Abstract
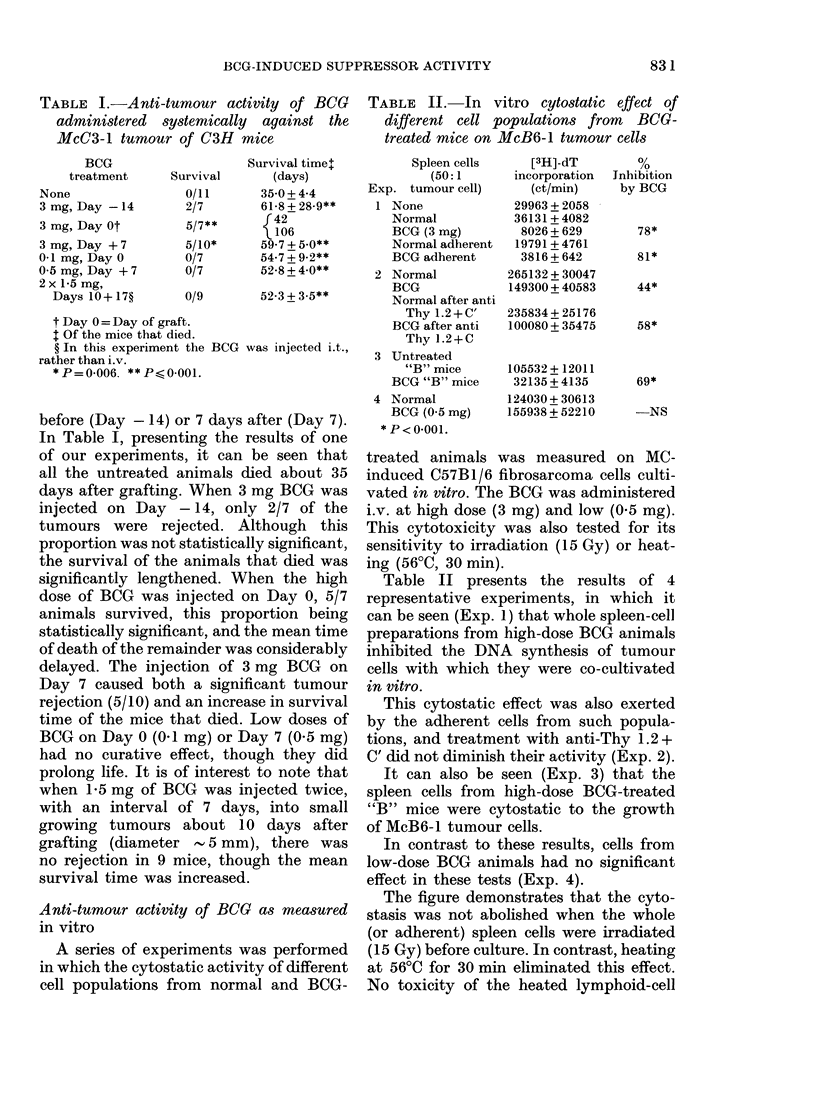
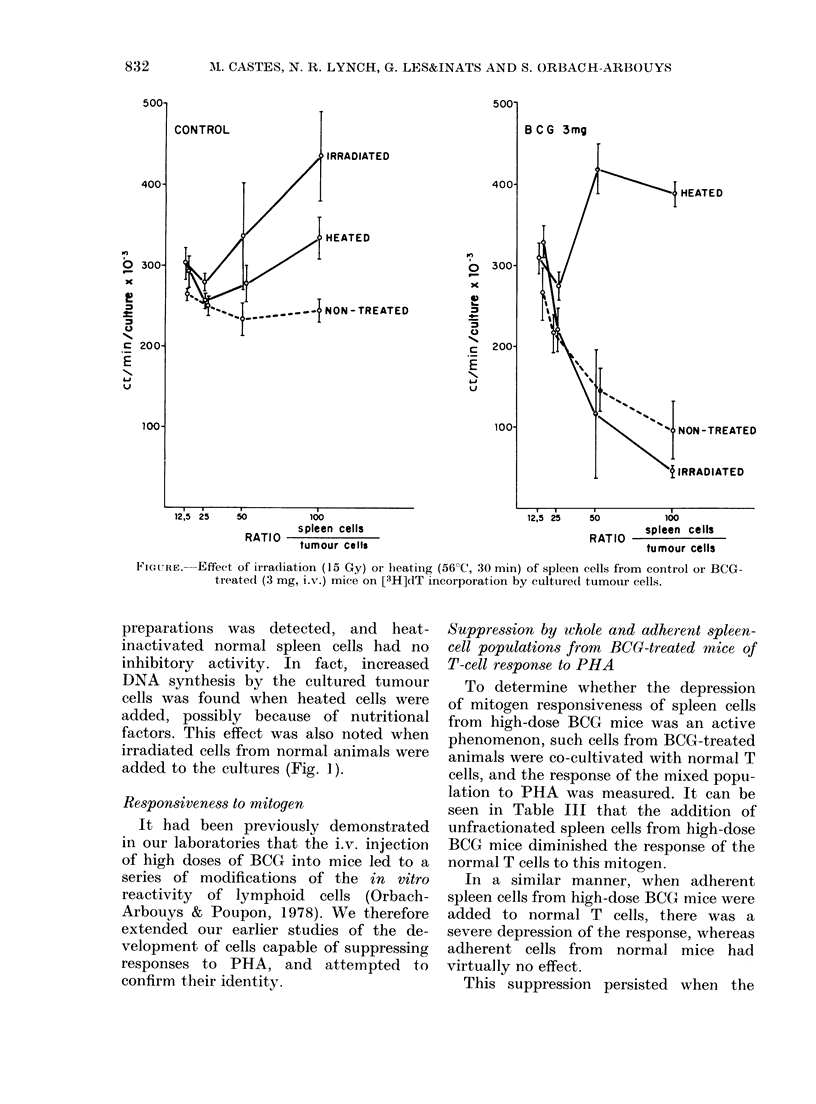
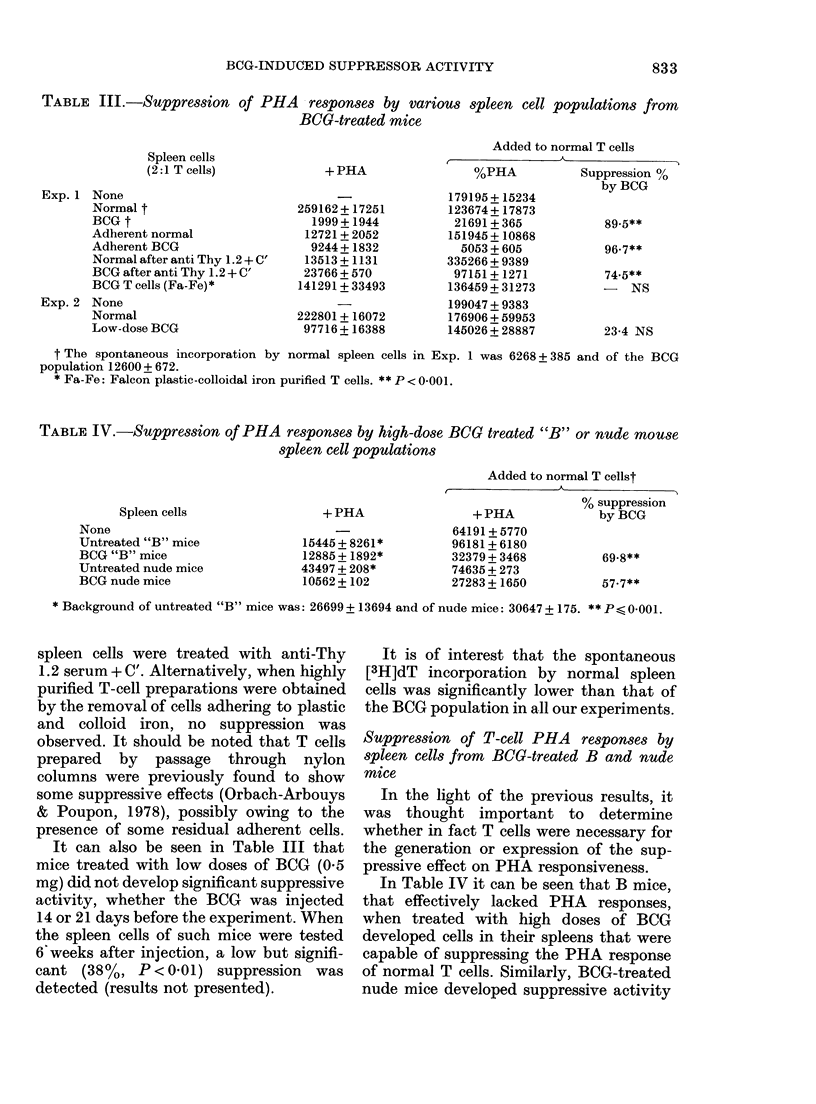
The i.v. injection of high doses (3 mg) of BCG into C3H mice bearing a transplantable 3-methylcholanthrene-induced fibrosarcoma caused the regression of a significant proportion. This effect was most evident when the BCG was injected on the day of the graft, or 7 days later. The injection of this agent either 14 days before the graft, or in low doses (0.1 or 0.5 mg), or directly into the tumour (i.t.) only prolonged the survival of the animals. Spleen cells from systemic high-dose BCG-treated mice were found to exert a strong nonspecific cytostatic effect in vitro that was not an artefact of the test conditions, and was not expressed by cells from low-dose animals. The cytostatic effect was shown to be caused by cells with the characteristics of macrophages, i.e. they were strongly adherent, unaffected by treatment with anti-Thy 1.2 + C', radioresistant but heat-sensitive, and were detected in BCG-treated "B" mice. The spleens of high-dose BCG-treated mice also contained suppressor cells that were capable of inhibiting the in vitro reactivity of normal T cells to PHA. Like the cytostatic effect, this suppressor activity was not detected in low-dose mice, and the cells responsible had the properties of macrophages; the effect was lost after the removal of adherent cells by sequential exposure to plastic and colloidal iron, but was conserved after treatment with anti-Thy 1.2 + C'. T-cell-deprived animals, such as "B" or nude mice, also developed suppressor-cell activity when treated with systemic high-dose BCG. Close parallels became evident between the in vivo anti-tumour activity of BCG, the in vitro cytostatic effect, and the suppressor-cell activity. We here discuss the possible role of suppressor cells in the mechanism of action of this agent.