Abstract
The immunogenicity and protective efficacy of recombinant lumazine synthase from Brucella spp. (rBLS) administered with different adjuvants was evaluated in mice. Mice were immunized with rBLS in the absence or the presence of aluminum hydroxide gel (BLS-Al), monophosphoryl lipid A (BLS-MPA), or incomplete Freund's adjuvant (BLS-IFA). rBLS per se induced a vigorous immunoglobulin G (IgG) response, with high titers of IgG1 as well as IgG2. All the adjuvants increased this response; the BLS-IFA formulation was the most effective at inducing BLS-specific IgG antibodies. In addition, after in vitro stimulation with rBLS, spleen cells from BLS-IFA-, BLS-Al-, or BLS-MPA-immunized mice proliferated and produced interleukin-2 (IL-2), gamma interferon (IFN-γ), IL-10, and IL-4, suggesting the induction of a mixed Th1-Th2 response. Immunization with rBLS protected mice against challenge with B. abortus 544. The levels of protection in the spleen were similar for all adjuvants, but only BLS-Al and BLS-IFA were effective in the liver. Our results indicate that BLS might be a useful candidate for the development of subunit vaccines against brucellosis, since it elicits antigen-specific cellular responses, with production of IFN-γ and protection, independently of the adjuvant formulation used.
Brucella abortus is a gram-negative, facultative intracellular bacterium that infects cattle and humans, provoking abortion and infertility in the former and undulant fever, endocarditis, arthritis, and osteomyelitis in the latter (37). Because of the serious economic and medical consequences of brucellosis, efforts have been made through the use of vaccines to prevent the infection (26).
In many countries, the control of bovine brucellosis is organized on the basis of vaccination with live attenuated B. abortus strain 19. Although efficacious, this vaccine has some disadvantages, such as the ability to cause disease in humans, the possibility of causing abortion when administered to pregnant cattle, and the diagnostic difficulty of distinguishing field infections from vaccinated animals (since the vaccine induces anti-O-polysaccharide side chain-specific antibodies) (7). While the latter concern has been solved with the development of rough strains (31), the need for a better vaccine for brucellosis eradication clearly exists (Rep. W. H. O. Meet., W. H. O. document EMC/ZDI/98.14, p. 40-43, World Health Organization, Geneva, Switzerland, 11-12 December 1997).
In contrast, killed vaccines are noninfectious but they are considered less efficacious than live ones at inducing protective immunity against intracellular pathogens. In addition, with whole-microorganism vaccines, the regulatory requirements for the exact specifications of vaccine composition and for the mechanisms to obtain immunity are difficult to meet. In this respect, recombinant subunit vaccines have numerous advantages: they are completely inert, their compositions are predetermined, their production can be better controlled, and their homogeneity is much higher. However, the success of these vaccines depends on the selection of the right antigen(s), adjuvant(s), and delivery system(s). By optimization of these factors, the immune response can be tailored against a specific pathogen (23, 35).
Immunity against Brucella requires cell-mediated mechanisms (3, 4, 13). In particular, Th1 immune responses characterized by production of gamma interferon (IFN-γ) are associated with protective immunity to Brucella (15, 24, 38). These responses are best stimulated by live vaccines or potentially by multiple injections of appropriate protective antigens in the presence of adjuvants which favor cell-mediated immune mechanisms. The difficulty is that few effective candidate antigens have yet been identified.
Numerous cell surface and intracellular components have been assessed as protective antigens. Until now, significant activity has been identified for only a few antigens: the L7/L12 ribosomal protein (28), the Cu-Zn superoxide dismutase (33), a 22.9-kDa protein (11), and the cytoplasmic protein p39 (2). While the protection afforded could be improved using a multiple subunit vaccine, it also remains possible that a more effective antigen or a better adjuvant might lead to protection with a monovalent subunit vaccine.
