Abstract
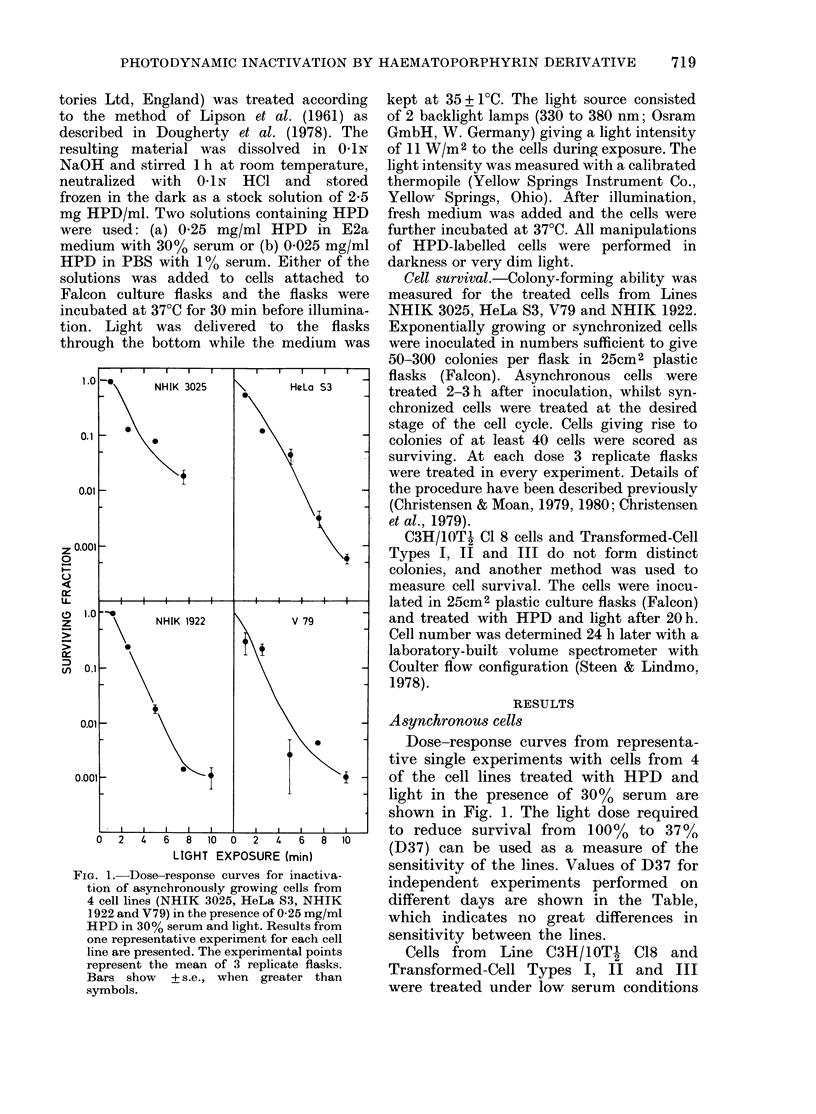
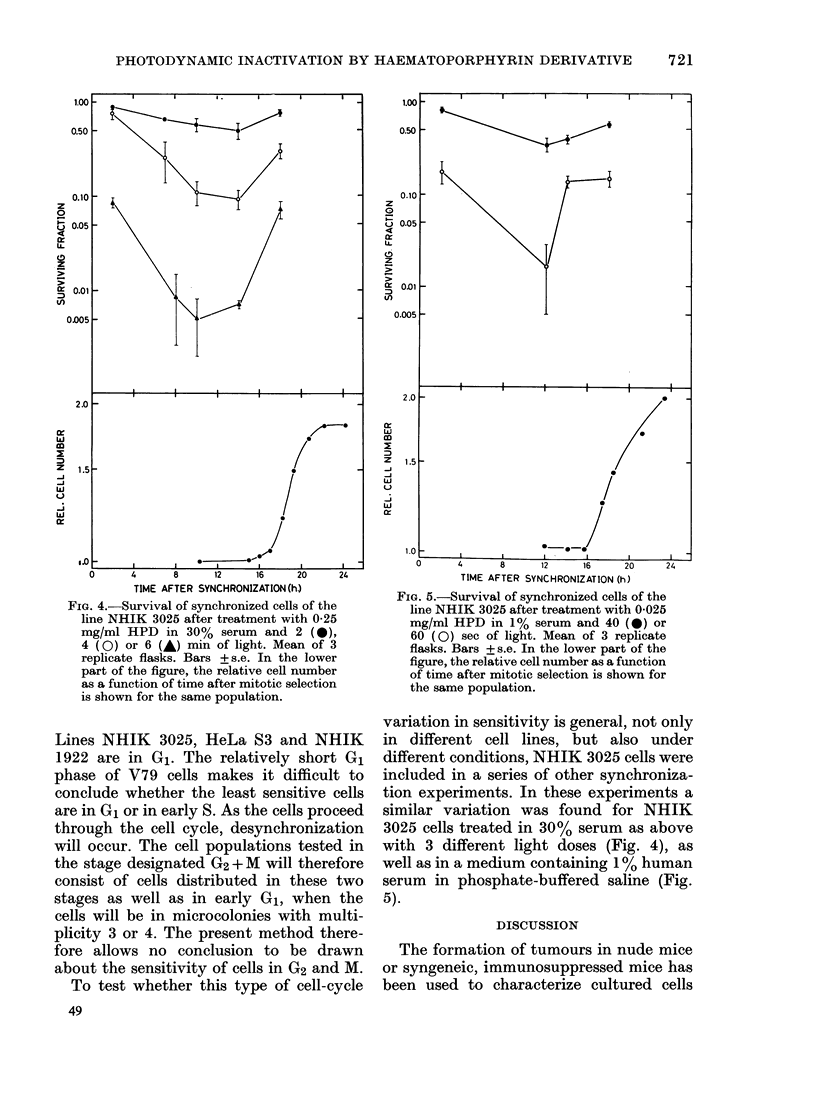
Phototherapy in the presence of haematoporphyrin derivative has been shown to have a preferential effect on malignant tumours when compared to normal tissue. This communication presents a comparison of the sensitivity to photochemotherapy in vitro of different cell lines. Asynchronous populations of cells were exposed to light in the presence of haematoporphyrin derivative, and found to be inactivated with a comparable efficiency. The lines were of human, Chinese-hamster or mouse origin and had different abilities to form tumours after heterotransplantation into nude mice or transplantation into syngeneic, immunosuppressed mice. Synchronized cells from 4 of the lines showed a similar variation in sensitivity to light throughout the cell cycle. Cells near the middle of interphase showed the highest sensitivity, whilst cells in early G1 were found to be least sensitive towards treatment with haematoporphyrin derivative and light.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnett R., Charalambides A. A., Jones K., Magnus I. A., Ridge R. J. The direct determination of porphyrin carboxylic acids. High-pressure liquid chromatography with solvent systems containing phase-transfer agents. Biochem J. 1978 Aug 1;173(2):693–696. doi: 10.1042/bj1730693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T., Moan J. Photodynamic inactivation of synchronized human cells in vitro in the presence of hematoporphyrin. Cancer Res. 1979 Sep;39(9):3735–3737. [PubMed] [Google Scholar]
- Christensen T., Moan J., Wibe E., Oftebro R. Photodynamic effect of haematoporphyrin throughout the cell cycle of the human cell line NHIK 3025 cultivated in vitro. Br J Cancer. 1979 Jan;39(1):64–68. doi: 10.1038/bjc.1979.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Gomer C. J., Weishaupt K. R. Energetics and efficiency of photoinactivation of murine tumor cells containing hematoporphyrin. Cancer Res. 1976 Jul;36(7 Pt 1):2330–2333. [PubMed] [Google Scholar]
- Dougherty T. J., Kaufman J. E., Goldfarb A., Weishaupt K. R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978 Aug;38(8):2628–2635. [PubMed] [Google Scholar]
