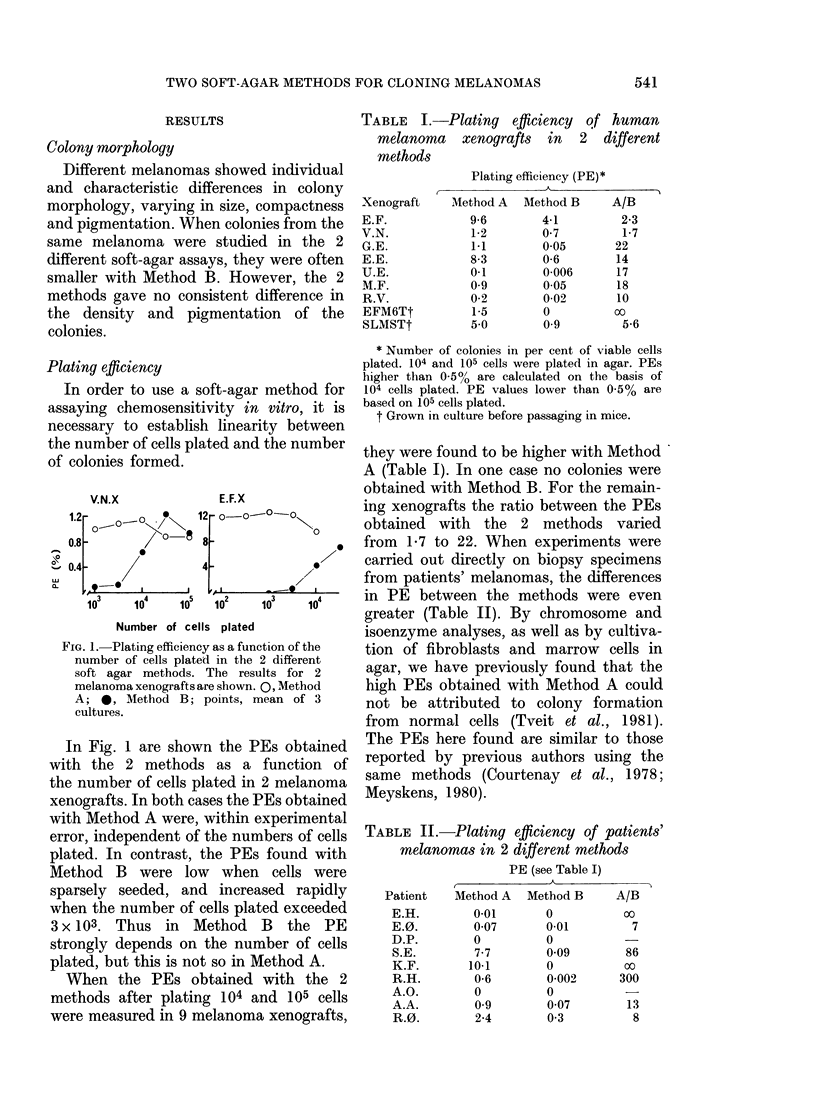
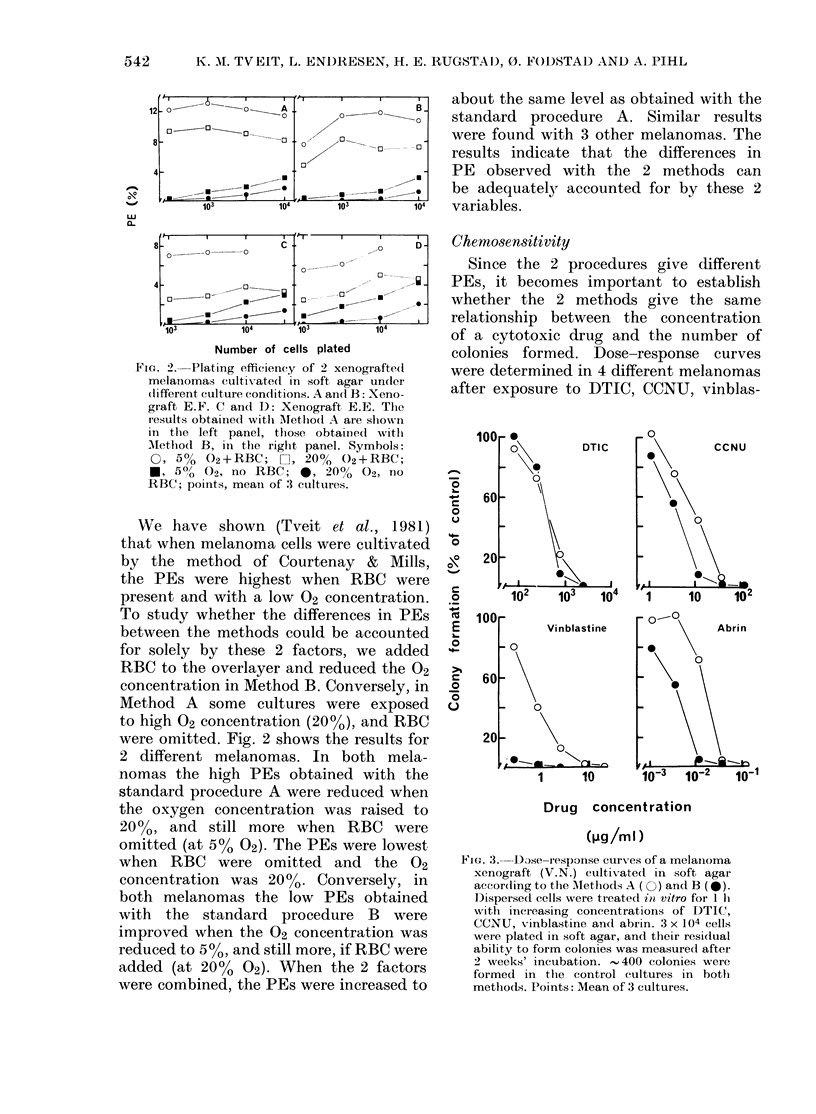
Two soft-agar methods for assaying chemosensitivity of human cancers in vitro were compared with respect to colony morphology, plating efficiency (PE) and chemosensitivity of human melanomas. In 9 xenografts and 9 patients' biopsy specimens Method A (essentially that of Courtenay & Mills, 1978) gave considerably higher PE that Method B (essentially that of Hamburger & Salmon, 1977) and, in contrast to Method B, the number of colonies was proportional to the number of cells plated. Evidence was obtained that the observed differences in PE could be attributed to the low O2 concentration and the presence of rat red blood cells in Method A. Colony morphology was similar in the 2 assays. When cells from 4 xenografted melanomas were treated in vitro with DTIC, CCNU, vinblastine and abrin, and the inhibition of colony formation was assayed concurrently in the 2 soft-agar methods, the tumour cells appeared to be more sensitive to 3 of the drugs in Method B than in A. The results demonstrate that chemosensitivity data obtained with the 2 assays cannot be directly compared.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Telfer P. A., Fry P. The effect of erythrocytes on mouse bone marrow colony development in vitro. Blood. 1971 Sep;38(3):353–359. [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Selby P. J., Smith I. E., Mills J., Peckham M. J. Growth of human tumour cell colonies from biopsies using two soft-agar techniques. Br J Cancer. 1978 Jul;38(1):77–81. doi: 10.1038/bjc.1978.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney L., Berman E. R. Oxygen toxicity: membrane damage by free radicals. Invest Ophthalmol. 1976 Oct;15(10):789–792. [PubMed] [Google Scholar]