In previous reports, investigators have demonstrated that the Brucella lumazine synthase (BLS), first identified as an 18-kDa cytoplasmic protein present in all Brucella species (19) and later shown to fold as a pentamer (10), is useful in the diagnosis of human and animal brucellosis (7, 8, 18, 19). Recently, Velikovsky et al. have shown that the injection in mice of plasmid DNA encoding BLS induces a Th1-specific immune response and protection against B. abortus challenge (34). In this study, we evaluated the immunogenicity and the protective efficacy of recombinant BLS (rBLS) administered in association with different adjuvants.
MATERIALS AND METHODS
Animals.
Female BALB/c mice (4 to 6 weeks old) (obtained from Instituto Nacional de Tecnología Agropecuaria, CICV, Castelar, Buenos Aires, Argentina) were acclimated and randomly distributed into experimental groups. Mice were kept in conventional animal facilities and received water and food ad libitum.
Bacteria.
Escherichia coli strain BL21(DE3) was used as a host for the expression of rBLS and was routinely grown at 37°C in Luria-Bertani broth or agar supplemented, when required, with 100 μg of ampicillin/ml. B. abortus strain 544 and B. melitensis strain H38S were grown in cultures in tryptose-soy agar supplemented with yeast extract (Merck, Buenos Aires, Argentina).
Cloning and expression of rBLS.
rBLS was cloned, expressed in E. coli, and purified as previously described (34). Purity was assessed by silver staining as reported elsewhere (10, 18). The protein preparation contained less than 0.05 endotoxin units per mg of protein, as assessed using a Limulus amebocyte lysate analysis kit (Sigma, St. Louis, Mo.).
Adjuvants and preparation of immunogens.
Aluminum hydroxide gel (Al) was kindly provided by Instituto Biológico Argentino S.A.I.C. An Al suspension (0.6 mg/ml) was mixed with rBLS and incubated for 30 min at room temperature. The Al-adsorbed antigen was washed, and the final pellet was resuspended in phosphate-buffered saline (PBS). Monophosphoryl lipid A (MPA) and incomplete Freund's adjuvant (IFA) (both from Sigma) were used according to the manufacturer's instructions.
Immunizations.
Mice were immunized intraperitoneally (i.p.) with 10 μg of rBLS in 200 μl of PBS or a different adjuvant. Control mice were injected with PBS alone. Each mouse was injected at days 0 and 15. Sera were obtained at 15, 30, 45, and 60 days after the first immunization. Mice used as the positive control group in the protection experiments were subcutaneously immunized with 8 × 108 heat-killed B. melitensis H38S bacteria in 200 μl of IFA. For comparison purposes, a control group was immunized with a plasmid carrying the BLS gene, as previously described (34).
rBLS ELISA.
Serum reactivities with rBLS were determined by indirect enzyme-linked immunosorbent assay (ELISA) as described previously (34). The cutoff value for the assay of 20 sera from nonimmunized mice assayed at a 1:100 dilution was calculated as the mean specific optical density plus 3 standard deviations. Serum titers were established as the reciprocal of the final serum dilution to yield an optical density value higher than the cutoff value.
Stimulation of cytokine production.
Spleen cell suspensions from immunized and control mice were prepared in RPMI 1640 (Gibco BRL, Life Technologies, Grand Island, N.Y.) supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10% fetal calf serum (complete medium). Cells were cultured at 4 × 106 cells/ml in duplicate with rBLS (5 μg/ml) or concanavalin A (ConA) (2.5 μg/ml) (Sigma). Cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and 95% air) for 24 h for cytokine gene expression or 48 h for cytokine secretion. At the end of the incubation, cells were centrifuged at 400 × g at 4°C and processed immediately for RNA extraction and supernatants were aliquoted and stored at −70°C until assayed for cytokine production.
In vitro blastogenesis.
