Abstract
Streptococcus pneumoniae is a leading cause of gram-positive sepsis, and lipoteichoic acid (LTA) may be important in causing gram-positive bacterial septic shock. Even though pneumococcal LTA is structurally distinct from the LTA of other gram-positive bacteria, the immunological properties of pneumococcal LTA have not been well characterized. We have investigated the ability of LTAs to stimulate human monocytes by using highly pure and structurally intact preparations of pneumococcal LTA and its two structural variants. The variants were pneumococcal LTA with only one acyl chain (LTA-1) and completely deacylated LTA (LTA-0). The target cells used in the study were peripheral blood mononuclear cells (PBMCs) and two model cell lines (CHO/CD14/TLR2 and CHO/CD14/TLR4) that express human CD25 protein in response to Toll-like receptor 2 (TLR2) and TLR4 stimulation, respectively. Intact pneumococcal LTA and LTA-1 stimulated PBMC and CHO/CD14/TLR2 cells in a dose-dependent manner but did not stimulate CHO/CD14/TLR4 cells. Pneumococcal LTA was about 100-fold less potent than Staphylococcus aureus LTA in stimulating the CHO/CD14/TLR2 cells and PBMCs. LTA-0 (or pneumococcal teichoic acid) stimulated neither CHO/CD14/TLR2 nor CHO/CD14/TLR4 cells even at high concentrations. Excess teichoic acid, LTA-0, antibodies to phosphocholine, or antibodies to TLR4 did not inhibit the LTA-induced TLR2 stimulation. However, antibodies to CD14, TLR1, or TLR2 suppressed tumor necrosis factor alpha (TNF-α) production by PBMCs in response to LTA or LTA-1. These results suggest that pneumococcal LTA with one or both acyl chains stimulates PBMCs primarily via TLR2 with the help of CD14 and TLR1.
Bacterial infections trigger both the adaptive and innate branches of the immune system in the host. By recognizing the antigens specific to a particular pathogen, the adaptive immunity provides highly effective antigen-specific protection. However, it becomes relevant only during the late phase of the infection. In contrast, innate immunity protects the host during the early phase of infection by using germ line-encoded receptors to recognize the structurally conserved molecular patterns present in many pathogens. The two groups of molecules are commonly called pathogen-associated molecular pattern (PAMP) molecules and the PAMP receptors (29). In addition to providing the initial protection to the host, innate immunity influences the subsequent development of adaptive immunity (24).
Accumulating evidence suggests that the Toll-like receptor (TLR) is a key PAMP receptor. Humans have 10 TLRs, each of which exists as a hetero- or homodimer complex on the cell surface. Each TLR appears to recognize different PAMP molecules (45). Although various PAMP molecules and PAMP receptors have been identified (20), the best-characterized PAMP and PAMP receptor pair is made up of gram-negative bacterial lipopolysaccharide (LPS) and TLR4. LPS is the endotoxin mainly responsible for gram-negative bacterial septic shock (9, 43). It is an amphiphilic molecule formed by linking a polysaccharide molecule to lipid A (39). Upon entering the host, LPS binds to LPS-binding proteins (LBP) in the serum (41) and then with soluble (or membrane) CD14 in the serum (or cell surface) (53). The LPS in the LPS-CD14-LBP complex is then transferred to the TLR4-MD-2 complex on the target cells in the host (42). LPS then triggers the target cells (macrophages and polymorphonuclear leukocytes), leading to activation of NF-κB and induction of cytokines (such as tumor necrosis factor alpha [TNF-α]), proinflammatory mediators, and cell adhesion molecules (34). The in vivo importance of TLR4 has been shown by the increased susceptibility of TLR4-deficient mice to gram-negative bacteria (21).
The lipoteichoic acid (LTA) of gram-positive bacteria is considered to be analogous to the LPS of gram-negative bacteria and shares many of its biochemical and physiological properties (17). Like LPS, LTA is an amphiphile that is formed by linking a hydrophilic polyphosphate polymer to a glycolipid (13). Its immunostimulatory potential had been controversial, because the LTA preparations used in earlier studies were either damaged or contaminated (16, 31). Recent studies using highly purified preparations of Staphylococcus aureus LTA have clearly shown that staphylococcal LTA can efficiently stimulate monocytes via TLR2 to produce TNF-α (12). Furthermore, staphylococcal LTA can synergize with peptidoglycan (PGN) to induce septic shock and multiorgan failure in rats (10, 25, 26, 32). These findings have supported the conclusion that LTA is responsible for gram-positive sepsis just as LPS is for gram-negative sepsis (17).
Along with S. aureus, Streptococcus pneumoniae is a common etiologic agent for gram-positive sepsis (1, 37, 51). S. pneumoniae is also a major cause of pneumonia, otitis media, and meningitis (50). Recently, it was shown that pneumococci activate the innate immune system via TLR2 (54), which is critically involved in pneumococcal meningitis (11). Pneumococcal LTA is a potent inducer of acute inflammation (38) and may be important in causing septic shock and/or other diseases, perhaps by stimulating TLR2 (27), as has been shown for staphylococcal LTA (12). However, the structures of the LTA from S. aureus and S. pneumoniae are quite different. Staphylococcal LTA, like other common LTAs, is composed of a polyphosphate polymer of 20 to 50 small repeating units of various sizes (about 130 to 320 Da) attached to a neutral glycolipid (30). Pneumococcal LTA, on the other hand, has a positively charged glycolipid and polyphosphate polymer that is formed by linking 6 to 8 large repeating units with 1,299 Da per repeating unit (3, 14). Therefore, to establish the role of pneumococcal LTA in pneumococcal infections, we have prepared highly purified pneumococcal LTAs and investigated their ability to stimulate cells via TLRs.
MATERIALS AND METHODS
Reagents.