- Fodstad O., Aass N., Pihl A. Assessment of tumour growth and of response to chemotherapy of human melanomas in athymic, nude mice. Br J Cancer Suppl. 1980 Apr;4:146–149. [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E., Kim M. B., Trent J. M., Soehnlen B. J., Alberts D. S., Schmidt H. J. Direct cloning of human ovarian carcinoma cells in agar. Cancer Res. 1978 Oct;38(10):3438–3444. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Colony formation in agar by murine plasmacytoma cells: potentiation by hemopoietic cells and serum. J Cell Physiol. 1973 Jun;81(3):397–410. doi: 10.1002/jcp.1040810312. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Soehnlen B. J., Saxe D. F., Casey W. J., Salmon S. E. In vitro clonal assay for human metastatic melanoma cells. Stem Cells. 1981;1(1):61–72. [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K., Ramanujam M., Sakamoto A. Sodium ascorbate potentiates the growth inhibitory effect of certain agents on neuroblastoma cells in culture. Proc Natl Acad Sci U S A. 1979 Feb;76(2):829–832. doi: 10.1073/pnas.76.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Sanford K. K., Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J Natl Cancer Inst. 1972 Dec;49(6):1705–1712. doi: 10.1093/jnci/49.6.1705. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Olsnes S., Pihl A. In vitro sensitivity of human melanoma xenografts to cytotoxic drugs. Correlation with in vivo chemosensitivity. Int J Cancer. 1980 Dec 15;26(6):717–722. doi: 10.1002/ijc.2910260604. [DOI] [PubMed] [Google Scholar]



