Abstract
It has been previously shown that one of the three meningococcal C conjugate (MCC) vaccines introduced in the United Kingdom proved highly immunogenic after the first dose of a three-dose schedule, with evidence of immune memory after dose 3. Thus, in infants a one- or two-dose schedule of this MCC vaccine, conjugated to tetanus toxoid (TT), may suffice. Healthy infants (n = 586) were randomized to receive either one (group 1), two (group 2), or three (group 3) doses of MCC-TT vaccine with a 10-μg polysaccharide booster given at 13 to 14 months of age. Serum bactericidal antibody (SBA) levels were measured by utilizing rabbit complement (rSBA), meningococcal C-specific immunoglobulin G (IgG), and avidity indices (AIs). For groups 1, 2, and 3, the percentages of infants with an rSBA level of ≥8 against strain C11 were 98.4, 100, and 99.4%, respectively. Infants in group 1 with prevaccination rSBA titers of ≥8 had post-primary MCC rSBA geometric mean titers (GMTs) significantly lower than those infants with prevaccination rSBA titers of <8. One dose of MCC-TT vaccine given to infants at 2 months of age yielded significantly lower SBA GMTs and geometric mean AIs (GMAIs) than two or three doses but elicited a significantly greater response after boosting, as reflected by rSBA levels and GMAI. This study provides the first evidence that the number of doses of MCC-TT used in infant immunization schedules could be decreased.
In November 1999, the United Kingdom introduced universal meningococcal C conjugate (MCC) vaccination at 2, 3, and 4 months of age, on the basis of safety and immunogenicity studies (15). A prelicensure study in 83 infants in the United Kingdom (18) who were given one of the three candidate MCC vaccines, a tetanus toxoid conjugate (MCC-TT) (14), at 2, 3, and 4 months of age demonstrated that the vaccine was highly immunogenic after a single dose at 2 months of age, with all infants achieving a putative protective antibody titer of ≥8 (bactericidal antibody levels in serum were measured with baby rabbit complement [rSBA]) (1a, 3). There was a further significant increase in antibody levels after a second dose of MCC-TT vaccine but no further increase after the third dose at 4 months of age. Antibody levels fell by 14 months of age, but booster antibody responses to meningococcal C polysaccharide and MCC vaccines provided evidence of successful priming for immunologic memory after the full course of three doses (18).
The excellent response to this MCC-TT vaccine among infants at 2 months of age and the modest response to the third dose of vaccine raise the possibility that a one- or two-dose schedule in infants may be sufficient to provide protection from meningococcal C disease. Demonstration of priming for immune memory by the one- or two-dose schedules suggests that these regims could also provide long-term protection. This could be assessed by examining antibody responses to meningococcal C polysaccharide vaccination in the second year of life since children do not normally respond to polysaccharide antigens at that age. In addition to measuring antibody responses, avidity maturation (which is characteristic of a T-cell-dependent antibody response) can be assessed after immunization with polysaccharide conjugate vaccines and then used as a surrogate marker for the induction of immune memory. The use of avidity indices (AIs) in this way has now been widely documented for MCC (4, 19, 20), Haemophilus influenzae type b (Hib) conjugate (9, 16), and pneumococcal conjugate (2) vaccines.
MATERIALS AND METHODS
Study population and vaccination schedule.
The study population consisted of healthy infants in Gloucestershire, Sheffield, and Fife, United Kingdom, who were eligible for their infant routine immunizations. Infants were excluded if they were >11 weeks of age, had a birth weight of ≤2 kg, had received another inactivated vaccine less than 2 weeks prior to enrollment or a live viral vaccine less than 4 weeks prior to enrollment, were immunodeficient or immunocompromised, or had a prior history of meningococcal disease. Infants were randomized to receive one, two, or three doses of MCC-TT conjugate vaccine at 2 months (group 1), at 2 and 4 months (group 2), or at 2, 3, and 4 months (group 3) of age, respectively. Randomization into the three groups was done by using a computer-generated block procedure.
Additional dose(s) of MCC-TT vaccine were offered to any infants who did not achieve an rSBA titer of ≥16 after completion of their respective priming regimens. The extra dose procedure ensured that infants were brought, at least, in line with the current schedule in the United Kingdom, i.e., if they were in the one-dose group they received two extra doses, and if they were in the two-dose group they received one extra dose. For the nonresponders in the three-dose group, a further dose was offered at the discretion of the site investigator. The additional dose(s) were given infants at 3 to 6 months of age, and a further blood sample was taken approximately 4 weeks later to confirm protection. Such infants were then withdrawn from the booster phase of the study.
Vaccines.
The MCC-TT vaccine was dispensed in single dose syringes in liquid suspension (lot 49803). Each 0.5-ml dose contained serogroup C meningococcal de-O-acetylated polysaccharide (10 μg), tetanus toxoid (up to 20 μg), and aluminum hydroxide (0.5 mg). The vaccine was given by intramuscular injection into the right anterolateral thigh.