Pneumococcal cell wall polysaccharide (C-PS [teichoic acid]) was purchased from Statens Serum Institut (Copenhagen, Denmark). Proteinase K, lysozyme, TEPC-15 (mouse immunoglobulin A [IgA], kappa), S. aureus PGN, and Escherichia coli 055:B5 LPS were obtained from Sigma Chemical Co. (St. Louis, Mo.). The E. coli LPS was repurified by phenol extraction before use (22). Porphyromonas gingivalis 33277 LPS was prepared as previously described (23). Mouse anti-human TLR2 (clone 2392; IgG1) and mouse anti-human CD14 (clone mem-18; IgG1) antibodies were obtained from Genentech Corp. (San Francisco, Calif.) and Caltag Laboratories (Burlingame, Calif.), respectively. Mouse anti-human TLR4 (clone HTA125; IgG2a), anti-human TLR1 (clone GD2.F4; IgG1), and all isotype-matched control antibodies (IgG1 and IgG2a) were purchased from eBioscience (San Diego, Calif.).
Purification of pneumococcal LTA.
Pneumococcal LTA was purified by the method used by Behr et al. (3) with an additional purification step: ion-exchange chromatography. Briefly, S. pneumoniae strain R36A was cultured overnight at 37°C in Todd-Hewitt broth (Difco, Detroit, Mich.) with 0.5% yeast extract. Cells were pelleted, resuspended in 0.05 M sodium acetate (pH 4.0), and disrupted by ultrasonication (Sonicator model W-220F). LTA was extracted from the lysate with a chloroform-methanol-water (1:1:0.9) mixture, and after phase separation, the aqueous phase containing LTA was collected. Lipid contaminants were removed from the aqueous phase by extraction with chloroform, and residual methanol was removed by evaporation. Then the LTA was adsorbed onto an octyl-Sepharose CL-4B column (2.5 by 20 cm) (Sigma) equilibrated in a mixture of 15% n-propanol and 0.05 M sodium acetate (pH 4.7). LTA was eluted from the column with a stepwise n-propanol gradient (20, 35, and 45%), and column fractions containing the LTA were pooled and dialyzed against water. LTA was further purified by Q-Sepharose ion-exchange chromatography (1 by 10 cm) (Fast Flow; Sigma), equilibrated in the 10 mM 2-amino-2-methyl-1-propanol-HCl buffer (pH 9.5) with 30% n-propanol. LTA was eluted from the column with a linear salt gradient (0 to 0.3 M NaCl in the equilibration buffer), and the eluate was collected in 5-ml aliquots. Each fraction was individually dialyzed against pyrogen-free water, evaporated under vacuum, and stored at −20°C.
Structure confirmation, quantitation, and determination of purity of LTAs.
The molecular identities of the LTA were determined by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry with Voyager DE Pro mass spectrometer (Applied Biosystems, Framingham, Mass.). Mass spectrometry of the LTA showed three major peaks, with molecular weights corresponding to approximately 7,270, 8,570, and 9,870 m/z units. These peaks correspond to LTA species containing 5, 6, and 7 tetra-saccharide repeating units, with 2 phosphocholine (PC) moieties per repeating unit. The molecular weights of these peaks did not change during the three stages of purification (data not shown), indicating that pneumococcal LTA was not altered or degraded during our multistep purification procedure. The concentrations of the LTA and its variants were determined by measuring their inorganic phosphorous contents (2). The purity of the LTAs was determined by measuring their protein and endotoxin contents by conventional silver staining after polyacrylamide gel electrophoresis and by Limulus amebocyte lysate (LAL) assay (BioWhittaker, Walkersville, Md.), respectively. DNA or RNA contamination was assessed by measuring UV absorbption at 260 and 280 nm.
Deacylation of the pneumococcal LTA.
Two variants of pneumococcal LTA were prepared by alkali hydrolysis: partially deacylated LTA (LTA-1; monoacylated) and completely deacylated LTA (LTA-0). LTA-0 was prepared by incubating the LTA in 0.1 N NaOH at 37°C for 2 h and by passing it through an octyl-Sepharose column to remove intact LTA and partially deacylated LTA. LTA-1 was prepared like LTA, but the Q-Sepharose ion-exchange chromatography was performed at pH 10.5 instead of 9.5. The structures of LTA-1 and LTA-0 were confirmed by MALDI-TOF mass spectrometry. LTA has two acyl groups attached to the glycerol backbone; the C18:1 group is attached to the C2 position, and the C16:0 group is attached to the C1 position. Hydrolytic removal of C18:1 or C16:0 should reduce the molecular mass by 265 or 239 Da, respectively. Mass spectrometry of LTA-0 showed three major peaks similar to those seen with LTA; however, the LTA-0 peaks were 504 m/z units lower than the LTA peaks. LTA-1 also had three major peaks; however, the LTA-1 peaks were about 264 m/z units lower than the LTA peaks. Furthermore, after alkali hydrolysis, the LTA-1 peaks lost an additional 239 m/z units. These mass spectrometric data indicate that LTA-1 lost one acyl moiety at the C2 position and that LTA-0 lost both acyl groups.
Purification of LTA from S. aureus.