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger A. M., Cooperband S. R., Green J. A. Culture conditions affecting induction and release of lymphocyte produced proliferation inhibitory factor (PIF). Cell Immunol. 1973 Jul;8(1):12–27. doi: 10.1016/0008-8749(73)90089-0. [DOI] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Calderon J., Williams R. T., Unanue E. R. An inhibitor of cell proliferation released by cultures of macrophages. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4273–4277. doi: 10.1073/pnas.71.11.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Jandinski J. The relationship of cell division to the generation of cytotoxic activity in mixed lymphocyte culture. J Exp Med. 1974 Dec 1;140(6):1712–1716. doi: 10.1084/jem.140.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- Finklestein J. Z., Tittle K. L., Imagawa D. T. Immunoprophylaxis and immunotherapy of leukaemia with B.C.G. Lancet. 1972 Oct 21;2(7782):875–876. doi: 10.1016/s0140-6736(72)92238-6. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Williams R. M., Benacerraf B. Specific and nonspecific antitumor immunity. II. Macrophage-mediated nonspecific effector activity induced by BCG and similar agents. J Natl Cancer Inst. 1975 Mar;54(3):709–720. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Blomgren H. Further evidence for autonomy of T cells mediating specific in vitro cytotoxicity: efficiency of very small amounts of highly purified T cells. Cell Immunol. 1973 Oct;9(1):127–141. doi: 10.1016/0008-8749(73)90174-3. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Fujimoto S., Sehon A. H. Regulation of the immune response to tumor antigens. III. Characterization of thymic suppressor factor(s) produced by tumor-bearing hosts. J Immunol. 1977 Aug;119(2):757–764. [PubMed] [Google Scholar]