Blastogenesis assays were performed in triplicate in round-bottomed microtiter plates (Nunclon; Nunc, Roskilde, Denmark). Splenocytes were grown in cultures at 2 × 105 cells/well and stimulated with rBLS (5 μg/ml) or ConA (2.5 μg/ml) in a final volume of 200 μl/well. Control cultures consisting of complete medium and cells were run simultaneously. Cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and 95% air) for 2 days (ConA) or 6 days (rBLS). At 18 h before harvesting, 1.0 μCi of [3H]thymidine (ICN Pharmaceuticals Inc., Costa Mesa, Calif.) in 25 μl of complete medium was added to each well. Cells were harvested onto glass-fiber mats, washed (using a harvester [Skatron Instruments Inc., Sterling, Va.]) with distilled water, and dried overnight at room temperature. The dried filters were placed into 3 ml of scintillation fluid, and radioactive incorporation was measured in a liquid scintillation counter (Beckman Instruments, Fullerton, Calif.). Results were expressed as stimulation index (S.I.) values (counts per minute of stimulated cultures divided by counts per minute of unstimulated cultures). S.I. values of >2 were considered to represent a specific response.
Detection of cytokine mRNA by semiquantitative RT-PCR.
Reverse transcriptase-PCR (RT-PCR) for cytokine gene expression was performed as previously described (34). Results were expressed in terms of severalfold increase over the mRNA levels of cells grown in cultures in the absence of antigen. Severalfold increases higher than 2 were considered to represent up-regulation of the investigated cytokine gene.
Cytokine ELISAs.
Interleukin-4 (IL-4), IL-10, and IFN-γ in culture supernatants were measured according to the instructions of the manufacturer (PharMingen, San Diego, Calif.) by sandwich ELISA using paired cytokine-specific monoclonal antibodies.
Protection experiments.
At 30 days after the last booster injection, mice were challenged i.p. with 4 × 104 CFU of B. abortus 544. Mice were killed by cervical dislocation 30 days after being challenged, and their spleens and livers were removed aseptically. Each spleen or liver was homogenized in a stomacher bag, serially diluted, plated on tryptose-soy agar, and incubated for 4 days at 37°C with 5% CO2. The number of CFU per spleen or liver was counted, and the results were represented as the mean log CFU ± standard deviation per group.
Statistical analysis of the data.
The CFU data were normalized and evaluated by one-way analysis of variance followed by Dunnett's post hoc test (InStat; GraphPad, San Diego, Calif.). The Kruskal-Wallis test and one-way analysis of variance were used to compare antibody and cellular responses, respectively.
RESULTS
rBLS induces a vigorous humoral response.
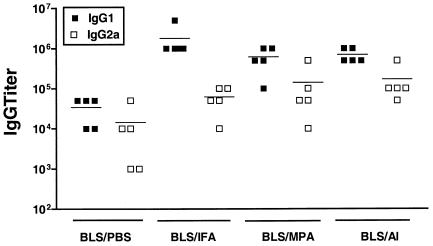
We examined the antibody response elicited by rBLS given alone or with different adjuvants. Mice were immunized with rBLS in the absence (BLS-PBS) or the presence of Al (BLS-Al), MPA (BLS-MPA), or IFA (BLS-IFA). Mice injected with PBS served as controls. The titers of anti-BLS immunoglobulin G (IgG) antibodies were measured by ELISA in sera from immunized mice. Immunization with BLS-PBS elicited an IgG response that was detectable at 15 days after the first immunization and increased after each injection to reach high titers (mean titer, 6,400) at 60 days postvaccination. These results indicate that BLS per se is a powerful immunogen that is able to induce high titers of specific antibodies, corroborating and extending the previous findings of Velikovsky et al. (34). As expected, a remarkable increase in the antibody titers was observed when mice were immunized with rBLS in the presence of different adjuvants, with the BLS-IFA formulation being the most effective at inducing BLS-specific IgG antibodies (mean titer, 143,750) and the BLS-Al and BLS-MPA formulations being the next most effective (mean titers, 57,500 and 47,500, respectively).
Mice immunized with BLS-PBS elicited high titers of specific IgG1 and IgG2a antibodies. IgG1 titers predominated over IgG2a titers (Fig. 1). This subclass profile was constant during the whole immunization schedule in spite of the repeated injections given (data not shown). Immunization with BLS-IFA, BLS-Al, and BLS-MPA increased both IgG1 and IgG2a titers by at least an order of magnitude, but as with BLS-PBS, IgG1 titers were higher than IgG2a titers (Fig. 1). These results indicate that mice immunized with BLS are prone to producing IgG1-specific antibodies and that different adjuvant formulations are unable to reverse this trend, even when the use of MPA as the adjuvant is able to preferentially induce IgG2a antibodies (25).