Three doses of the other standard United Kingdom pediatric vaccines (diphtheria-tetanus-whole-cell pertussis [DTwP] and Hib) were given concomitantly into the anterolateral aspect of the left thigh at 2, 3, and 4 months of age to all infants. The DTwP-Hib vaccine used was Pasteur Merieux ACT-HIBDTP. During the primary regimen, 33 infants received one or more doses of an acellular pertussis-containing vaccine; 5 of these infants (2 each from groups 1 and 2 with 1 from group 3) received acellular vaccine exclusively. Oral polio vaccine was from local supplies and was also administered concomitantly to infants at 2, 3, and 4 months of age.
A single 0.1-ml challenge dose (containing 10 μg of C polysaccharide) of a licensed unconjugated serogroups A and C (A/C) polysaccharide vaccine (SmithKline Beecham AC Vax) was given by intramuscular injection at 13 to 14 months of age into the right thigh. For parents consenting to routine mumps-measles-rubella vaccination, vaccine from a local stock was administered into the contralateral thigh at the same time.
Blood samples.
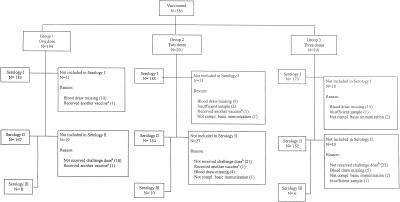
Individuals were bled prior to and 4 weeks after completion of the primary and challenge dose series for assessment of antibodies to meningococcal C polysaccharide. The schedule for vaccination and blood sampling is shown in Fig. 1. Blood samples (1 to 4 ml) were separated, divided into aliquots at the field centers, and then stored frozen at −20°C or below. Aliquots of separated sera were dispatched, in dry ice, to the PHLS Meningococcal Reference Unit, Manchester, United Kingdom, for meningococcal serogroup C serum bactericidal antibody (SBA) and serogroup C-specific IgG. A further aliquot was sent to the Institute of Child Health, London, United Kingdom, for measurement of meningococcal serogroup C-specific IgG AIs.
FIG. 1.
Compliance with sampling. Serology I, after primary immunization; serology II, 1 month after challenge; serology III, 7 to 14 days after challenge. Superscripts: a, excluded due to receiving another inactivated vaccine <2 weeks prior to enrolement or a live viral vaccine <4 weeks prior to enrollment; b, the number of subjects with an C11 rSBA titer of <16 post-MCC is included in the number of subjects who did not receive the challenge dose (this included six subjects in group 1, three subjects in group 2, and one subject in group 3).
For individuals for whom further doses of MCC vaccine were recommended, an MCC antibody test was offered 4 weeks after revaccination. These individuals were not included in the postchallenge analysis.
In order to investigate the early antibody responses postchallenge, a small subset of children were bled 7 to 14 days after administration of the challenge dose. The time intervals were 7 to 8 days for groups 1 and 3 and 7 to 14 days for group 2.
Antibody assays.
All sera were assayed blind by serum bactericidal assay as described by Maslanka et al. (13) with baby rabbit serum (Pel-Freeze, Inc., Rodgerson, Ariz.) as an exogenous complement source (rSBA). The strains used were the O-acetylation-positive (Oac+) C11 strain (phenotype C:16:P1.7-1,1) and two clinical isolates representing a prevalent epidemic strain in the UK: Oac+ M97.250926 (C:2a:P1.5,2) and the de-O-acetylated (Oac−) strain L91 543 (C:2a:P1.5). SBA titers were expressed as the reciprocal of the final serum dilution yielding ≥50% killing after 60 min. For the purposes of advising on the need for revaccination, evidence of an antibody response to MCC vaccine was defined as a postvaccination rSBA titer of ≥16 against strain C11, since at the time the proposed correlate of protection of an rSBA titer of ≥8 (3, 22) had not been validated (Andrews et al., unpublished).
IgG antibody was measured by using the standard enzyme-linked immunosorbent assay (ELISA) with both Oac+ and Oac− polysaccharide as previously described (5, 7). The antigen for the ELISAs comprised either naturally (i.e., not chemically derived) Oac− (Centre for Applied Microbiology and Research, Porton Down, United Kingdom) or Oac+ (Aventis Pasteur, Lyon, France) serogroup C polysaccharide. The calibration factor of the standard CDC1992 serum for serogroup C-specific IgG used in both Oac− and Oac+ assays was 24.1 μg/ml (11).
