Abstract
Quorum-sensing systems are critical regulators of the expression of virulence factors of various organisms, including Pseudomonas aeruginosa. Las and Rhl are two major quorum-sensing components, and they are regulated by their corresponding autoinducers, N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL). Recent progress has demonstrated the potential of quorum-sensing molecules, especially 3-oxo-C12-HSL, for modulation of the host immune system. Here we show the specific ability of 3-oxo-C12-HSL to induce apoptosis in certain types of cells. When bone marrow-derived macrophages were incubated with synthetic 3-oxo-C12-HSL, but when they were incubated not C4-HSL, significant loss of viability was observed in a concentration (12 to 50 μM)- and incubation time (1 to 24 h)-dependent manner. The cytotoxic activity of 3-oxo-C12-HSL was also observed in neutrophils and monocytic cell lines U-937 and P388D1 but not in epithelial cell lines CCL-185 and HEp-2. Cells treated with 3-oxo-C12-HSL revealed morphological alterations indicative of apoptosis. Acceleration of apoptosis in 3-oxo-C12-HSL-treated cells was confirmed by multiple criteria (caspases 3 and 8, histone-associated DNA fragments, phosphatidylserine expression). Structure-activity correlation experiments demonstrated that the fine structure of 3-oxo-C12-HSL, the HSL backbone, and side chain length are required for maximal activity. These data suggest that Pseudomonas 3-oxo-C12-HSL specifically promotes induction of apoptosis, which may be associated with 3-oxo-C12-HSL-induced cytotoxicity in macrophages and neutrophils. Our data suggest that the quorum-sensing molecule 3-oxo-C12-HSL has critical roles in the pathogenesis of P. aeruginosa infection, not only in the induction of bacterial virulence factors but also in the modulation of host responses.
Pseudomonas aeruginosa is an opportunistic pathogen that causes a wide range of acute and chronic infections, including sepsis and wound and pulmonary infections (27). In particular, this organism is a major cause of pulmonary damage and death in patients with cystic fibrosis, diffuse panbronchiolitis, and other forms of bronchiectasis (13, 45, 46). Colonization of the respiratory tract with P. aeruginosa leads to an exuberant inflammatory response in the airways that is characterized by large numbers of neutrophils (1). Although the mechanisms by which the organism evades neutrophil defenses are unclear, P. aeruginosa persists in the tissues, resulting in chronic colonization and infection of the lungs of patients.
This organism is known to produce a variety of virulence factors, such as pigment, protease, and exotoxins. The synthesis of these factors is regulated by a cell-to-cell signaling mechanism referred to as quorum sensing (24), which was originally described in Vibrio fischeri as a LuxR/LuxI-type system (17). Las and Rhl are known to be two major quorum-sensing components in P. aeruginosa, and this mechanism enables bacteria to coordinately turn genes on and off in a density-dependent manner by the production of small diffusible molecules called autoinducers (42). P. aeruginosa predominately produces two autoinducers, N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL) (25, 26). The expression of these autoinducer-regulated virulence factors directly contributes to bacterial colonization and dissemination, which may determine the course and outcome of the disease in individuals infected with P. aeruginosa.
Recently, it has become apparent that Pseudomonas HSLs are not only important in the regulation of bacterial virulence genes but also interact with eukaryotic cells and modulate immune responses (7, 30, 40). Stimulation of human lung structural cells, such as fibroblasts and bronchial epithelial cells, with C12-HSL induces production of the chemokine interleukin-8 (IL-8) (34). More recently, it has been reported that 3-oxo-C12-HSL induced cyclooxygenase 2 and prostaglandin E2 production in human lung fibroblasts, suggesting a pivotal role for 3-oxo-C12-HSL in inflammation (36). Also, other investigators have reported inhibition of IL-12 in peritoneal exudate cells (40) and stimulation of gamma interferon in T cells by 3-oxo-C12-HSL (35). These data demonstrate that the Pseudomonas autoinducer 3-oxo-C12-HSL is a potent immunomodulator of multiple different eukaryotic cells and that production of 3-oxo-C12-HSL may greatly affect the ability of the bacterium to cause disease.
Neutrophils play a critical role in the phagocytosis and killing of P. aeruginosa and subsequently die through the onset of apoptosis. The physiological process of apoptosis at the site of infection is regulated not only by host factors but also by pathogens themselves (50). For examples, Fan and collaborators have reported inhibition of apoptosis in Chlamydia-infected cells through blockade of mitochondrial cytochrome c release and caspase activation (10). On the other hand, type III secretion systems, specific machinery that delivers a set of bacterial factors into host cells, are a critical apparatus for induction of apoptosis in several organisms, including P. aeruginosa (12, 16, 18, 48). In Pseudomonas-induced apoptosis, a variety of virulence determinants, such as pyocyanin (41), exoenzyme S (18), and cell surface porin (3), have been reported to manipulate the host apoptosis cascade. Although inappropriate induction of eukaryotic cell apoptosis could impair host defenses and favor bacterial survival and persistence, which bacterial factor(s) is responsible for and the precise pathogenesis of pathogen-induced apoptosis in the lungs of patients with chronic P. aeruginosa infections are still poorly understood.