Highly purified LTA was isolated from S. aureus (ATCC 6538) by n-butanol extraction, as previously described (30). Briefly, the cells were cultured aerobically in tryptic soy broth (Difco, Detroit, Mich.) for 16 h at 37°C in a shaking incubator. The cells were harvested, suspended in 0.1 M sodium citrate buffer (pH 4.7), and disintegrated by ultrasonication (Sonicator model W-220F). The cells were then mixed with an equal volume of n-butanol by stirring them for 30 min at room temperature. After centrifugation at 13,000 × g for 20 min, the aqueous phase was evaporated, dialyzed against pyrogen-free water, and equilibrated with 0.1 M sodium acetate buffer containing 15% n-propanol (pH 4.7). The LTA was first purified by hydrophobic interaction chromatography on an octyl-Sepharose CL-4B (Sigma) column (2.5 by 20 cm). The column was eluted with a stepwise n-propanol gradient (100 ml of 20% n-propanol, 200 ml of 35% n-propanol, and 100 ml of 45% n-propanol). Then the column fractions containing LTA were pooled after an inorganic phosphate assay, and the pool was dialyzed against water. The LTA-containing fractions were further subjected to DEAE-Sepharose ion-exchange chromatography (2.5 by 9.5 cm) (Fast Flow; Sigma), equilibrated in the 0.1 M sodium acetate buffer (pH 4.7) containing 30% n-propanol. The column was eluted with 300 ml of a linear salt gradient (0 to 1 M NaCl in the equilibration buffer), and the eluate was collected in 10-ml aliquots. The quantity of the purified LTA was determined by inorganic phosphate assay.
Cell lines and culture condition.
Two NF-κB reporter cell lines (54), CHO/CD14/TLR2 and CHO/CD14/TLR4, coexpressing CD14 and TLR2 or TLR4, respectively, were kindly provided by Douglas Golenbock (Boston Medical Center, Boston, Mass.). The cell lines have the gene encoding membrane CD25 with the human E-selectin promoter, which has NF-κB binding sites. The cells were grown in Ham's F-12 medium (GIBCO-BRL, Rockville, Md.) supplemented with 10% defined fetal bovine serum (HyClone, Logan, Utah), 1 mg of G418 per ml (Calbiochem, La Jolla, Calif.), and 400 U of hygromycin B per ml (Calbiochem, La Jolla, Calif.) at 37°C and 5% CO2. Flow cytometry was used to determine the expression of the genes for CD14, TLR2, and TLR4 introduced into CHO/CD14/TLR2 and CHO/CD14/TLR4 cells (data not shown). When the cells were 70% confluent, various stimuli (E. coli or P. gingivalis LPS and S. aureus PGN, LTA, or LTA variants) were added. After 16 h, the cells were washed once with phosphate-buffered saline (PBS) and detached with 2 mM EDTA in PBS. The cells were then labeled on ice with fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD25 (Becton Dickinson, San Diego, Calif.), and 104 cells were analyzed on a FACSCalibur flow cytometer with CellQuest acquisition analysis software (Becton Dickinson, San Diego, Calif.). In some experiments, LTA was further treated with lysozyme (100 μg/ml) at 37°C for 50 min and then heat denatured at 100°C for 10 min or treated with proteinase K (100 μg/ml) at 55°C for 50 min in a buffer containing 10 mM Tris-HCl, 5 mM EDTA, and 50 mM NaCl (pH 8.0).
Stimulation of human PBMCs.
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood by isolating the buffy coat from the blood and then removing contaminating erythrocytes with a Histopaque density gradient. Monocytes were isolated from the PBMCs by removing non-monocytic cells with an indirect magnetic isolation kit (Miltenyi Biotec, Auburn, Calif.) and with monoclonal hapten-conjugated CD3, CD7, CD19, CD45RA, CD56, and IgE antibodies from Miltenyi Biotec. This procedure routinely resulted in >95% pure CD14+ cells by flow cytometry.
Human monocytes were suspended in RPMI 1640 supplemented with 10% fetal bovine serum, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 2 mM l-glutamine, 20 mM HEPES, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. The monocyte suspension was placed in 96-well plates (200 μl per well) and was stimulated with LTA (or its variants) for 20 h. The amount of TNF-α in the culture supernatant was determined with a human TNF-α enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go kit (eBioscience) according to the manufacturer's protocol. To assess the functional role of CD14, TLR1, TLR2, or TLR4 in TNF-α production, cells were incubated with 10 μg of anti-CD14, anti-TLR1, anti-TLR2, or anti-TLR4 monoclonal antibodies or isotype-matched control antibodies per ml for 40 min before addition of LTA (or its variants).
RESULTS
Highly purified pneumococcal LTA stimulated TLR2 but not TLR4.
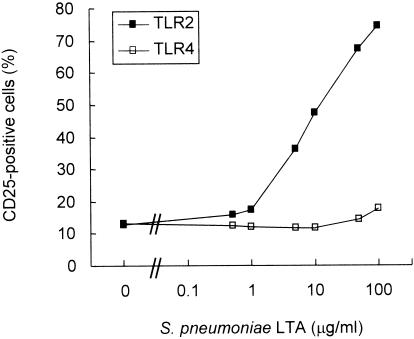
To examine the ability of pneumococcal LTA to stimulate cells via TLR, highly pure and structurally intact pneumococcal LTA was isolated from strain R36A. The pneumococcal LTA preparation was then added to the two NF-κB reporter cell lines (CHO/CD14/TLR2 and CHO/CD14/TLR4), and the percentage of CD25+ cells was determined by flow cytometry after 16 h of culture. Prior to the exposure to any stimulants, about 10 to 15% of the CHO/CD14/TLR2 or CHO/CD14/TLR4 cells expressed detectable amounts of CD25. As expected, E. coli LPS (1 μg/ml) induced the expression of CD25 on CHO/CD14/TLR4 cells as did P. gingivalis LPS (1 μg/ml) on CHO/CD14/TLR2 cells (data not shown). Upon exposure to 100 μg of pneumococcal LTA per ml, about 74% of CHO/CD14/TLR2 cells expressed CD25 (Fig. 1). In contrast, the fraction of CD25+ cells among CHO/CD14/TLR4 cells remained low (about 10%), even after exposure to a high concentration of pneumococcal LTA. The slight increase at 100 μg/ml was not reproducible, but the basic pattern of response was reproducible when the experiments were repeated with several different preparations of pneumococcal LTA. These results suggested that pneumococcal LTA signals TLR2 but not TLR4.
