Abstract
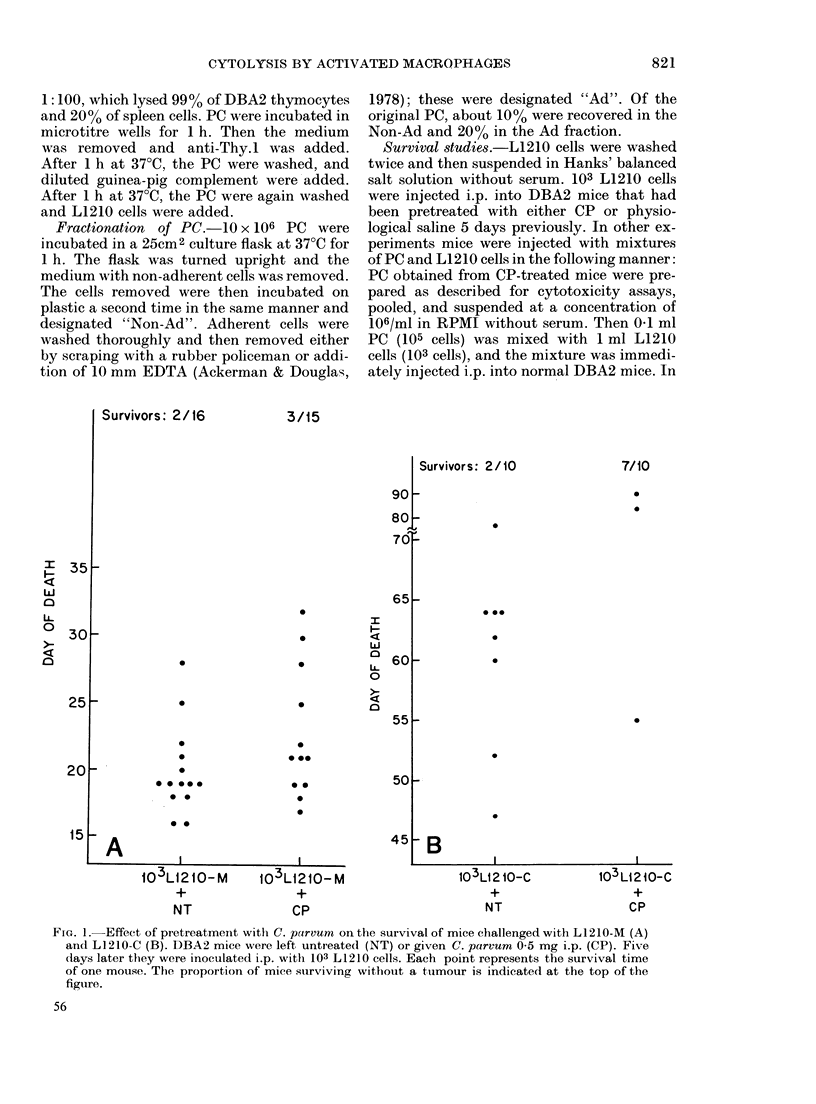
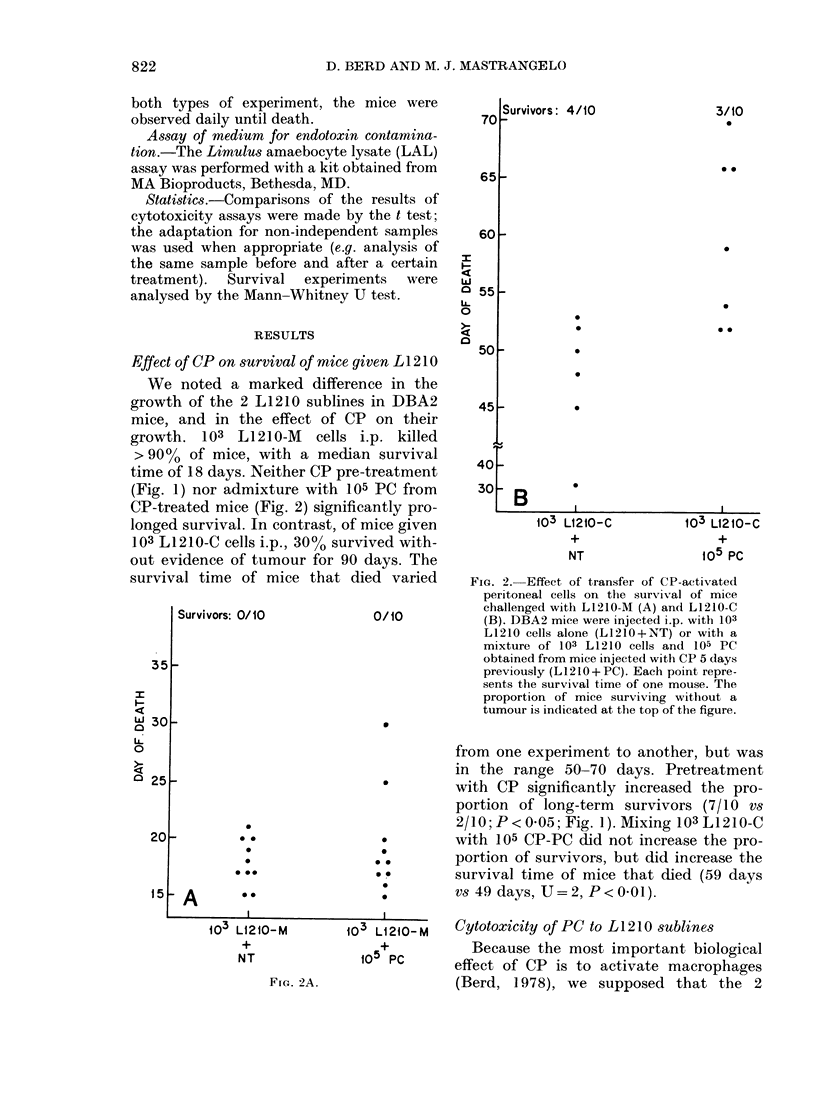
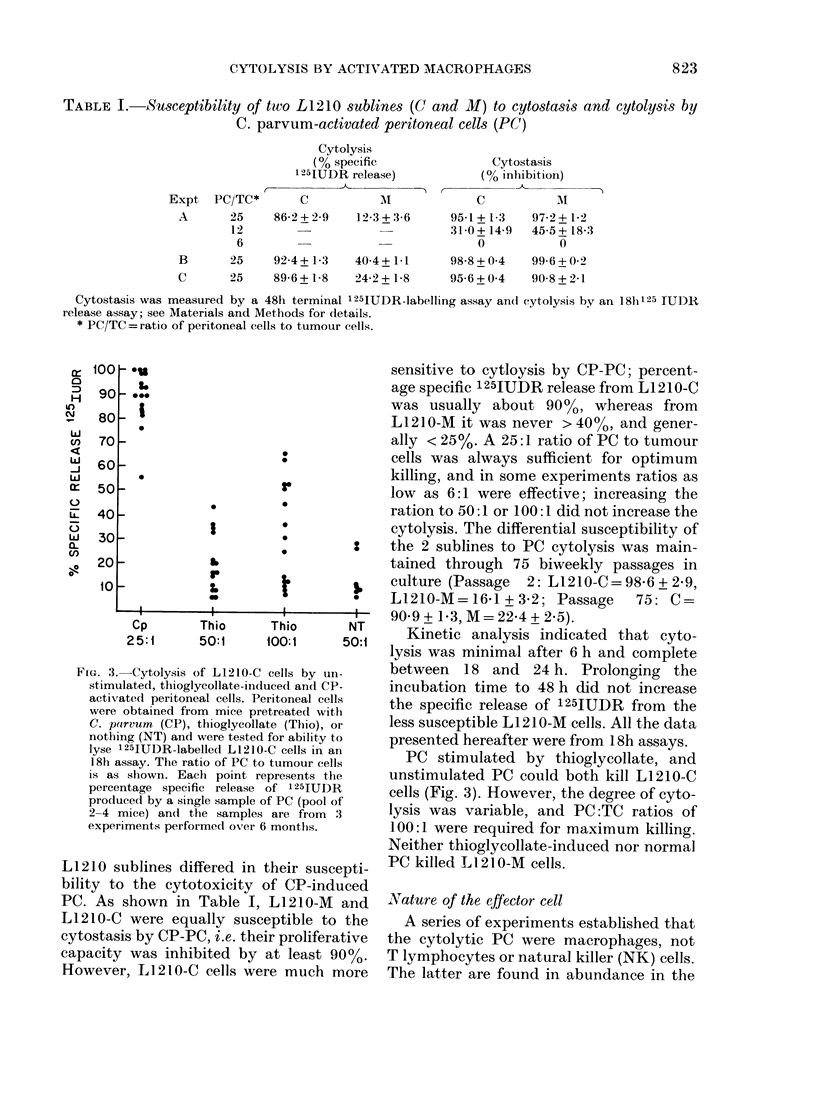
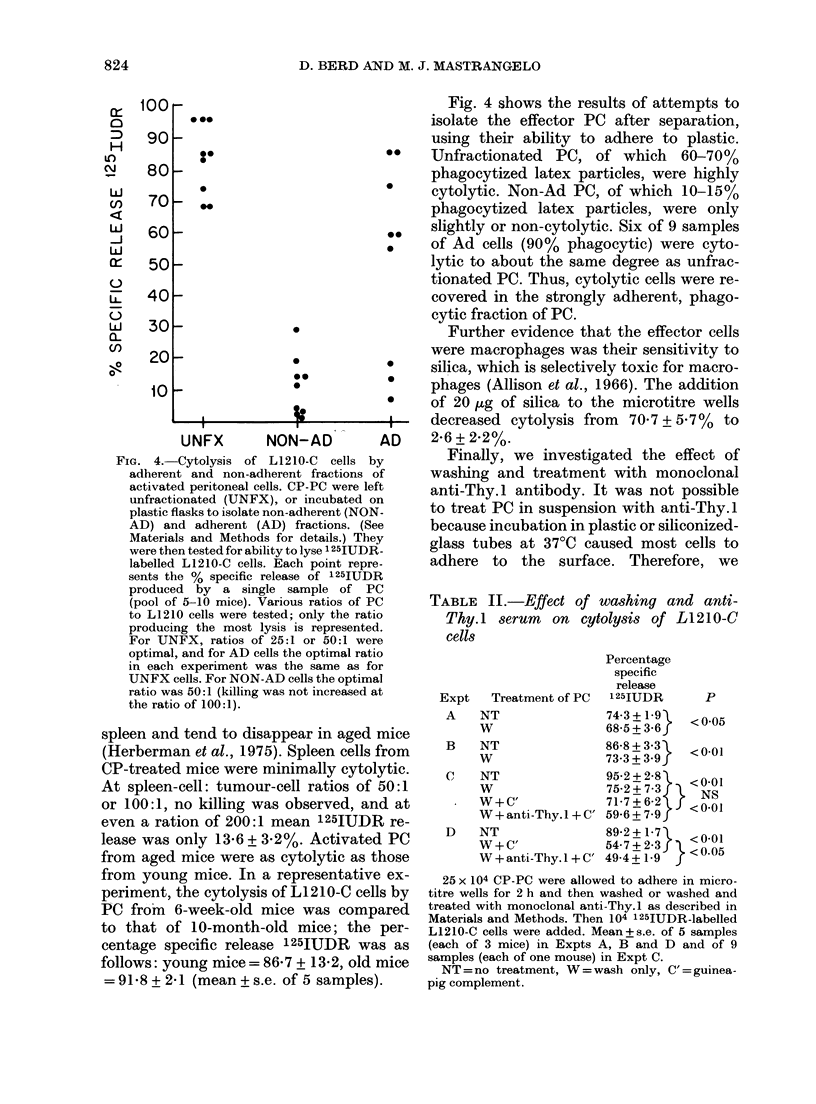
We observed the growth of 2 sublines of leukaemia L1210 in histocompatible DBA2 mice given 10(3) cells i.p. and studied the protective effect of Corynebacterium parvum (CP). The growth of subline L1210-M was unaffected by pretreatment with CP or admixture with 10(5) peritoneal cells (PC) from CP-treated mice. In contrast, the growth of subline L1210-C was inhibited; CP pretreatment increased the proportion of long-term survivors (70% vs 20%) and admixture with CP-PC prolonged the survival time (59 days vs 49 days; P less than 0.05). In vitro experiments indicated that Sublines M and C were equally sensitive to cytostasis by CP-PC, as measured in a terminal labelling assay (greater than 90% inhibition of proliferation). However, subline C was much more