Here we investigated the potential of Pseudomonas autoinducers and their analogues to induce apoptosis in several types of cells. Our data demonstrate that 3-oxo-C12-HSL accelerated apoptosis in macrophages and neutrophils but not in epithelial cells. The structure-activity correlation experiments show that the effect of 3-oxo-C12-HSL is strictly specific to this molecule. The roles and significance of apoptosis induction by a bacterial autoinducer, especially in a setting of persistent pulmonary P. aeruginosa infection, are discussed.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Charles River Japan (Kanagawa, Japan). All mice were housed under specific-pathogen-free conditions at the animal care facility of the Toho University School of Medicine. Mice were 6 to 8 weeks old at the start of the experiments.
Culture conditions for BM-derived macrophages.
Macrophages were derived from the bone marrow (BM) exudates of mouse femurs as described previously (38). BM cells were cultured in RPMI 1640 medium (Gibco, Grand Island, N.Y.) containing 20% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, Utah), 30% L-cell-conditioned medium as a source of macrophage colony-stimulating factor at 37°C in a humidified atmosphere containing 5% CO2. After 6 to 7 days of incubation, loosely adherent cells were collected by chilling the dish on ice and gently washed with ice-cold phosphate-buffered saline. Cells were collected by centrifugation and plated in tissue culture wells in RPMI 1640 medium containing 10% FBS. Macrophages were cultured for an additional day prior to the start of an experiment.
Preparation of cell lines and mouse neutrophils.
U-937 (human macrophage), P388D1 (mouse macrophage), CCL-185 (human lung epithelial), and HEp-2 (human epithelial) cells were obtained from the American Type Culture Collection and maintained in RPMI 1640 medium supplemented with 10% FBS. Cells were passed every 4 to 5 days by using 0.05% trypsin with 0.1% EDTA to dissociate adherent cells. To obtain enriched populations of neutrophils, the peritoneal cavity was washed with heparinized phosphate-buffered saline 20 h after administration of a peritoneal injection of 2% thioglycolate (2 ml). After centrifugation, collected cells were resuspended in RPMI 1640 medium containing 10% FBS and incubated in 5% CO2 for 2 h at 37°C. Nonadherent cells were resuspended in RPMI 1640 medium containing 10% FBS and used for subsequent experiments as neutrophils (47). These cells were judged to be more than 90% neutrophils, as assessed by light microscopy of Diff-Quick-stained cytospin preparations.
Assessment of cell viability.
Viability of cells treated with Pseudomonas HSL was measured by using TetraColor ONE (Seikagaku Kogyo, Tokyo, Japan) in accordance with the manufacturer's instructions (22). At the indicated time points, 10 μl of this reagent was added to each well and incubated for an additional 4 h. The absorbance at 450 nm was monitored, and percent viability of cells was calculated by comparison to that of nontreated control cells.
Synthesis of Pseudomonas HSLs and their analogues.
C4-HSL, 3-oxo-C12-HSL, and several HSL analogues were synthetically produced as follows (Fig. 1). For compounds 1 and 2, pentanoic acid; ethyl 3-oxoheptanoate, which was converted to 3-oxoheptanoic acid by an ordinary hydrolysis reaction and crystallized from ether-n-hexane; and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride were obtained from Tokyo Kasei Kogyo Ltd. (Tokyo, Japan). To a solution of S-(−)-α-amino-γ-butyrolactone hydrobromide (1 mmol; Sigma, St. Louis, Mo.) in water (1 ml) neutralized with triethylamine (153 μl) was added a solution of carboxylic acid (1.1 mmol) in a mixture of dioxane (14 ml), water (2 ml), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (400 mg). The mixture was stirred at room temperature for 1.5 to 2 h and then evaporated. The residue was diluted with ethyl acetate, washed with 5% HCl-5% NaHCO3-saturated NaCl, dried over anhydrous Na2SO4, and evaporated. The residue was recrystallized from acetone-ether.
FIG. 1.
Structures of the Pseudomonas HSLs and analogues used in this study.
An acyl compound, 3-oxododecanoic acid, was prepared by treatment of 2-undecanone with 1 equivalent of lithium bis(trimethylsilyl)amide in tetrahydrofuran at −78°C for 1 h, followed by addition of excess crushed dry ice. 3-Oxo-C12-l-HSL and compound 4 (3-oxo-C12-d-HSL) were synthesized by coupling of l- or d-HSL hydrobromide and 3-oxododecanoic acid with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, 1-hydroxybenzotriazole, and triethylamine in dichloromethane, respectively. C4-HSL or compound 3 (C12-HSL) was synthesized under the same reaction conditions used to make 3-oxo-C12-HSL, by using butyric acid or dodecanoic acid as an acyl substrate, respectively. However, synthesis of C4-HSL was carried out without 1-hydroxybenzotriazole. Synthesis of compound 5 (3-oxo-C12-Ala-OMe) was also accomplished by coupling of 3-oxododecanoic acid and l-alanine methyl ester hydrochloride under the same conditions. Synthesized C4-HSL and 3-oxo-C12-HSL are structurally and functionally identical to the natural molecules produced by P. aeruginosa. Each lot of HSLs and the analogues was shown to be pure by HPLC and 1H nuclear magnetic resonance experiments. No detectable levels of endotoxin were found in preparations of these substances with a Limulus amebocyte lysate assay.