FIG. 1.
Pneumococcal LTA induces NF-κB-dependent CD25 expression via TLR2. CHO/CD14/TLR2 or CHO/CD14/TLR4 cells were treated with pneumococcal LTA at the indicated concentrations (0 to 100 μg/ml) for 16 h. Cellular activation of TLR-dependent NF-κB was determined by flow cytometry with measurement of CD25 reporter gene expression.
Impurities in the LTA preparation are not responsible for stimulation.
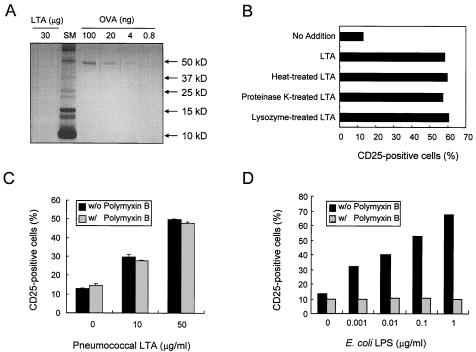
Even though we have used multiple independent steps for purification, it is possible that our LTA preparations have a minor impurity. A potential explanation for the low potency is that the observed stimulatory activity is mediated not by pneumococcal LTA but by a minor contaminant in the preparation. We have therefore carefully examined our preparations for contaminants. Contamination by DNA and RNA was not demonstrable inasmuch as our concentrated pneumococcal LTA preparations (1 mg/ml) displayed no significant UV absorption at 260 and 280 nm. Protein contamination was assessed by polyacrylamide gel electrophoresis and silver staining. The silver staining was capable of visualizing 4 ng of ovalbumin per lane but revealed no bands, even when 30 μg of LTA was loaded per lane (Fig. 2A). These data demonstrate that our LTA preparation contained less than 0.01% (wt/wt) protein. Furthermore, boiling or treatment with proteinase K or lysozyme did not reduce the ability of our LTA to signal through TLR2 (Fig. 2B). These results strongly suggested the absence of lipoprotein in our LTA preparation.
FIG. 2.
TLR2 stimulation by the pneumococcal LTA is not from impurities in the LTA preparation. (A) Thirty micrograms of pneumococcal LTA was subjected to 15% polyacrylamide gel electrophoresis (PAGE) followed by silver staining. Ovalbumin (OVA) was used as a protein standard. (B) Ten micrograms of pneumococcal LTA or LTA treated with heat, proteinase K, or lysozyme was added to CHO/CD14/TLR2 cells, and then the cells were incubated for 16 h. The cells were stained with FITC-labeled anti-CD25 monoclonal antibody and subjected to flow cytometric analysis for the expression of the NF-κB-dependent reporter gene CD25. (C) CHO/CD14/TLR2 cells were incubated with pneumococcal LTA (10 or 50 μg/ml) in the presence (w/) or absence (w/o) of polymyxin B (50 μg/ml) for 16 h, and the TLR2-dependent NF-κB activation was determined by flow cytometric analysis of CD25. (D) CHO/CD14/TLR4 cells were incubated with reextracted LPS of E. coli E055:B5 (0.001 to 1 μg/ml) in the presence or absence of polymyxin B (50 μg/ml) for 16 h, and TLR4-dependent NF-κB activation was determined by flow cytometric analysis of CD25 expression.
Since LPS is ubiquitous and can stimulate cells via TLR2 (23, 52), our pneumococcal LTA was also investigated for LPS contaminants. Studies with a LAL assay suggested that our pneumococcal LTA preparations have less than 5 pg of endotoxin per mg of LTA. Furthermore, the TLR2-stimulatory activity of our sample is resistant to polymyxin B (Fig. 2C), whereas 50 μg of polymyxin B per ml completely blocked the biological activity of E. coli LPS (0.001 to 1 μg/ml) in TLR4 stimulation (Fig. 2D). All of these studies taken together suggest that our observation is not due to contaminant molecules.
Pneumococcal LTA induces modest production of TNF-α by human PBMCs.
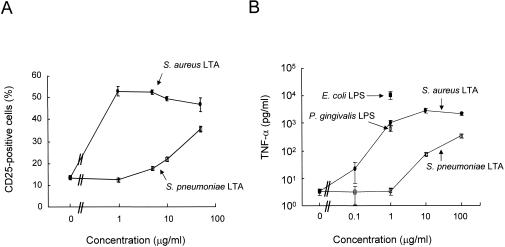
Since the pneumococcal LTA appeared to be less potent in stimulating TLR2 than S. aureus LTA based on published reports (40), we directly compared the potency of both LTAs by using the NF-κB reporter cell line responsive to TLR2 (Fig. 3A). The fraction of CD25+ cells increased from about 12% to about 50% after being exposed to 1 μg of the staphylococcal LTA per ml. The fraction of CD25+ cells did not increase any further, even when the amount of staphylococcal LTA was increased up to 50 μg/ml. In contrast, when the cells were exposed to pneumococcal LTA, the fraction of CD25+ cells gradually increased with increasing concentration of pneumococcal LTA. The CD25+ cell fraction remained at the background level when the cells were exposed to 1 μg of pneumococcal LTA per ml, became about 20% at 10 μg/ml, and reached about 40% at 50 μg/ml.
FIG. 3.
Pneumococcal LTA is less potent than staphylococcal LTA. (A) CHO/CD14/TLR2 cells were treated with LTA from S. aureus (solid circles) or LTA from S. pneumoniae (open squares) for 16 h. At the end of the incubation period, TLR2-dependent NF-κB activation was determined by flow cytometric analysis of CD25. (B) Human PBMCs were treated with the indicated amount of staphylococcal LTA (solid circle), pneumococcal LTA (open square), or 1 μg of P. gingivalis (open circles) or E. coli E055:B5 LPS (solid squares) per ml for 15 h. Cell-free culture media were collected and assayed for TNF-α by sandwich ELISA.