Antibody avidity was measured by an elution ELISA with the chaotrope, thiocyanate, as described previously (8) and modified for the meningococcal C IgG assay (20). Briefly, sera were diluted in buffer containing 10 mM phosphate-buffered saline, 0.01% Brij 35, and 5% newborn bovine serum to a final concentration of 0.5 μg/ml and then incubated on an antigen-coated plate overnight at 4°C. The plates were washed, and ammonium thiocyanate diluted in the serum buffer at various concentrations from 0 to 1 M was added to the wells. After 15 min at room temperature, the plates were washed, and antibody was detected by using polyclonal horseradish peroxidase-labeled goat antibody to human IgG incubated for 2 h. After a washing step, the assay was developed by using the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine dihydrochloride in citric acid-phosphate buffer and stopped after 30 min with 2 M H2SO4. The absorbance was then read at 450 nm. The percentage reduction of absorbance in the presence of ammonium thiocyanate compared to serum with no chaotrope was plotted against thiocyanate concentration. An AI was generated by plotting the log of the percent reduction against the thiocyanate concentration and calculating the amount of thiocyanate required to reduce the absorbance in a given serum by 50%. Meningococcal C IgG antibody avidity was evaluable in subjects with antibody levels of ≥0.6 μg/ml, and an AI of 50 was the considered the lower limit of the assay. Values of <50 were assigned a value of 25 for computational purposes.
Statistical analysis.
Geometric mean titers (GMTs) and concentrations with 95% confidence intervals (95% CI) were calculated for each group at each sampling time. Titers were log transformed, and analysis of variance and normal error regression were used to compare groups and examine the effects of (i) age and weight at first vaccination, (ii) sex, (iii) interval from vaccination to bleed, and (iv) prevaccination or prebooster titers. Differences between titers at different time points were analyzed by using paired t tests. This was a per protocol analysis.
Written informed consent was obtained for all participants in the trial from their parents or guardians as appropriate. The trial was conducted according to International Committee for Harmonisation Guidelines for Good Clinical Practice and with appropriate quality assurance. Ethical approval was obtained from the PHLS and appropriate local research ethics committees.
RESULTS
Compliance with sampling.
A total of 586 healthy infants (age range, 6 to 11 weeks) were recruited between November 1999 and January 2001. Of these, 194, 201, and 191 infants were recruited into groups 1, 2, and 3, respectively. The numbers of subjects from whom blood samples were available are shown in Fig. 1. A subset of 24 children, comprising 8, 10, and 6 children for groups 1, 2, and 3, respectively, were bled 7 to 14 days after the challenge dose at 12 to 13 months of age.
Antibody responses to MCC vaccines. (i) Effect of sex, age at first vaccination, and weight at first vaccination.
With two exceptions, there was no discernible association of these variables with any of the antibody responses. The first exception was a small apparent association of age with post-primary anti-Oac− serogroup C-specific IgG (8% decrease in antibody level per week of increasing age at first vaccination; P = 0.01). The second exception was a small apparent association of weight with post-primary anti-Oac+ serogroup C-specific IgG (19% increase in antibody level per kilogram at first vaccination; P = 0.01).
(ii) Serogroup C serum bactericidal titers.
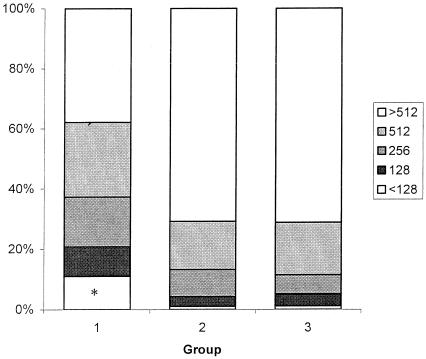
Serum bactericidal titers against the three strains are shown in Table 1. After administration of MCC, the group 1 rSBA GMTs for both Oac+ strains were lower than those for groups 2 and 3 (P < 0.001). No significant differences were seen between groups 2 and 3 (P = 0.68 and P = 0.97). For groups 1, 2, and 3, the percentages of infants with an rSBA level of ≥8 against strain C11 were 98.4, 100, and 99.4%, respectively. The percentages of responders at different cutoffs between <128 and >512 are given in Fig. 2. There were no significant differences in rSBA GMT between the three groups post-MCC when the Oac− strain was used (P = 0.20).
TABLE 1.