ELISAs for MIP-2 and MCP-1.
Macrophage inflammatory protein 2 (MIP-2) is a human homologue of IL-8 and an important chemokine for neutrophils, whereas macrophage chemotactic protein 1 (MCP-1) is a strong macrophage-monocyte chemotactic factor. Levels of MIP-2 and MCP-1 in the culture supernatant of cells treated with or without Pseudomonas HSL were determined with a commercially available enzyme-linked immunosorbent assay (ELISA) kit (DuoSet ELISA development system; R&D Systems) in accordance with the manufacturer's directions. This ELISA method consistently detected murine MIP-2 and MCP-1 concentrations greater than 20 to 50 pg/ml. The ELISA did not cross-react with other chemokines and cytokines, such as keratinocyte-derived chemokine, MIP-1α, RANTES, IL-1, IL-2, IL-6, or tumor necrosis factor alpha.
Assessment of apoptosis.
Cells were treated with or without HSL and collected at the indicated time point. After cytocentrifuge preparations, cells were stained with Diff-Quick, and cellular and nuclear morphology was examined. To evaluate induction of apoptosis, levels of histone-associated DNA fragments and caspase 3 and 8 activities were determined in cells exposed to C4-HSL or 3-oxo-C12-HSL. DNA fragmentation was quantified by measurement of histone-associated DNA fragments with an ELISA kit (Cell Death Detection ELISAplus; Roche Diagnostics GmbH). Caspase 3 and 8 activities were determined by colorimetric assays (R&D Systems), in which caspase-specific peptides conjugated to the color reporter molecule p-nitroanilide were used. The data are expressed as fold increases compared to the values of control cells (n = 5).
Flow cytometry.
Annexin V binding to cells was performed with an apoptosis detection kit (BD Bioscience, San Jose, Calif.). Briefly, 105 cells treated with or without HSLs for 4 h were labeled with annexin V-fluorescein isothiocyanate and propidium iodide for 15 min at 25°C in the dark and analyzed by two-color flow cytometry (FACScan flow cytometer; Becton Dickinson, San Jose, Calif.) with CellQuest analysis software (Becton Dickinson) as previously described (14).
Statistical analysis.
Statistical significance was determined with the unpaired, two-tailed alternate Welsh t test and the nonparametric Mann-Whitney test. Calculations were performed with InStat for Macintosh (GraphPad Software, San Diego, Calif.).
RESULTS
Effects of Pseudomonas HSLs on production of the chemokines MIP-2 and MCP-1 in BM-derived macrophages.
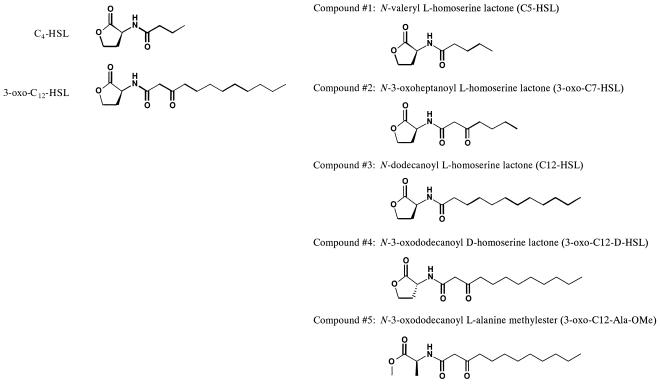
BM-derived macrophages were incubated with various concentrations (0.4 to 50 μM) of C4-HSL or 3-oxo-C12-HSL for 24 h, and then levels of MCP-1 and MIP-2 in the culture supernatants were examined (Fig. 2). We did not observe any induction of MIP-2 and MCP-1 by C4-HSL at the concentrations examined. In contrast, significant and concentration-dependent induction of MIP-2 was demonstrated by 3-oxo-C12-HSL. Interestingly, 50 μM 3-oxo-C12-HSL induced a rapid drop in MIP-2 production in BM-derived macrophages. On the other hand, the effects of 3-oxo-C12-HSL on MCP-1 were different. A significant reduction in MCP-1 was demonstrated in cells treated with 3-oxo-C12-HSL from a concentration of 10 μM. These results are consistent with previous reports demonstrating specific effects of 3-oxo-C12-HSL on the induction of neutrophil-chemotactic factor IL-8 in human fibroblasts and epithelial cells (7, 34). Also, our data show that 3-oxo-C12-HSL, but not C4-HSL, suppressed MCP-1 and MIP-2 at relatively higher concentrations.
FIG. 2.
Effects of C4-HSL and 3-oxo-C12-HSL on MIP-2 and MCP-1 production in BM-derived macrophages. BM-derived macrophages were incubated with or without increasing concentrations of Pseudomonas C4-HSL (closed columns) or 3-oxo-C12-HSL (open columns) for 18 h. Levels of MIP-2 (a) and MCP-1 (b) in culture supernatants were then determined by ELISA (n = 5). *, P < 0.05 compared with a nontreated control group.
Loss of viability of BM-derived macrophages caused by 3-oxo-C12-HSL.