To determine if the difference in response to the two LTAs was related to the use of CHO cell lines, we stimulated PBMCs with LTAs from both bacteria and determined the resulting production of TNF-α (Fig. 3B). A large amount of TNF-α (1,000 pg/ml) was induced with 1 μg of staphylococcal LTA per ml, and when the amount of the LTA increased to 100 μg/ml, TNF-α production increased only slightly. In contrast, when the PBMCs were stimulated with pneumococcal LTA, only a modest amount of TNF-α was induced, even with high concentrations of pneumococcal LTA. For instance, only 75 pg of TNF-α per ml was induced with 10 μg of pneumococcal LTA per ml, and only 350 pg of TNF-α per ml was induced with 100 μg of LTA per ml. Thus, pneumococcal LTA is 100-fold less potent than staphylococcal LTA. As a control, the PBMCs were also stimulated with LPS from P. gingivalis and E. coli, which are well-known TLR2- and TLR4-stimulating agents, respectively (12, 23, 49). Our PBMC stimulation system was normally responsive, since the PBMCs produced a large (and expected) amount (640 pg/ml) of TNF-α in response to P. gingivalis LPS (1 μg/ml) and an even larger amount (10,000 pg/ml) in response to E. coli LPS (1 μg/ml).
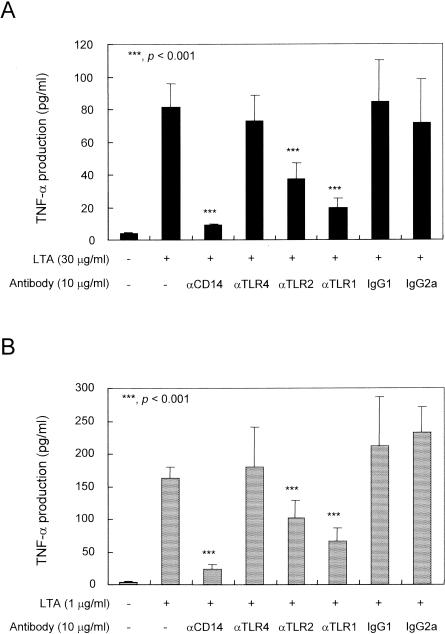
To investigate if the pneumococcal LTA and staphylococcal LTA differ in their requirements for stimulation of TLR, we examined the need for CD14 and TLR1, which were shown to be important for efficient signaling by TLR2 (36, 47, 48) (Fig. 4). Antibodies to CD14, TLR1, or TLR2 (but not those to TLR4 or isotype-matched control antibodies) reduced the TNF-α production by PBMCs in response to pneumococcal LTA. Similarly, antibodies to CD14, TLR1, or TLR2 significantly reduced the staphylococcal LTA-induced production of TNF-α. Taken together, both types of LTA share several additional receptor molecules required for the stimulation of PBMCs.
FIG. 4.
Monoclonal antibodies to CD14, TLR1, or TLR2 inhibited the LTA-induced TNF-α production in human PBMCs. Human PBMCs were treated with anti-TLR1, anti-TLR2, anti-TLR4, or anti-CD14 antibody for 40 min prior to stimulation with 30 μg of pneumococcal LTA per ml (A) or 1 μg of staphylococcal LTA per ml (B) for 20 h. The levels of TNF-α were determined by sandwich ELISA. Data are expressed as means ± standard deviations. *, **, and ***, P < 0.05, <0.01, and <0.001, respectively. Statistical significance between groups was evaluated by analysis of variance.
An acyl chain is essential in TLR2 stimulation by the pneumococcal LTA.
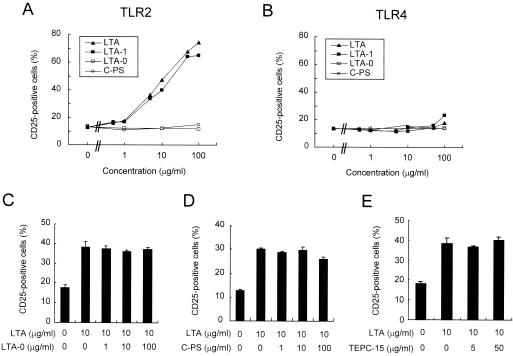
Pneumococcal LTA expresses PC and can bind other receptors such as platelet-activating factor receptor (PAFR) (8). To examine if PC moiety of the pneumococcal LTA is important in stimulating the cells, we next examined the molecular moieties necessary for the stimulation. For these studies, two LTA variants were produced: LTA-1 with only one acyl chain and LTA-0 with no acyl chains. LTA-1 stimulated the TLR2-sensitive cell line as well as LTA, but LTA-0 did not stimulate the TLR2-sensitive cell line at all (Fig. 5A). Also, pneumococcal teichoic acid, which is almost identical to LTA-0 in structure (15), did not elicit any significant stimulation of cells via TLR2 or TLR4 (Fig. 5A and B). Furthermore, a large amount (100 μg/ml) of C-PS or LTA-0 was unable to inhibit the stimulation of CHO/CD14/TLR2 cells by a relatively small amount of LTA (10 μg/ml) (Fig. 5C and D). Moreover, a PC-specific antibody, TEPC-15 (50 μg/ml), did not inhibit LTA-induced stimulation of cells via TLR2 (Fig. 5E). These results demonstrate that the stimulation of CHO/CD14/TLR2 cells by the pneumococcal LTA requires at least one acyl chain, but the polyphosphate region may not be critical.
FIG. 5.