- Forbes I. J., Cowled P. A., Leong A. S., Ward A. D., Black R. B., Blake A. J., Jacka F. J. Phototherapy of human tumours using haematoporphyrin derivative. Med J Aust. 1980 Nov 1;2(9):489–493. doi: 10.5694/j.1326-5377.1980.tb100708.x. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S., Williams L. J., Jr Heterotransplantation of human malignant tumors in "nude" thymusless mice. II. Malignant tumors induced by injection of cell cultures derived from human solid tumors. J Natl Cancer Inst. 1974 Mar;52(3):921–930. doi: 10.1093/jnci/52.3.921. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Smith D. M. Photoinactivation of Chinese hamster cells by hematoporphyrin derivative and red light. Photochem Photobiol. 1980 Sep;32(3):341–348. doi: 10.1111/j.1751-1097.1980.tb03772.x. [DOI] [PubMed] [Google Scholar]
- Kelly J. F., Snell M. E., Berenbaum M. C. Photodynamic destruction of human bladder carcinoma. Br J Cancer. 1975 Feb;31(2):237–244. doi: 10.1038/bjc.1975.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. F., Snell M. E. Hematoporphyrin derivative: a possible aid in the diagnosis and therapy of carcinoma of the bladder. J Urol. 1976 Feb;115(2):150–151. doi: 10.1016/s0022-5347(17)59108-9. [DOI] [PubMed] [Google Scholar]
- Kessel D. Effects of photoactivated porphyrins at the cell surface of leukemia L1210 cells. Biochemistry. 1977 Jul 26;16(15):3443–3449. doi: 10.1021/bi00634a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Transport and binding of hematoporphyrin derivative and related porphyrins by murine leukemia L1210 cells. Cancer Res. 1981 Apr;41(4):1318–1323. [PubMed] [Google Scholar]
- Kohn K., Kessel D. On the mode of cytotoxic action of photo-activated porphyrins. Biochem Pharmacol. 1979 Aug 15;28(16):2465–2470. doi: 10.1016/0006-2952(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Moan J., Pettersen E. O., Christensen T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br J Cancer. 1979 Apr;39(4):398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J., Smedshammer L., Christensen T. Photodynamic effects on human cells exposed to light in the presence of hematoporphyrin. pH effects. Cancer Lett. 1980 Jun;9(4):327–332. doi: 10.1016/0304-3835(80)90025-7. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Gray M. J., Silberman L., Lipson R. L. Identification of neoplastic versus normal cells in human cervical cell culture. Obstet Gynecol. 1974 May;43(5):635–639. [PubMed] [Google Scholar]
- Musser D. A., Wagner J. M., Weber F. J., Datta-Gupta N. The effect of tumor localizing porphyrins on the conversion of fibrinogen to fibrin. Res Commun Chem Pathol Pharmacol. 1979 Nov;26(2):357–382. [PubMed] [Google Scholar]