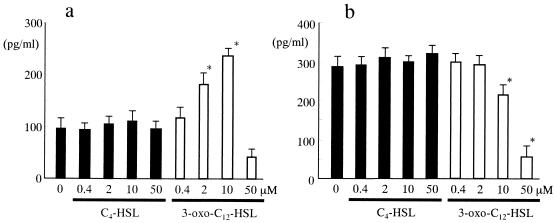
The reduction of chemokines MCP-1 and MIP-2 by 50 μM 3-oxo-C12-HSL prompted us to examine the change in the viability of cells under these experimental conditions. In cells treated with 3-oxo-C12-HSL, aggregation of cells (20 μM) and detachment and shrinkage of cells (100 μM) were observed (data not shown). Consistent with the chemokine induction data, no change in morphology was seen in cells treated with C4-HSL, even at a concentration of 100 μM (data not shown). For quantitative analysis, changes in cell viability were determined and compared to the viability of control cells 1 to 24 h after addition of 50 μM C4-HSL or 3-oxo-C12-HSL (Fig. 3a). No change in viability was seen in cells treated with C4-HSL during the course of the experiment. In contrast, 3-oxo-C12-HSL drastically induced loss of viability. Cell viability decreased to less than 50% at 1 h and to nearly 10% at 6 h after addition of 3-oxo-C12-HSL. At 2 h of incubation, significant loss of viability was observed from 12 μM 3-oxo-C12-HSL in a concentration-dependent manner. These data demonstrate that Pseudomonas 3-oxo-C12-HSL, but not C4-HSL, induces loss of viability in BM-derived macrophages.
FIG. 3.
Effects of C4-HSL and 3-oxo-C12-HSL on the viability of BM-derived macrophages. (a) BM-derived macrophages were incubated with 50 μM C4-HSL (closed circle) or 3-oxo-C12-HSL (open circle) for 1 to 24 h. Cell viability was examined at each time point and compared to that of nontreated cells (n = 5-7). (b) After incubation with increasing concentrations of 3-oxo-C12-HSL for 2 h, cell viability was examined and compared to that of nontreated control cells (n = 5-7). *, P < 0.05 compared with nontreated control group.
Sensitivity of various types of cells to Pseudomonas HSL.
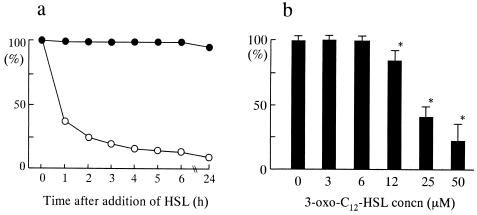
Next, we examined whether the effects of 3-oxo-C12-HSL on cell viability are specific to BM-derived macrophages. Several types of cells (neutrophils and U-937, P388D1, CCL-185, and HEp-2 cells) were incubated with C4-HSL or 3-oxo-C12-HSL (50 μM) for 2 and 24 h, and then cell viability was compared to that of the same types of cells incubated without HSL (Fig. 4). CCL-185 and HEp-2 are cell lines derived from epithelial cells, whereas U-937 and P388D1 are monocytic cells. We did not observe any loss of viability in the cells examined in the presence of 50 μM C4-HSL. In contrast, 3-oxo-C12-HSL induced significant loss of viability in neutrophils and U-937 and P388D1 cells, but not in CCL-185 and HEp-2 cells. Morphological changes, such as aggregation and shrinkage of cells, were observed in neutrophils and macrophage cell lines, but not in epithelial cell lines (data not shown). In particular, neutrophils were shown to be the most sensitive cells to the cytotoxic effects of 3-oxo-C12-HSL. We observed loss of viability in neutrophils from a concentration of 6 μM 3-oxo-C12-HSL, in a concentration-dependent fashion (data not shown). Our data show that certain types of cells, such as macrophages, monocytes, and neutrophils, may be sensitive to the effects of Pseudomonas 3-oxo-C12-HSL.
FIG. 4.
Sensitivities of various cells to Pseudomonas HSLs. Cells were incubated with 50 μM C4-HSL (closed columns) or 3-oxo-C12-HSL (open columns) for 2 or 24 h, and then cell viability at each time point was examined and compared to that of nontreated cells (n = 5 to 7). Panels: a, neutrophils; b, U-937 cells; c, P388D1 cells; d, CCL-185 cells; e, HEp-2 cells. *, P < 0.05 compared with nontreated control group.
Apoptosis markers in cells treated with Pseudomonas HSL.