Lipid moieties of pneumococcal LTA are critical in TLR2 stimulation. CHO/CD14/TLR2 or CHO/CD14/TLR4 cells were treated with pneumococcal LTA, LTA-1 (monoacylated), LTA-0 (delipidated), or C-PS at the indicated concentrations for 16 h (A and B). Likewise, CHO/CD14/TLR2 was stimulated with pneumococcal LTA in the presence of LTA-0, C-PS, or anti-PC antibody (TEPC-15) at the indicated amounts for competition experiments (C, D, and E). The cells were stained with FITC-labeled anti-CD25 monoclonal antibody and subjected to flow cytometric analysis for the expression of the NF-κB-dependent reporter gene CD25.
Pneumococcal LTA-1 requires CD14, TLR1, and TLR2 to stimulate PBMCs.
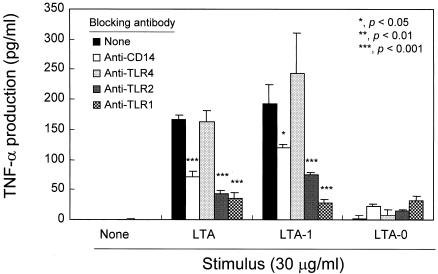
To determine whether LTA molecules with different numbers of acyl chains require different stimulation conditions, we stimulated human PBMCs with LTA, LTA-1, or LTA-0 in the presence of blocking antibodies against CD14, TLR1, TLR2, or TLR4. As expected, PBMCs readily produced TNF-α in response to LTA or LTA-1, but not LTA-0. The anti-CD14, anti-TLR1, or anti-TLR2 antibodies significantly (P < 0.001) inhibited TNF-α secretion by PBMCs stimulated with LTA. The same antibodies also significantly (P < 0.05) inhibited LTA-1 activation of cells (Fig. 6). To the contrary, pretreatment with anti-TLR4 antibody (Fig. 6) or with irrelevant isotype control antibodies (data not shown) had no effect on TNF-α production by LTA- or LTA-1-stimulated PBMCs. These results indicate that stimulation of PBMCs by LTA or LTA-1 requires CD14, TLR1, and TLR2 but not TLR4.
FIG. 6.
Pneumococcal LTA and LTA-1 require CD14, TLR1, and TLR2 for TNF-α production in human PBMCs. Human PBMCs were treated with anti-TLR1, anti-TLR2, anti-TLR4, or anti-CD14 antibody for 40 min prior to stimulation with 30 μg of pneumococcal LTA and its variants per ml for 20 h. The levels of TNF-α were determined by sandwich ELISA. Data are expressed as means ± standard deviations. *, **, and ***, P < 0.05, <0.01, and <0.001, respectively. Statistical significance between groups was evaluated by analysis of variance.
DISCUSSION
Because the pneumococcal cell wall elicits strong inflammatory responses, pneumococcal cell wall components, including LTA, have been examined for their ability to elicit inflammatory cytokines. One study found that pneumococcal LTA was not so efficient in inducing TNF-α production but was highly efficient in inducing interleukin-1 (IL-1) production (38). In contrast, another study reported that pneumococcal LTA did not induce production of the two cytokines, even at 50 μg/ml (4). In yet another study, pneumococcal LTA was about 10-fold less potent than staphylococcal LTA in inducing (IL-12) p40 gene expression (6). The discrepancies among these previous studies could be due to contaminants or molecular degradations of the LTA preparations used for the studies, because several recent studies have clearly shown that even minor contaminants (or degradations) can profoundly influence the biological properties of an LTA preparation (16, 31).
In this study, we used highly purified and intact preparations of pneumococcal LTA to show that pneumococcal LTA is about 100-fold less potent than staphylococcal LTA in stimulating human PBMCs to produce TNF-α. The acyl chain of LTA is an important part of this mechanism, since PBMCs could be stimulated with partially deacylated LTA but not with completely deacylated LTA. The stimulation of PBMCs by pneumococcal LTA as well as staphylococcal LTA appears to occur primarily via TLR2/TLR1 with the help of CD14, inasmuch as the stimulation could be suppressed with antibodies to TLR2, CD14, or TLR1 but not with antibodies to TLR4 or C-PS. Involvement of TLR6 could not be tested, because the reagent was not available for our study.
It is very unlikely that our observations were due to contaminants, since our pneumococcal LTA preparations were highly purified. Nevertheless, we have further excluded this possibility by making a concerted effort to detect the presence of specific biologically active contaminants in our LTA preparations. Endotoxin contamination was unlikely, because we did not detect its presence by LAL test, because the activity that we observed was resistant to polymyxin B, and because the endotoxin contaminants, if present, would probably have stimulated TLR4. Macrophage stimulation by staphylococcal PGN is TLR1 independent (48). While we need to investigate the TLR1 requirement with pneumococcal PGN, the stimulation of our pneumococcal LTA is TLR1 dependent. Consequently, pneumococcal PGN may not be responsible for our observation. Lipoprotein contamination was also unlikely, because the activity that we observed was resistant to proteinase K treatment and heating at 100°C. Finally, monoacyl LTA was also as stimulatory as native (diacyl) LTA. Since monoacyl LTA was purified under conditions different from those used to purify the native LTA, it is unlikely that both preparations of pneumococcal LTA have the same biologically active contaminant.
Recently, Schroder et al. reported that their preparation of pneumococcal LTA requires TLR2, just as we have observed (40). In contrast, however, their preparation of pneumococcal LTA was as potent as staphylococcal LTA in stimulating cytokine production. At present, several explanations are possible for this difference. First, the difference may be in the assay system. The difference could be subtle, since the overall efficiency of stimulating the target cells by LPS depends on the cooperation of many molecules, including albumin (18). Second, the pneumococcal LTAs used in the two studies were isolated from two different strains and may have different structures inasmuch as microheterogeneity among pneumococcal LTAs exists. Pneumococcal LTA may have only one PC in every repeating unit instead of two (55). In addition, some repeating units have no PC moieties, and pneumococcal strains differ in the number of such PC-free units (unpublished information). Third, the difference may still be due to contaminants or molecular degradations. It is important to investigate these issues, because staphylococci and pneumococci are the two leading causes of gram-positive septic shock and because LTA may be important in the pathogenesis of septic shock.