SBA GMTs before and after administration of MCC vaccine and before and after a booster of A/C polysaccharide vaccine (10-μg dose) according to study group
| Strain | Study group | Prevaccination
|
1 mo post-MCC
|
Prechallenge
|
1 mo postchallenge
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n (%) SBA ≥8 | SBA GMT (95% CI) | n | n (%) SBA ≥8 | SBA GMT (95% CI) | n | n (%) SBA ≥8 | SBA GMT (95% CI) | n | n (%) SBA ≥8 | SBA GMT (95% CI) | ||
| CI1 (Oac+) | 1 | 183 | 10 (5.5) | 2.4 (2.2-2.6) | 182 | 179 (98.4) | 460.2 (369.0-574.0) | 144 | 63 (43.8) | 10.4 (7.5-14.3) | 166 | 164 (98.8) | 6,376.6 (4,887.5-8,319.4) |
| 2 | 188 | 11 (5.9) | 2.4 (2.2-2.7) | 188 | 188 (100) | 1,325.5 (1,115.4-1,575.1) | 147 | 88 (59.9) | 20.7 (14.6-29.4) | 157 | 156 (99.4) | 3,556.2 (2,699.9-4,684.1) | |
| 3 | 172 | 10 (5.8) | 2.6 (2.3-2.9) | 173 | 172 (99.4) | 1,405.2 (1,164.2-1,696.1) | 141 | 99 (70.2) | 21.5 (15.8-29.2) | 143 | 140 (97.9) | 2,945.9 (2,116.1-4,101.1) | |
| M97.250926 (Oac+) | 1 | 183 | 4 (2.2) | 2.2 (2.1-2.4) | 182 | 172 (98.4) | 210.0 (164.0-269.0) | 144 | 65 (45.1) | 10.0 (7.5-13.4) | 166 | 164 (98.8) | 5,374.0 (3,957.6-7,297.3) |
| 2 | 187 | 8 (4.3) | 2.3 (2.1-2.5) | 188 | 186 (98.9) | 721.4 (593.1-877.5) | 147 | 86 (58.5) | 16.9 (12.2-23.6) | 157 | 157 (100) | 2,634.0 (1,959.3-3,541.2) | |
| 3 | 172 | 8 (4.7) | 2.4 (2.2-2.6) | 188 | 171 (98.8) | 726.3 (590.0-894.1) | 141 | 98 (70.0) | 22.9 (16.7-31.4) | 143 | 138 (96.5) | 2,245.9 (1,593.4-3,165.8) | |
| L91 543 (Oac−) | 1 | 183 | 13 (7.1) | 2.6 (2.3-2.8) | 182 | 181 (99.5) | 836.8 (684.3-1,023.4) | 144 | 60 (41.7) | 9.1 (6.7-12.2) | 166 | 164 (98.8) | 4,306.5 (3,241.1-5,722.1) |
| 2 | 187 | 22 (11.8) | 2.7 (2.4-3.1) | 188 | 187 (99.5) | 954.7 (805.3-1,131.9) | 147 | 83 (56.5) | 16.1 (11.6-22.3) | 157 | 155 (98.7) | 2,140.2 (1,643.5-2,787.1) | |
| 3 | 173 | 21 (12.1) | 2.9 (2.5-3.3) | 172 | 170 (98.8) | 1075.2 (870.3-1,328.4) | 141 | 88 (62.9) | 16.4 (12.2-22.0) | 142 | 139 (97.9) | 2,078.2 (1,537.4-2,809.4) | |
FIG. 2.
Percentage of responders at different cutoff values in the serum bactericidal assay after vaccination with either one dose (group 1), two doses (group 2), or three doses (group 3) of the MCC vaccine. ✽, For group 1, subjects with SBA titers of <128 comprised 11% of this group. Eight infants (4.4%) had titers of 64; four (2.2%) and eight (4.4%) infants had titers of <32.
Further dose(s) of MCC vaccine were given to the seven infants (six from group 1 and one from group 3) who had C11 rSBA titers of <16 post-MCC. Of these, four infants, all from group 1, had rSBAs of ≥8 in the prevaccination sample (titers of 8, 16, 32, and 32, respectively). After the administration of extra dose(s) of vaccine, rSBA titers of ≥64 were seen in all six subjects from whom a blood sample was available. There were no significant differences between the groups in prevaccination titers. However, infants in group 1 with prevaccination levels of ≥8 had post-primary GMTs of 30, 16, and 222 for C11, M97.250926, and L91 543, respectively, compared to GMTs of 540, 222, and 951 for infants with prevaccination levels of <8. These differences are statistically significant (P < 0.001). No such significant differences were found for groups 2 and 3. There was no significant observed association between prevaccination titers in group 1 and postchallenge antibody titers.
In pre- to post-primary series, ≥4-fold rises were seen in 95.6% (174 of 182), 99.5% (187 of 188), and 98.8% (170 of 172) of infants in groups 1, 2, and 3, respectively. Of the 11 infants without ≥4-fold rises, 6 had rSBA titers to C11 of >8 after administration of MCC. The remaining infant who had a C11 rSBA of <16 in the post-primary series had a fourfold rise in rSBA from 2 to 8. The other five infants who failed to show ≥4-fold rises all had rSBA levels of ≥32 for C11 after administration of MCC.