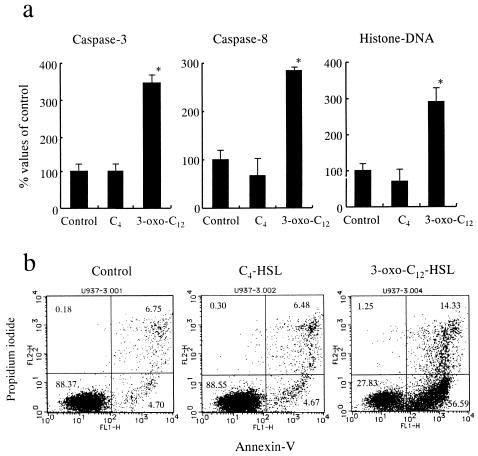
To explore the mechanisms of loss of viability, we examined the morphology of cells treated with C4-HSL or 3-oxo-C12-HSL (Fig. 5). When neutrophils were incubated with 50 μM C4-HSL, we did not observe any change in morphology. In contrast, this concentration of 3-oxo-C12-HSL induced alterations in morphology characterized by nuclear fragmentation and chromatin condensation, which are indicative of apoptosis. We observed similar morphological changes in U-937 cells when they were exposed to 3-oxo-C12-HSL, but not when they were exposed to C4-HSL. The observation of these morphological changes caused by 3-oxo-C12-HSL prompted us to examine apoptotic markers in these cells. U-937 cells were incubated with 50 μM C4-HSL or 3-oxo-C12-HSL for 4 h, and then apoptotic markers, such as caspases 3 and 8 and histone-associated DNA fragments, were examined in cell lysates (Fig. 6a). C4-HSL treatment did not induce these apoptotic factors. In contrast, a significant increase in these factors was demonstrated in cells treated with 3-oxo-C12-HSL. Furthermore, phosphatidylserine expression on the cell surface, which is a specific marker of cells entering apoptosis, was examined by flow cytometry (Fig. 6b). Approximately 5% of both the control and C4-HSL-treated cells were judged to be entering apoptosis on the basis of their surface expression of positive annexin V and negative propidium iodine. In contrast, more than 50% of the 3-oxo-C12-HSL-treated cells were apoptotic. Taken together, these data strongly suggest that Pseudomonas 3-oxo-C12-HSL, but not C4-HSL, accelerates apoptosis, which may be associated with the loss of viability seen in macrophages and neutrophils when they were exposed to 3-oxo-C12-HSL.
FIG. 5.
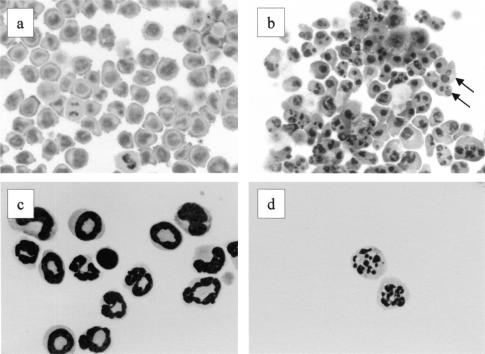
Morphology of cells exposed to 3-oxo-C12-HSL. Cells were incubated with or without 50 μM 3-oxo-C12-HSL for 4 h. After cytospin preparation, cells were stained with Diff-Quick and then cell morphology was examined. Panels: a, nontreated U-937 cells; b, C12-HSL-treated U-937 cells; c, nontreated neutrophils; d, 3-oxo-C12-HSL-treated neutrophils. Arrows indicate fragmentation of nuclei.
FIG. 6.
Changes in apoptotic markers after incubation with Pseudomonas HSLs. (a) U-937 cells were incubated with or without 50 μM C4-HSL or 3-oxo-C12-HSL for 4 h. Cell lysates were then collected and examined for caspase 3 and 8 activities and levels of histone-associated DNA fragments (n = 5 to 6). *, P < 0.05 compared with the nontreated control group. (b) U-937 cells were treated with or without 50 μM C4-HSL or 3-oxo-C12-HSL for 4 h, and then expression of annexin V-fluorescein isothiocyanate and propidium iodide was examined as described in Materials and Methods.
Effects of several HSL analogues on cell viability.
Next, we examined whether this HSL-induced cytotoxicity is structurally specific to the 3-oxo-C12-HSL molecule and which part of the 3-oxo-C12-HSL structure is critical for this phenomenon. To determine the molecular basis of this activity, we synthesized a set of HSL analogues on which structural alterations were performed (Fig. 1). Cells were incubated with or without these compounds, and viability was examined (Fig. 7). In these experiments, we did not observe any change in viability caused by any of these compounds, except 3-oxo-C12-HSL. These data suggest that the fine structure of 3-oxo-C12-HSL, in addition to the HSL backbone and the length of side chains, may be important for the maximal ability of this molecule to express cytotoxic activity.
FIG. 7.
Effects of Pseudomonas HSL analogues on cell viability. U-937 cells were incubated with 50 μM Pseudomonas C4-HSL, 3-oxo-C12-HSL, and their analogues for 4 h. Cell viability was then examined and compared to that of nontreated cells (n = 5).
DISCUSSION
The present data demonstrate that 3-oxo-C12-HSL, an autoinducer of the Las quorum-sensing system, specifically induces apoptosis in neutrophils and macrophages. Since apoptosis is a critical event for immunological and inflammatory processes, dysregulation of this physiological cascade may greatly favor bacteria for their survival and persistence. These results further reinforce the current concept that bacterial HSLs not only regulate bacterial virulence factors but also modulate eukaryotic cell functions, suggesting that this molecule has a pivotal role in the pathogenesis of P. aeruginosa infection (36).