Whatever the explanation may turn out to be, we believe that the LTA from each gram-positive bacterium may have many different inflammatory properties and should be assessed individually. Such differences were clearly shown with LPS from gram-negative bacteria. For instance, LPS from most gram-negative bacterial species interacts with TLR4, but LPS from P. gingivalis (23) and leptospira (52) interacts with TLR2. LPS from Neisseria meningitidis may even stimulate both TLR2 and TLR4 (35). LPS from Rhodobacter capsulatus is inhibitory for TLR4 activity (28). Furthermore, unlike other LTAs, pneumococcal LTA can provide an additional inflammatory signal via PAFR with its PC moiety. Several studies have suggested that this is a significant stimulation pathway (5, 7, 8). Since PAFR is more abundantly expressed on activated cells, pneumococcal LTA may provide inflammatory stimulation via TLR at the early phase and then via PAFR at the late phase.
Our studies revealed that TLR1 is an important coreceptor for pneumococcal LTA. This is not surprising, because TLR1 or TLR6 is frequently a coreceptor for TLR2. What is surprising is that LTA-1 also required TLR1 and was equally as functional as the normal LTA in inducing TNF-α production. This was unexpected, because the number of acyl groups often influences the coreceptors or the cellular products. A mycoplasmal lipopeptide with two acyl groups (MALP-2) requires TLR6, whereas MALP-2 with an additional palmitoyl group does not (33, 46). When one acyl group is removed from MALP-2, the lipoprotein becomes about 100 times less potent than the native MALP-2 in stimulating murine macrophages to secrete nitric oxide (33). Another example is found with Enterococcus hirae, which produces two types of LTA: one with two acyl chains and another with four acyl chains. Tetra-acyl LTA is more potent than diacyl-LTA in inducing the production of cytokines such as IL-6, TNF-α, and gamma interferon (44). Thus, despite our findings, we are further investigating the two pneumococcal LTA preparations for potential differences in their biological properties (e.g., NO synthesis).
Pneumococcal LTA has been shown to be highly immunogenic and is more immunogenic than pneumococcal teichoic acid (19). It is possible that pneumococcal LTA may be more immunogenic than simple pneumococcal teichoic acid because LTA may stimulate dendritic cells via TLR. The stimulated dendritic cells may then facilitate the B cells to produce antibodies. For this situation, even relatively inefficient stimulation of dendritic cells by the LTA may be sufficient, because relatively large amounts of LTA would be present at the immunization site. Further understanding of how pneumococcal LTA stimulates the immune system may help the development of a vaccine that can elicit antibodies against bacterial capsular polysaccharides.
Acknowledgments
We gratefully appreciate Genentech Corporation (San Francisco, Calif.) for supplying the necessary reagents to complete these studies.
This work was funded by AI-31473 (M.H.N.), DE 09081 (S.M.M.), and DE 14215 (S.M.M.). Seung Hyun Han is supported by a fellowship from the International Vaccine Institute in Seoul, Korea.
Editor: A. D. O'Brien
REFERENCES
- 1.Ahmed, A. J., J. A. Kruse, M. T. Haupt, P. H. Chandrasekar, and R. W. Carlson. 1991. Hemodynamic responses to gram-positive versus gram-negative sepsis in critically ill patients with and without circulatory shock. Crit. Care Med. 19:1520-1525. [DOI] [PubMed] [Google Scholar]
- 2.American Public Health Association, American Water Works Association, and Water Environment Federation. 1995. Stannous chloride method, p. 4-112-4-113. In A. D. Eaton, L. S. Clesceri, and A. E. Greenberg (ed.), Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, D.C.