- Ohara H., Terasima T. Variations of cellular sulfhydryl content during cell cycle of HeLa cells and its correlation to cyclic change of x-ray sensitivity. Exp Cell Res. 1969 Nov;58(1):182–185. doi: 10.1016/0014-4827(69)90133-5. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., FISHER H. W. Clonal growth in vitro of human cells with fibroblastic morphology; comparison of growth and genetic characteristics of single epithelioid and fibroblast-like cells from a variety of human organs. J Exp Med. 1957 Jul 1;106(1):145–158. doi: 10.1084/jem.106.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. O., Bakke O., Lindmo T., Oftebro R. Cell cycle characteristics of synchronized and asynchronous populations of human cells and effect of cooling of selected mitotic cells. Cell Tissue Kinet. 1977 Nov;10(6):511–522. doi: 10.1111/j.1365-2184.1977.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Rofstad E. K., Pettersen E. O., Lindmo T., Oftebro R. The proliferation kinetics of NHIK 1922 cells in vitro and in solid tumours in athymic mice. Cell Tissue Kinet. 1980 Mar;13(2):163–171. doi: 10.1111/j.1365-2184.1980.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Sandberg S., Romslo I. Porphyrin-induced photodamage at the cellular and the subcellular level as related to the solubility of the porphyrin. Clin Chim Acta. 1981 Jan 22;109(2):193–201. doi: 10.1016/0009-8981(81)90334-x. [DOI] [PubMed] [Google Scholar]
- Saxholm H. J. The oncogenic potential of three different 7,12-dimethylbenz(a)anthracene transformed C3H/10T1/2 cell clones at various passages and the importance of the mode of immunosuppression. Eur J Cancer. 1979 Apr;15(4):515–526. doi: 10.1016/0014-2964(79)90087-2. [DOI] [PubMed] [Google Scholar]
- Schothorst A. A., De Haas C. A., Suurmond D. Photochemical damage to skin fibroblasts caused by protoporphyrin and violet light. Arch Dermatol Res. 1980;268(1):31–42. doi: 10.1007/BF00403884. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Lindmo T. Cellular and nuclear volume during the cell cycle of NHIK 3025 cells. Cell Tissue Kinet. 1978 Jan;11(1):69–81. doi: 10.1111/j.1365-2184.1978.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Utsumi H., Elkind M. M. Photodynamic cytotoxicity of mammalian cells exposed to sunlight-simulating near ultraviolet light in the presence of the carcinogen 7,12-dimethylbenz(a)anthracene. Photochem Photobiol. 1979 Aug;30(2):271–278. doi: 10.1111/j.1751-1097.1979.tb07146.x. [DOI] [PubMed] [Google Scholar]