The features of chronic P. aeruginosa infection in the lungs of cystic fibrosis patients are predominantly characterized by a massive influx of neutrophils into the airway. Several bacterial components or products, such as flagellin (7), pyocyanin (41), and nitrite reductase (21), are responsible for the induction of neutrophil chemotactic factors. Direct evidence of the involvement of quorum-sensing molecules in neutrophil influx was first reported by DiMango and collaborators, who have demonstrated that diverse Pseudomonas gene products, including the autoinducer 3-oxo-C12-HSL, stimulate IL-8 production in respiratory epithelial cells (7). Recently, it has been reported that 3-oxo-C12-HSL-mediated IL-8 production in fibroblasts and epithelial cells is transcriptionally regulated by NF-κB and activator protein 2 (34). Our data are in accordance with these reports and further demonstrate the specific ability of 3-oxo-C12-HSL to induce the chemokine MIP-2 (a murine homologue of IL-8) in murine BM-derived macrophages. In contrast, although the meaning and significance of the finding are not known, we observed suppression of MCP-1 by 3-oxo-C12-HSL. This is in contrast to the result obtained with the murine skin injection model, in which a more-than-sixfold increase in MCP-1 mRNA was demonstrated (35). Other investigators have reported the involvement of 3-oxo-C12-HSL in the balance between Th1 and Th2 by demonstrating suppression of IL-12 in macrophages and promotion of immunoglobulin G1 and immunoglobulin E production in spleen cells (40). Taken together, these data strongly suggest that the Pseudomonas autoinducer 3-oxo-C12-HSL is a potent immunomodulator of multiple different eukaryotic cells.
In patients with chronic P. aeruginosa infections of the lungs, the organism is found in microcolonies or in a biofilm that consists of clusters of bacteria embedded in a matrix of polysaccharide (19, 32). Although the concentration of autoinducers in the lungs of P. aeruginosa-infected patients remains unknown, C4-HSL and 3-oxo-C12-HSL, in addition to mRNAs for these autoinducers and autoinducer-regulated virulence factors, were detected in the sputum of these patients (8, 37). These data indicate that a functional cell-to-cell signaling mechanism occurs in the airways of these patients. It has been shown that biofilms of P. aeruginosa grown in vitro can produce approximately 600 μM 3-oxo-C12-HSL, a concentration that is significantly higher than what has previously been measured in planktonic cultures (4). Considering the fact that most of the P. aeruginosa bacteria are present in a biofilm in the lungs of patients with chronic infections, the concentration of autoinducers, at least in some areas of the active site of infection, may be equivalent to or higher than 12 to 50 μM, concentrations at which we consistently observed induction of apoptosis in susceptible cells. However, the concentration that eukaryotic cells in the lungs would actually be exposed to remains an important question. Recently, several investigators have reported that sequential activation of quorum-sensing systems is critical for P. aeruginosa biofilm differentiation and maturation (6, 23). Experiments to determine whether lower concentrations of 3-oxo-C12-HSL act in concert with other bacterial products, such as biofilm matrix molecules, are ongoing in our laboratory.
Apoptosis is important in the normal resolution phase of inflammation, since it leads to functional down-regulation (44) and to the recognition and clearance of apoptotic neutrophils by macrophages (31). Ingestion of apoptotic neutrophils triggers macrophages to produce antiinflammatory cytokines and suppresses the generation of proinflammatory mediators (9, 20). Since apoptotic death is less proinflammatory, inappropriate induction of apoptosis rather than necrosis could confer a further advantage on an invading pathogen. A wide range of pathogens have been reported to interfere with the apoptosis cascade as a survival strategy by means of an array of pathogen-encoded virulence determinants (11, 43). Recently, Usher and colleagues have reported induction of neutrophil apoptosis by Pseudomonas pyocyanin and suggested it as a potential mechanism of persistent infection (41). In addition, a variety of cells, including macrophages, epithelial cells, fibroblasts, and T cells, have been shown to be targets of P. aeruginosa-induced apoptosis (2, 12, 18). The present study showed that macrophages and neutrophils are susceptible, while epithelial cells are resistant, to 3-oxo-C12-HSL-induced cytotoxicity. However, the susceptibility of these cells to apoptosis at sites of infection, especially in the airways of cystic fibrosis patients, may be more complicated because bacterial components and products induce apoptosis-inhibiting factors, such as granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (29). Although the precise mechanisms of 3-oxo-C12-HSL-mediated apoptosis remain unknown, a clear elevation of caspase 3 and 8 activities in susceptible cells was observed. An increase in caspase 8 activity may be important because this enzyme play a critical role as the initiator caspase in the extrinsic apoptotic cascade (28). The effects of 3-oxo-C12-HSL on an upstream molecule(s) in the extrinsic pathway, such as Fas and the Fas-associated death domain, may be potential candidates for research into mechanisms of 3-oxo-C12-HSL-induced apoptosis. In this regard, Jendrossek and colleagues have reported that P. aeruginosa infection triggered up-regulation of Fas expression in epithelial cells and that a deficiency in Fas or Fas ligand prevented Pseudomonas-induced apoptosis (15).