Before polysaccharide challenge, rSBA titers in all samples had declined but stayed higher than prevaccination. Prechallenge rSBA GMTs for group 1 for C11 (GMT = 10.4, 95% CI = 7.5 to 14.3) remained lower than for groups 2 (GMT = 20.7; 95% CI = 14.6 to 29.4) and 3 (GMT = 21.5; 95% CI = 15.8 to 29.2) (P < 0.02). After polysaccharide challenge there were no differences in rSBA GMTs between groups 2 and 3 (P = 0.37, 0.48, and 0.89 for strains C11, M97.250926, and L91 543, respectively). However, GMTs were always significantly higher in group 1 than groups 2 and 3 (P < 0.01). For all groups and strains, rSBA GMTs were higher after challenge than after MCC (P < 0.001).
For the subset of children bled 7 to 14 days after challenge, the C11 rSBA GMTs were 6,888.6 (95% CI = 3,507.1 to 13,530.6), 4,390 (95% CI = 1,450.4 to 13,287.7), and 1,290.2 (95% CI = 208.8 to 7,970.3) for groups 1, 2, and 3, respectively. These GMTs are similar to those seen in children bled 1 month postchallenge. Likewise, for L91 543 the titers were similar in the children bled at 7 to 14 days and those bled at 1 month. However, for M97.250926 the titers were, on average, 62% lower in those bled early (P = 0.02). In infants bled early, there were no significant differences between the groups for any of the rSBA assays. This is probably due to the small numbers in this subset.
(iii) Serogroup C-specific IgG antibody responses.
Serogroup C-specific IgG responses are shown in Table 2. Geometric mean concentrations (GMCs) after administration of MCC were higher as determined by the Oac− antigen assay than as determined by the Oac+ antigen assay (P < 0.001). At 1 month after administration of MCC, groups 1 and 2 showed lower concentrations in the Oac+ polysaccharide assay than group 3 (P < 0.001). There was no difference between groups 1 and 2 (P = 0.55). In the Oac− polysaccharide assay, group 2 showed concentrations lower than group 1 (P < 0.001) who showed lower concentrations than group 3 (P = 0.02).
TABLE 2.
Geometric mean serogroup C-specific IgG antibody concentrations measured using O-acetylated and de-O-acetylated serogroup C polysaccharide assays and GMAIs before and after administration of MCC vaccine and before and after a challenge dose of A/C polysaccharide vaccine (10-μg dose) according to study groupa
| Assay | Study group | Prevaccination
|
1 mo post-MCC
|
Prechallenge
|
1 mo postchallenge
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n (%) with Oac+ <0.6 | GMC or GMAI (95% CI) | n | n (%) with Oac+ <0.6 | GMC or GMAI (95% CI) | n | n (%) with Oac+ <0.6 | GMC or GMAI (95% CI) | n | n (%) with Oac+ <0.6 | GMC or GMAI (95% CI) | ||
| Oac− | 1 | 181 | NA | 0.3 (0.2-0.3) | 183 | NA | 24.0 (21.1-27.3) | 147 | NA | 1.0 (0.8-1.1) | 166 | NA | 13.5 (11.6-15.6) |
| 2 | 187 | NA | 0.3 (0.2-0.3) | 188 | NA | 15.9 (14.5-17.3) | 149 | NA | 1.5 (1.3-1.8) | 158 | NA | 10.2 (8.7-11.9) | |
| 3 | 172 | NA | 0.2 (0.2-0.3) | 173 | NA | 28.5 (26.0-31.3) | 141 | NA | 1.8 (1.6-2.1) | 142 | NA | 11.1 (9.7-12.8) | |
| Oac+ | 1 | 181 | NA | 0.2 (0.2-0.3) | 183 | NA | 8.3 (7.3-9.5) | 147 | NA | 0.8 (0.7-0.9) | 166 | NA | 25.4 (21.4-30.3) |
| 2 | 187 | NA | 0.2 (0.2-0.2) | 188 | NA | 8.7 (7.9-9.7) | 149 | NA | 1.3 (1.1-1.5) | 158 | NA | 17.5 (14.7-20.8) | |
| 3 | 172 | NA | 0.2 (0.2-0.2) | 173 | NA | 13.3 (12.0-14.7) | 141 | NA | 1.4 (1.2-1.6) | 142 | NA | 18.1 (15.2-21.6) | |
| GMAI | 1 | 177 | 137 (77.4) | 91.1 (75.9-109.5) | 180 | 4 (2.2) | 63.3 (60.6-66.1) | 105 | 22 (21.0) | 111.8 (101.8-122.9) | 161 | 3 (1.9) | 186.9 (174.2-200.6) |
| 2 | 184 | 139 (75.5) | 106.8 (92.3-123.6) | 185 | 0 (0) | 83.4 (79.0-88.0) | 131 | 16 (12.2) | 114.2 (105.5-123.6) | 150 | 0 (0) | 162.3 (151.9-173.4) | |
| 3 | 173 | 138 (79.8) | 88.6 (69.7-112.5) | 170 | 0 (0) | 78.9 (75.6-82.3) | 121 | 8 (6.6) | 106.6 (99.3-114.5) | 137 | 1 (0.7) | 150.6 (141.3-160.4) | |
NA, Not applicable; that is, GMAI cannot be measured in subjects with serogroup C-specific IgG levels of <0.6 μg/ml.