sensitive to cytolysis (18h 125IUDR-release assay) by CP-PC; percentage specific release from L1210-C was at least 90%, whilst from L1210-M it was generally less than 25%. The differential susceptibility of the 2 sublines to cytolytic PC was maintained through 75 passages in culture. The effector cells were considered to be macrophages, because they were adherent, phagocytic, and sensitive to silica. Cytolysis was unrelated to endotoxin contamination, because it was not inhibited by polymyxin B, and was inhibited by pre-incubating PC in culture medium for 24 or 48 h before adding target cells. Thus the relevance of nonspecific macrophage-mediated cytotoxicity in vitro to tumour resistance in vivo may depend on the strength of the cytotoxic reaction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Booth C. G., Spencer F. Lack of correlation between in vivo rejection of syngeneic fibrosarcomas and in vitro non-specific macrophage cytotoxicity. Br J Cancer. 1978 Nov;38(5):583–590. doi: 10.1038/bjc.1978.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974 May;34(5):1074–1078. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Macrophage nonimmunologic recognition: target cell factors related to contact inhibition. Science. 1973 May 25;180(4088):868–870. doi: 10.1126/science.180.4088.868. [DOI] [PubMed] [Google Scholar]
- Keller R. Cytostatic elimination of syngeneic rat tumor cells in vitro by nonspecifically activated macrophages. J Exp Med. 1973 Sep 1;138(3):625–644. doi: 10.1084/jem.138.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Susceptibility of normal and transformed cell lines to cytostatic and cytocidal effects exerted by macrophages. J Natl Cancer Inst. 1976 Feb;56(2):369–374. doi: 10.1093/jnci/56.2.369. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Stevenson M. M. Macrophage function in tumor-bearing mice: tumoricidal and chemotactic responses of macrophages activated by infection with Mycobacterium bovis, strain BCG. J Immunol. 1977 Jun;118(6):2176–2181. [PubMed] [Google Scholar]
- Morahan P. S., Kaplan A. M. Macrophage activation and anti-tumor activity of biologic and synthetic agents. Int J Cancer. 1976 Jan 15;17(1):82–89. doi: 10.1002/ijc.2910170112. [DOI] [PubMed] [Google Scholar]
- Peters L. J., McBride W. H., Mason K. A., Hunter N., Basić I., Milas L. In vivo transfer of antitumor activity by peritoneal exudate cells from mice treated with Corynebacterium parvum: reduced effect in irradiated recipients. J Natl Cancer Inst. 1977 Sep;59(3):881–887. doi: 10.1093/jnci/59.3.881. [DOI] [PubMed] [Google Scholar]
- Svet-Moldavsky G. J., Liozner A. L., Mkheidze D. M., Sokolov P. P., Bykovsky A. P. Tumor-induced skin heterogenization. II. Virus causing the phenomenon. J Natl Cancer Inst. 1970 Sep;45(3):475–484. [PubMed] [Google Scholar]