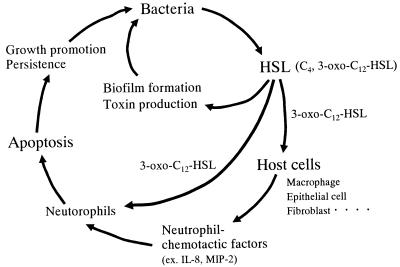
We propose a mechanism of chronic P. aeruginosa infection of the lungs involving quorum-sensing systems (Fig. 8). P. aeruginosa produces HSLs, which induce the production of a panel of virulence factors. Activation of the quorum-sensing cascade also promotes biofilm formation at the site of infection, which makes conditions more favorable for bacterial persistence in the lungs. Bacterial autoinducers, especially 3-oxo-C12-HSL, stimulate several types of cells, such as epithelial cells, fibroblasts, and macrophages, to induce production of neutrophil chemotactic factors (IL-8 in humans and MIP-2 in mice). Migrated neutrophils are triggered to produce several toxic substances for killing of bacteria, but these molecules, as well as bacterial virulence factors, also cause the tissue destruction that is a hallmark of the lungs of cystic fibrosis patients. In those lungs, at least where bacteria are actively producing autoinducers and autoinducer-regulated virulence factors, cells may be exposed to these bacterial factors. Because of their short life span, neutrophils start to enter apoptosis, and this process may be accelerated by the presence of bacterial factors, such as 3-oxo-C12-HSL, as described in this report. Apoptotic neutrophils, in addition to secreted mucus and other cell debris, may become nutrients for the growth of bacteria and a niche for their survival. Taken together, our data support the notion that Pseudomonas quorum-sensing systems contribute to bacterial colonization of and persistence in the lungs through bifunctional ways, i.e., (i) modulation of bacterial functions and biofilm formation and (ii) dysregulation of host immune systems.
FIG. 8.
Bifunctional roles of 3-oxo-C12-HSL in the pathogenesis of chronic Pseudomonas lung infection. ex., for example.
Now that the pathological roles of the quorum-sensing system in P. aeruginosa infection are known, quorum-sensing system inhibition by interference with the activation cascade or, more specifically, by suppression of autoinducer production has been considered an attractive therapeutic strategy. Recently, we have reported that the macrolide antibiotic azithromycin strongly suppressed the Pseudomonas quorum-sensing system, especially production of 3-oxo-C12-HSL (39). Also, the use of autoinducer analogs as competitors is currently being explored (5, 33, 49). In this regard, our data obtained from a set of HSLs demonstrate that the fine structure of 3-oxo-C12-HSL, in addition to the HSL backbone and side chain length, may be important for maximal activity, which suggests a specific binding molecule or site in cells as a target for 3-oxo-C12-HSL. Given that 3-oxo-C12-HSL-induced apoptosis plays a crucial role in the pathogenesis of P. aeruginosa infection, the search for and identification of a eukaryotic molecular target for 3-oxo-C12-HSL is a promising research subject.
In conclusion, the present data demonstrate that the Pseudomonas autoinducer 3-oxo-C12-HSL specifically induced apoptosis in critical cell populations, i.e., macrophages and neutrophils. Induction of apoptosis in phagocytes may thus be another powerful weapon used as a host defense evasion strategy by this organism. Further understanding of the cellular mechanisms of 3-oxo-C12-HSL-induced cell death may provide new insight into the mechanisms of persistence of this organism in the lungs and may also give rise to therapeutic strategies aimed at preserving the host responses to this serious infection.
Acknowledgments
We thank Hajime Hashimoto for the helpful suggestion and critical discussion.
Editor: B. B. Finlay
REFERENCES
- 1.Baltimore, R. S., C. D. Christie, and G. J. Smith. 1989. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis: implications for the pathogenesis of progressive lung deterioration. Am. Rev. Respir. Dis. 140:1650-1661. [DOI] [PubMed] [Google Scholar]
- 2.Bruno, T. F., D. E. Woods, and C. H. Mody. 2000. Exoenzyme S from Pseudomonas aeruginosa induces apoptosis in T lymphocytes. J. Leukoc. Biol. 67:808-816. [DOI] [PubMed] [Google Scholar]
- 3.Buommino, E., F. Morelli, S. Metafora, F. Rossano, B. Perfetto, A. Baroni, and M. A. Tufano. 1999. Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect. Immun. 67:4794-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlton, T. S., R. de Nys, A. Netting, N. Kumar, M. Hentzer, M. Givskov, and S. Kjelleberg. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ. Microbiol. 2:530-541. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra, S. R., C. Harty, D. S. Hooi, M. Daykin, P. Williams, G. Telford, D. I. Pritchard, and B. W. Bycroft. 2003. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-l-homoserine lactone as immune modulators. J. Med. Chem. 46:97-104. [DOI] [PubMed] [Google Scholar]
- 6.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 7.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson, D. L., R. Endersby, A. Kirkham, K. Stuber, D. D. Vollman, H. R. Rabin, I. Mitchell, and D. G. Storey. 2002. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 70:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadok, V. A., D. L. Bratton, A. Konowal, P. W. Freed, J. Y. Westcott, and P. M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Investig. 101:890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassme, H., V. Jendrossek, and E. Gulbins. 2001. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 6:441-445. [DOI] [PubMed] [Google Scholar]