As for the SBA, there were no differences between the groups in the prevaccination IgG concentrations. However, high prevaccination concentrations were significantly associated with lower priming concentrations in group 1 but not in groups 2 and 3. The effect seen was always a reduced response with increasing prevaccination concentrations. In group 1, a 10-fold increase in prevaccination IgG concentration gave an average reduction in the serogroup C-specific IgG post-primary concentration of 15% in the Oac− assay and 17% in the Oac+ assay.
Prechallenge, all GMCs had declined, although they remained above prevaccination levels in all groups. The prechallenge GMCs were lower in group 1 than in groups 2 and 3 in both assays (P < 0.001). After challenge, groups 2 and 3 showed similar GMCs (P = 0.79) but lower GMCs than group 1 (P < 0.01) in the Oac+ assay. In the Oac− assay, groups 2 and 3 also showed similar GMCs (P = 0.41). However, only group 2 had a significantly lower GMC than group 1 (P < 0.01). For all groups, the GMCs in the Oac+ assay were higher after challenge than after administration of MCC (P < 0.001), whereas the converse was seen—higher (P < 0.001) GMCs after administration of MCC than after challenge—in the Oac− assay.
For the subset of children bled 7 to 14 days after challenge, the serogroup C-specific Oac+ IgG GMC were 13.6 (95% CI = 3.5 to 53.0), 23.9 (95% CI = 11.6 to 49.3), and 8.6 (95% CI = 3.1 to 24.1) for groups 1, 2, and 3, respectively. These GMCs were similar to those seen in children bled 1 month postchallenge.
(iv) Serogroup C-specific IgG AIs.
The serogroup C-specific IgG geometric mean AIs (GMAIs) are shown in Table 2. Prevaccination, the AIs could not be measured in 414 samples due to low serogroup C-specific IgG antibody concentrations, whereas prechallenge this number was only 4. No significant association between prevaccination antibody concentration and post-primary AIs was discernible.
After administration of MCC, the GMAI was lower for group 1 (mean age, 3 months) than for groups 2 and 3 (mean age, 5 months) (P < 0.001). There was no significant difference between groups 2 and 3 (P = 0.10). By the time of the prechallenge dose there were no significant difference(s) in GMAI between the groups (P = 0.45), reflecting a greater increase in AI between the post-primary sample and the prechallenge sample in group 1 than in groups 2 and 3. The GMAI for all three groups increased post-MCC to prechallenge to postchallenge (P < 0.001). Postchallenge, group 1 GMAIs were higher than those observed in groups 2 and 3 (P < 0.005). There was no significant difference between groups 2 and 3 (P = 0.13).
Among the subset of children bled 7 to 14 days postchallenge, the serogroup C-specific GMAIs were 235.5 (95% CI = 112.2 to 494.3), 188.4 (95% CI = 144.3 to 246.1), and 147.9 (95% CI = 109.0 to 200.6) for groups 1, 2, and 3, respectively. These GMCs were similar to those seen in children bled 1 month postchallenge.
DISCUSSION
The infant immunization schedule for meningococcal conjugate vaccination in the United Kingdom is three doses administered at 2, 3, and 4 months of age. During the prelicensure studies it was shown that the MCC-TT vaccine was highly immunogenic after a single dose, but priming for immunologic memory was not investigated after only one or two doses (18). It would be beneficial if the number of doses of MCC vaccine in the infant immunization schedule could be reduced, particularly at a time when pneumococcal conjugate vaccines are being licensed elsewhere and may soon need to be incorporated into an already complex infant immunization schedule, adding another injection to the two already administered at each visit.
After a single dose of MCC-TT vaccine administered at 2 months of age, 98.4% of infants had C11 rSBA titers of ≥8, while after two doses at 2 and 4 months or three doses at 2, 3, and 4 months 100 and 99.4% of infants had rSBA titers of ≥8, respectively. In infants who did not achieve SBA titers of ≥16, prevaccination SBA titers were often found to be elevated. This is of potential concern since over the forthcoming years more potential mothers will have been vaccinated and may thus be capable of transmitting maternal meningococcal serogroup C antibodies to their offspring. To ensure adequate individual protection, children not reaching an rSBA of ≥16 (the threshold used for revaccination in the present study) were revaccinated with MCC, retested, and then excluded from further investigations. Therefore, their response to a challenge dose of polysaccharide was not assessed. Thus, it is not known whether these children developed immunologic memory even though their primary rSBA responses were relatively low.
A ≥4-fold increase in rSBA titer between pre- and post-primary MCC series has been used to predict protection (5). In the present study, 11 of 544 (2%) infants did not have ≥4-fold rises, and 6 of these (of a total of only 7) also had post-MCC rSBA titers of <16.