- Ha T. Y., Waksman B. H., Treffers H. P. The thymic suppressor cell. I. Separation of subpopulations with suppressor activity. J Exp Med. 1974 Jan 1;139(1):13–23. doi: 10.1084/jem.139.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Zbar B., Rapp H. J. Histopathology of tumor regression after intralesional injection of Mycobacterium bovis. I. Tumor growth and metastasis. J Natl Cancer Inst. 1972 May;48(5):1441–1455. [PubMed] [Google Scholar]
- Hibbs J. B., Jr Activated macrophages as cytotoxic effector cells. Transplantation. 1975 Jan;19(1):77–81. doi: 10.1097/00007890-197501000-00015. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Crandall C. A., Crandall R. B. T-dependent suppression of the primary antibody response to sheep erythrocytes in mice infected with Trichinella spiralis. Cell Immunol. 1976 Nov;27(1):102–110. doi: 10.1016/0008-8749(76)90158-1. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Lespinats G., Poupon M. F. Cytostatic effect of spleen cells of tumor-bearing mice on syngenetic tumor cells. Cancer Res. 1977 Jun;37(6):1727–1732. [PubMed] [Google Scholar]
- Lynch N. R., Castes M., Astoin M., Salomon J. C. Mechanism of inhibition of tumour growth by aspirin and indomethacin. Br J Cancer. 1978 Oct;38(4):503–512. doi: 10.1038/bjc.1978.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Mathé G., Halle-Pannenko O., Bourut C. Immune manipulation by BCG administered before or after cyclophosphamide for chemo-immunotherapy of L1210 leukaemia. Eur J Cancer. 1974 Oct;10(10):661–666. doi: 10.1016/0014-2964(74)90005-x. [DOI] [PubMed] [Google Scholar]
- Mekori T., Sher S., Robinson E. Suppression of the mitogenic response to phytohemagglutinin in malignant neoplasia: correlation with clinical stage and therapy. J Natl Cancer Inst. 1974 Jan;52(1):9–12. doi: 10.1093/jnci/52.1.9. [DOI] [PubMed] [Google Scholar]
- Morton D., Eilber F. R., Malmgren R. A., Wood W. C. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970 Jul;68(1):158–164. [PubMed] [Google Scholar]