- 12.Hauser, A. R., and J. N. Engel. 1999. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoiby, N. 1994. Diffuse panbronchiolitis and cystic fibrosis: East meets West. Thorax 49:531-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homburg, C. H., M. de Haas, A. E. von dem Borne, A. J. Verhoeven, C. P. Reutelingsperger, and D. Roos. 1995. Human neutrophils lose their surface Fc gamma RIII and acquire annexin V binding sites during apoptosis in vitro. Blood 85:532-540. [PubMed] [Google Scholar]
- 15.Jendrossek, V., H. Grassme, I. Mueller, F. Lang, and E. Gulbins. 2001. Pseudomonas aeruginosa-induced apoptosis involves mitochondria and stress-activated protein kinases. Infect. Immun. 69:2675-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jesenberger, V., K. J. Procyk, J. Yuan, S. Reipert, and M. Baccarini. 2000. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 192:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman, M. R., J. Jia, L. Zeng, U. Ha, M. Chow, and S. Jin. 2000. Pseudomonas aeruginosa mediated apoptosis requires the ADP-ribosylating activity of exoS. Microbiology 146:2531-2541. [DOI] [PubMed] [Google Scholar]
- 19.Lam, J., R. Chan, K. Lam, and J. W. Costerton. 1980. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 28:546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meagher, L. C., J. S. Savill, A. Baker, R. W. Fuller, and C. Haslett. 1992. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J. Leukoc. Biol. 52:269-273. [PubMed] [Google Scholar]
- 21.Oishi, K., B. Sar, A. Wada, Y. Hidaka, S. Matsumoto, H. Amano, F. Sonoda, S. Kobayashi, T. Hirayama, T. Nagatake, and K. Matsushima. 1997. Nitrite reductase from Pseudomonas aeruginosa induces inflammatory cytokines in cultured respiratory cells. Infect. Immun. 65:2648-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto, O., S. Fujiwara, M. Abe, and Y. Sato. 1999. Dermatopontin interacts with transforming growth factor beta and enhances its biological activity. Biochem. J. 337:537-541. [PMC free article] [PubMed] [Google Scholar]
- 23.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 25.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 28.Roy, S., and D. W. Nicholson. 2000. Cross-talk in cell death signaling. J. Exp. Med. 192:21-26. [PubMed] [Google Scholar]
- 29.Saba, S., G. Soong, S. Greenberg, and A. Prince. 2002. Bacterial stimulation of epithelial G-CSF and GM-CSF expression promotes PMN survival in CF airways. Am. J. Respir. Cell Mol. Biol. 27:561-567. [DOI] [PubMed] [Google Scholar]
- 30.Saleh, A., C. Figarella, W. Kammouni, S. Marchand-Pinatel, A. Lazdunski, A. Tubul, P. Brun, and M. D. Merten. 1999. Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone inhibits expression of P2Y receptors in cystic fibrosis tracheal gland cells. Infect. Immun. 67:5076-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savill, J. S., A. H. Wyllie, J. E. Henson, M. J. Walport, P. M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Investig. 83:865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 33.Smith, K. M., Y. Bu, and H. Suga. 2003. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 10:81-89. [DOI] [PubMed] [Google Scholar]
- 34.Smith, R. S., E. R. Fedyk, T. A. Springer, N. Mukaida, B. H. Iglewski, and R. P. Phipps. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-κB and activator protein-2. J. Immunol. 167:366-374. [DOI] [PubMed] [Google Scholar]
- 35.Smith, R. S., S. G. Harris, R. Phipps, and B. Iglewski. 2002. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184:1132-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, R. S., R. Kelly, B. H. Iglewski, and R. P. Phipps. 2002. The Pseudomonas autoinducer N-(3-oxododecanoyl)homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169:2636-2642. [DOI] [PubMed] [Google Scholar]
- 37.Storey, D. G., E. E. Ujack, H. R. Rabin, and I. Mitchell. 1998. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 66:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tateda, K., R. Comte, J. C. Pechere, T. Kohler, K. Yamaguchi, and C. Van Delden. 2001. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usher, L. R., R. A. Lawson, I. Geary, C. J. Taylor, C. D. Bingle, G. W. Taylor, and M. K. Whyte. 2002. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J. Immunol. 168:1861-1868. [DOI] [PubMed] [Google Scholar]
- 42.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 44.Whyte, M. K., L. C. Meagher, J. MacDermot, and C. Haslett. 1993. Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 150:5124-5134. [PubMed] [Google Scholar]
- 45.Wilson, R., and R. B. Dowling. 1998. Lung infections. 3. Pseudomonas aeruginosa and other related species. Thorax 53:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood, R. E. 1976. Pseudomonas: the compromised host. Hosp. Pract. 11:91-100. [DOI] [PubMed] [Google Scholar]
- 47.Yasui, K., Y. Sekiguchi, M. Ichikawa, H. Nagumo, T. Yamazaki, A. Komiyama, and H. Suzuki. 2002. Granulocyte macrophage-colony stimulating factor delays neutrophil apoptosis and primes its function through Ia-type phosphoinositide 3-kinase. J. Leukoc. Biol. 72:1020-1026. [PubMed] [Google Scholar]
- 48.Yuk, M. H., E. T. Harvill, P. A. Cotter, and J. F. Miller. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-κB activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991-1004. [DOI] [PubMed] [Google Scholar]
- 49.Zhu, J., J. W. Beaber, M. I. More, C. Fuqua, A. Eberhard, and S. C. Winans. 1998. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J. Bacteriol. 180:5398-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zychlinsky, A., and P. Sansonetti. 1997. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J. Clin. Investig. 100:493-495. [DOI] [PMC free article] [PubMed] [Google Scholar]