After a single dose of MCC-TT vaccine, lower rSBA GMTs against the Oac+ strains were seen 1 month after MCC-TT vaccination than in those who received two or three doses. Conversely, after a challenge dose of 10 μg of A/C polysaccharide, higher rSBA GMTs were seen in those who received only a single dose of MCC-TT vaccine as opposed to those who received two or three doses. As already observed with other conjugate vaccines, the quantity of antigen used for the primary series is positively correlated with post-primary rSBA levels but negatively correlated with the magnitude of the booster response (1, 6, 21).
A similar relationship between quantity of antigen and antibody response was also noted with the AIs. It should be mentioned that group 1 infants were bled at 3 months of age, whereas groups 2 and 3 were bled at 5 months of age. In infancy, this difference of 2 months may account in part for the lower GMAIs seen for group 1 cohort as opposed to groups 2 and 3.
For serogroup C-specific IgG in the standard ELISA, this trend was not as clear. After polysaccharide challenge, the one dose group had higher IgG levels with Oac+ polysaccharide than subjects who received two or three doses but for the post-primary MCC-TT vaccination, the three-dose group had higher titers than the one- or two-dose groups, with no difference observed between the one- and two-dose groups. The reasons for this result may lie in the fact that the ELISA measures all antibody to the C polysaccharide, whether functional or not. For this reason it is not generally considered the “gold standard” assay for meningococcal C serology. For assays performed with the Oac− polysaccharide, the IgG levels for those who had received one or three doses were higher than those who had received two doses after MCC-TT vaccination, with no difference postchallenge between all three groups. The reasons why these Oac− IgG levels do not fall into the same pattern are not clear.
Overall, the conjugate vaccine induced more IgG binding to Oac− polysaccharide compared to Oac+ polysaccharide, suggesting that two groups of epitopes are being recognized, the common polysaccharide backbone epitopes and unique Oac− epitopes created or exposed by the absence of the O-acetyl side chain. After the challenge dose of polysaccharide vaccine, which is O acetylated, the pattern was reversed and more IgG bound to the Oac+ polysaccharide than to the Oac− polysaccharide, indicating that there was a greater booster effect on Oac+-specific responses. Bactericidal activity was also higher against the Oac− strain than against the two Oac+ strains after MCC-TT vaccination. This may reflect the increased susceptibility of this strain to antibody-dependent complement-mediated lysis due to the lack of Oac groups exposing epitopes to the more functional backbone-specific antibodies.
The present study provides evidence that the number of doses of MCC-TT vaccine used in the infant immunization schedule could be decreased to two or possibly one dose(s). Further studies are required to investigate whether a reduced schedule of other MCC vaccines would be satisfactory. It should also be noted that if different concomitantly administered infant vaccines are used this reduced schedule would have to be reevaluated. The fact that the quantity of antigen used for the primary series is positively correlated with post-primary rSBA levels but negatively correlated with the magnitude of the booster response is important since both anticapsular antibody and immunologic memory play a role in immunity to N. meningitidis, as is also true for Hib (12). The recent observations of a resurgence of Hib disease and a decline in efficacy in the United Kingdom (17) among a population of children immunized with a three-dose vaccine course known reliably to indue immune memory (10) suggest that absolute levels of circulating antibody may be of more importance than previously thought.
Acknowledgments
We thank all of the study personnel (Elizabeth Drummond, Susan Chalmers, and Carol Young) and general practioners (M. Bell, A. Gray, G. Burt, J. Burt, B. Krishnaswamy, N. Hyndman, J. Philips, D. Taylor, P. Chue Hong, G. Barker, G. Brown, D. Bee, W. Carr, G. McLaren, H. Gordon, E. Wallace, and R. Lendrum) in Fife and their staffs, the study nurses in Gloucestershire (Rhonwen Morris, Diana Webb, Anne Maher, Gail Breeze, Wendy Nedoma, Anne Stevens, and Amanda Harris), and the study nurses in Sheffield (Lynn Seymour, Gillian Race, Lynne Shaw, Roger Burkinshaw, and Saiqa Butt) who recruited, vaccinated, and followed up the study children.
Financial support was provided by the United Kingdom Department of Health and by Baxter BioScience, Columbia, Md.