- Olsson L., Ebbesen P., Kiger N., Florentin I., Mathé G. The antileukemic effect of systemic non-specific BCG-immunostimulation vs systemic specific immunostimulation with irradiated isogeneic leukemic cells. Eur J Cancer. 1978 Apr;14(4):355–362. doi: 10.1016/0014-2964(78)90205-0. [DOI] [PubMed] [Google Scholar]
- Orbach-Arbouys S., Castes M. Augmentation of immune responses after methotrexate administration. Immunology. 1979 Feb;36(2):265–269. [PMC free article] [PubMed] [Google Scholar]
- Orbach-Arbouys S., Castes M. Suppression of T-cell responses to histocompatibility antigens by BCG pre-treatment. Immunology. 1980 Feb;39(2):263–268. [PMC free article] [PubMed] [Google Scholar]
- Poupon M. F., Kolb J. P., Lespinats G. Evidence for splenic suppressor cells in C3H/He, T-cell-deprived C3H/He, and nude mice bearing a 3-methylcholanthrene-induced fibrosarcoma. J Natl Cancer Inst. 1976 Dec;57(6):1241–1247. doi: 10.1093/jnci/57.6.1241. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T. Depression of delayed-type hypersensitivity by Corynebacterium parvum: mandatory role of the spleen. Cell Immunol. 1974 Aug;13(2):251–263. doi: 10.1016/0008-8749(74)90243-3. [DOI] [PubMed] [Google Scholar]
- Stadecker M. J., Calderon J., Karnovsky M. L., Unanue E. R. Synthesis and release of thymidine by macrophages. J Immunol. 1977 Nov;119(5):1738–1743. [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L., Noworolski J., Mayhew B. Contact sensitivity to picryl chloride: the occurrence of B suppressor cells in the lymph nodes and spleen of immunized mice. Cell Immunol. 1976 Aug;25(2):266–278. doi: 10.1016/0008-8749(76)90117-9. [DOI] [PubMed] [Google Scholar]
- van der Gaag R., McCullagh P. Influence of secondary inoculum of tumour cells on growth of primary tumour. Br J Cancer. 1978 Jan;37(1):86–91. doi: 10.1038/bjc.1978.13. [DOI] [PMC free article] [PubMed] [Google Scholar]