- 3.Behr, T., W. Fischer, J. Peter-Katalinic, and H. Egge. 1992. The structure of pneumococcal lipoteichoic acid. Eur. J. Biochem. 207:1063-1075. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabellos, C., D. E. MacIntyre, M. Forrest, M. Burroughs, S. Prasad, and E. Tuomanen. 1992. Differing roles for platelet-activating factor during inflammation of the lung and subarachnoid space. The special case of Streptococcus pneumoniae. J. Clin. Investig. 90:612-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleveland, M. G., J. D. Gorham, T. L. Murphy, E. Tuomanen, and K. M. Murphy. 1996. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect. Immun. 64:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cundell, D. R., C. Gerard, I. Idanpaan-Heikkila, E. I. Tuomanen, and N. P. Gerard. 1996. PAF receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv. Exp. Med. Biol. 416:89-94. [DOI] [PubMed] [Google Scholar]
- 8.Cundell, D. R., N. P. Gerard, C. Gerard, I. Idanpaan-Heikkila, and E. I. Tuomanen. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 9.Danner, R. L., R. J. Elin, J. M. Hosseini, R. A. Wesley, J. M. Reilly, and J. E. Parillo. 1991. Endotoxemia in human septic shock. Chest 99:169-175. [DOI] [PubMed] [Google Scholar]
- 10.De Kimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359-10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 12.Ellingsen, E., S. Morath, T. Flo, A. Schromm, T. Hartung, C. Thiemermann, T. Espevik, D. Golenbock, D. Foster, R. Solberg, A. Aasen, and J. Wang. 2002. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med. Sci. Monit. 8:BR149-BR156. [PubMed] [Google Scholar]
- 13.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-302. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, W. 1994. Teichoic acid and lipoglycans. New Compr. Biochem. 27:199-215. [Google Scholar]
- 15.Fischer, W., T. Behr, R. Hartmann, J. Peter-Katalinic, and H. Egge. 1993. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoic acid (C-polysaccharide). Eur. J. Biochem. 215:851-857. [DOI] [PubMed] [Google Scholar]
- 16.Gao, J. J., Q. Xue, E. G. Zuvanich, K. R. Haghi, and D. C. Morrison. 2001. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect. Immun. 69:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsburg, I. 2002. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2:171-179. [DOI] [PubMed] [Google Scholar]
- 18.Gioannini, T. L., D. Zhang, A. Teghanemt, and J. P. Weiss. 2002. An essential role for albumin in the interaction of endotoxin with lipopolysaccharide-binding protein and sCD14 and resultant cell activation. J. Biol. Chem. 277:47818-47825. [DOI] [PubMed] [Google Scholar]
- 19.Goebel, W. F., and M. H. Adams. 1943. The immunological properties of the heterophile antigen and somatic polysaccharide of pneumococcus. J. Exp. Med. 77:435-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon, S. 2002. Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927-930. [DOI] [PubMed] [Google Scholar]
- 21.Hagberg, L., D. E. Briles, and C. S. Eden. 1985. Evidence for separate genetic defects in C3H/HeJ and C3HeB/FeJ mice, that affect susceptibility to gram-negative infections. J. Immunol. 134:4118-4122. [PubMed] [Google Scholar]
- 22.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 23.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaisho, T., and S. Akira. 2002. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 1589:1-13. [DOI] [PubMed] [Google Scholar]
- 25.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kengatharan, K. M., S. J. De Kimpe, and C. Thiemermann. 1996. Role of nitric oxide in the circulatory failure and organ injury in a rodent model of gram-positive shock. Br. J. Pharmacol. 119:1411-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438-444. [DOI] [PubMed] [Google Scholar]
- 28.Loppnow, H., P. Libby, M. Freudenberg, J. H. Krauss, J. Weckesser, and H. Mayer. 1990. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect. Immun. 58:3743-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 30.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morath, S., A. Geyer, I. Spreitzer, C. Hermann, and T. Hartung. 2002. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect. Immun. 70:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morath, S., A. Stadelmaier, A. Geyer, R. R. Schmidt, and T. Hartung. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 195:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morr, M., O. Takeuchi, S. Akira, M. M. Simon, and P. F. Muhlradt. 2002. Differential recognition of structural details of bacterial lipopeptides by Toll-like receptors. Eur. J. Immunol. 32:3337-3347. [DOI] [PubMed] [Google Scholar]
- 34.Morrison, D. C., and J. L. Ryan. 1987. Endotoxins and disease mechanisms. Annu. Rev. Med. 38:417-432. [DOI] [PubMed] [Google Scholar]
- 35.Netea, M. G., M. van Deuren, B. J. Kullberg, J. M. Cavaillon, and J. W. Van der Meer. 2002. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 23:135-139. [DOI] [PubMed] [Google Scholar]
- 36.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollack, M. M., A. I. Fields, and U. E. Ruttimann. 1985. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit. Care Med. 13:454-459. [DOI] [PubMed] [Google Scholar]
- 38.Riesenfeld-Orn, I., S. Wolpe, J. F. Garcia-Bustos, M. K. Hoffmann, and E. Tuomanen. 1989. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect. Immun. 57:1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rietschel, E. T., U. Schade, M. Jensen, H. W. Wollenweber, O. Luderitz, and S. G. Greisman. 1982. Bacterial endotoxins: chemical structure, biological activity and role in septicaemia. Scand. J. Infect. Dis. 31(Suppl.):8-21. [PubMed] [Google Scholar]
- 40.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of S. pneumoniae and S. aureus activates immune cells via Toll-like receptor (TLR)-2, LPS binding protein (LBP) and CD14 while TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 41.Schumann, R. R., S. R. Leong, G. W. Flaggs, P. W. Gray, S. D. Wright, J. C. Mathison, P. S. Tobias, and R. J. Ulevitch. 1990. Structure and function of lipopolysaccharide binding protein. Science 249:1429-1431. [DOI] [PubMed] [Google Scholar]
- 42.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suffredini, A. F., R. E. Fromm, M. M. Parker, M. Brenner, J. A. Kovacs, R. A. Wesley, and J. E. Parrillo. 1989. The cardiovascular response of normal humans to the administration of endotoxin. N. Engl. J. Med. 321:280-287. [DOI] [PubMed] [Google Scholar]
- 44.Takada, H., Y. Kawabata, R. Arakaki, S. Kusumoto, K. Fukase, Y. Suda, T. Yoshimura, S. Kokeguchi, K. Kato, T. Komuro, N. Tanaka, M. Saito, T. Yoshida, M. Sato, and S. Kotani. 1995. Molecular and structural requirements of a lipoteichoic acid from Enterococcus hirae ATCC 9790 for cytokine-inducing, antitumor, and antigenic activities. Infect. Immun. 63:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi, O., and S. Akira. 2001. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 1:625-635. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 49.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 50.Tuomanen, E. I., R. Austrian, and H. R. Masure. 1995. Pathogenesis of pneumococcal infection. N. Engl. J. Med. 332:1280-1284. [DOI] [PubMed] [Google Scholar]
- 51.Veterans Administration Systemic Sepsis Cooperative Study Group. 1987. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N. Engl. J. Med. 317:659-665. [DOI] [PubMed] [Google Scholar]
- 52.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 53.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 55.Zhang, J. R., I. Idanpaan-Heikkila, W. Fischer, and E. I. Tuomanen. 1999. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol. Microbiol. 31:1477-1488. [DOI] [PubMed] [Google Scholar]