Editor: D. L. Burns
REFERENCES
- 1.Ahman, H., H. Käyhty, A. Vuorela, O. Leroy, and J. Eskola. 1999. Dose dependency of antibody response in infants and children to pneumococcal polysaccharides conjugated to tetanus toxoid. Vaccine 17:2726-2732. [DOI] [PubMed] [Google Scholar]
- 1a.Andrews, N., R. Borrow, and E. Miller. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 2.Anttila, M., J. Eskola, H. Ahman, and H. Käyhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: a re-evaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow, R., D. Goldblatt, N. Andrews, P. Richmond, J. Southern, and E. Miller. 2001. Influence of prior meningococcal C polysaccharide vaccine on the response and generation of memory following meningococcal C conjugate vaccine in young children. J. Infect. Dis. 184:377-380. [DOI] [PubMed] [Google Scholar]
- 5.Burrage, M., A. Robinson, R. Borrow, N. Andrews, J. Southern, J. Findlow, S. Martin, C. Thornton, D. Goldblatt, M. Corbel, D. Sesardic, K. Cartwright, P. Richmond, and E. Miller. 2002. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 70:4946-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campagne, G., A. Garba, P. Fabre, A. Schuchat, R. Ryall, D. Boulanger, M. Bybel, G. Carlone, P. Briantais, B. Ivanoff, B. Xerri, and J. P. Chippaux. 2000. Safety and immunogenicity of three doses of a Neisseria meningitidis A+C diphtheria conjugate vaccine in infants from Niger. Pediatr. Infect. Dis. J. 19:144-150. [DOI] [PubMed] [Google Scholar]
- 7.Gheesling, L. L., G. M. Carlone, L. Pais, L. B. Pais, P. F. Holder, S. E. Maslanka, B. D. Plikaytis, M. Achtman, P. Densen, C. E. Frasch, H. Käyhty, J. P. Mays, L. Nencioni, C. Peeters, D. C. Phipps, J. T. Poolman, E. Rosenqvist, G. R. Siber, B. Thiesen, J. Tai, C. M. Thompson, P. P. Vella, and J. D. Wenger. 1994. Multicenter comparison of Neisseria meningitidis serogroup C anticapsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J. Clin. Microbiol. 32:1475-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldblatt, D. 1997. Simple solid phase assays of avidity, p. 31-51. In M. W. Turner and A. P. Johnson (ed.), Immunochemistry 2: a practical approach. IRL/Oxford University Press, Oxford, United Kingdom.
- 9.Goldblatt, D., A. Pinto Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 177:1112-1115. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt, D., P. Richmond, E. Millard, C. Thornton, and E. Miller. 1999. The induction of immunologic memory after vaccination with Haemophilus influenzae type b conjugate and acellular pertussis-containing diphtheria, tetanus, and pertussis vaccine combination. J. Infect. Dis. 180:538-541. [DOI] [PubMed] [Google Scholar]
- 11.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference. Clin. Diagn. Lab. Immunol. 2:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas, A. H., and D. M. Granoff. 2001. Imperfect memory and the development of Haemophilus influenzae type B disease. Pediatr. Infect. Dis. J. 20:235-239. [DOI] [PubMed] [Google Scholar]
- 13.Maslanka, S. E., L. L. Gheesling, D. E. LiButti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. N. Devi, C. E. Frasch, J. C. Huang, P. Kris-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. A. M. Peeters, S. Quataert, J. Y. Tai, and G. M. Carlone. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michon, F., C. H. Huang, E. K. Farley, L. Hronowski, J. Di, and P. C. Fusco. 2000. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev. Biol. 103:151-160. [PubMed] [Google Scholar]
- 15.Miller, E., D. Salisbury, and M. Ramsay. 2001. Planning, registration, and implementation of an immunization campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 20:S58-S67. [DOI] [PubMed] [Google Scholar]
- 16.Pichichero, M. E., T. Voloshen, D. Zajac, and S. Passador. 1999. Avidity maturation of antibody to Haemophilus influenzae type b (Hib) after immunization with diphtheria-tetanus-acellular pertussis-Hib-hepatitis B combined vaccine in infants. J. Infect. Dis. 180:1390-1393. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay, M. E., J. McVernon, N. J. Andrews, P. T. Heath, and M. P. Slack. 2003. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis. 188:481-485. [DOI] [PubMed]
- 18.Richmond, P., R. Borrow, J. Findlow, S. Martin, C. Thornton, K. Cartwright, and E. Miller. 2001. Evaluation of de-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming and bactericidal activity against O-acetylated and de-O-acetylated serogroup C strains. Infect. Immun. 69:2378-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160-163. [DOI] [PubMed] [Google Scholar]
- 20.Richmond, P., D. Goldblatt, P. C. Fusco, J. D. S. Fusco, I. Heron, S. Clark, R. Borrow, and F. Michon. 1999. Safety and immunogenicity of a new Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in healthy adults. Vaccine 18:641-646. [DOI] [PubMed] [Google Scholar]
- 21.Richmond, P. C., E. Miller, R. Borrow, S. Clark, F. Sadler, A. J. Fox, N. T. Begg, R. Morris, and K. A. V. Cartwright. 1999. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J. Infect. Dis. 179:1569-1572. [DOI] [PubMed] [Google Scholar]
- 22.Trotter, C., R. Borrow, N. Andrews, and E. Miller. 2003. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine 21:1094-1098. [DOI] [PubMed] [Google Scholar]