FIG. 1.
Characterization of antibody isotype profiles of mice immunized with rBLS. Mice (five per group) were immunized with rBLS in the absence (BLS-PBS) or the presence of BLS-Al, BLS-MPA, or BLS-IFA. Antibodies were evaluated by ELISA at 60 days after the last immunization in sera from immunized mice. Each symbol represents the antibody titer from an individual mouse, and the horizontal lines indicate the geometric means. Data are representative of two separate experiments.
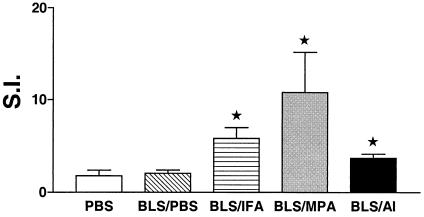
Injection of rBLS plus adjuvant induces T-cell proliferation.
To evaluate the cellular immune response induced by the different immunization protocols, we tested the ability of spleen cells from PBS-, BLS-PBS-, BLS-IFA-, BLS-Al-, or BLS-MPA-immunized mice to proliferate in vitro with rBLS. Cells from mice injected with BLS-IFA, BLS-MPA, and BLS-Al showed a significant (P < 0.05) antigen-specific proliferative response (S.I. = 5.81, 10.80, and 3.71, respectively) compared with splenocytes from PBS-immunized mice (S.I. = 1.78) (Fig. 2). In contrast, no significant response was detected in spleen cells from mice injected with BLS-PBS (S.I. = 2.06). The differences in proliferative response were not due to differences in background responses, because the unstimulated (control) cultures did not differ significantly among the four groups (data not shown).
FIG. 2.
Proliferative responses of spleen cells from mice immunized with BLS. Mice (five per group) were immunized with rBLS in the absence (BLS-PBS) or the presence of BLS-Al, BLS-MPA, or BLS-IFA. Mice immunized with PBS were used as controls. Spleen cells from immunized mice (2 × 105 cells/well) were stimulated with rBLS (0.5 μg/ml) for 5 days, and incorporation of [3H]thymidine was measured. The S.I. corresponds to the count per minute of stimulated cells divided by the count per minute of unstimulated cells. Results are expressed as the means ± standard errors of the means for five individual mice. Data are representative of three separate experiments. ★, significantly different from PBS-immunized group result (P < 0.05).
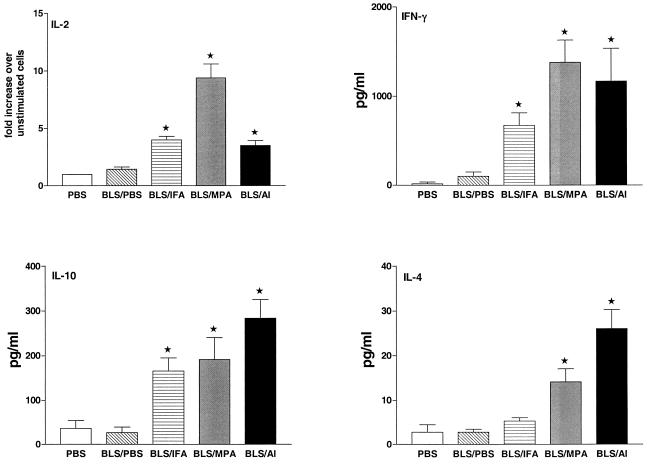
BLS induces a mixed Th1-Th2 cellular immune response.
To get further information on the types of immune responses induced by the different immunization protocols, we used RT-PCR and ELISA to investigate cytokine gene expression and secretion in spleen cells from PBS-, BLS-PBS-, BLS-IFA-, BLS-Al-, or BLS-MPA-immunized mice. rBLS induced a significant (P < 0.05) up-regulation of the expression of the IL-2 gene in cells from BLS-IFA-, BLS-MPA-, and BLS-Al-immunized mice. No up-regulation was observed in cells from BLS-PBS-injected animals (Fig. 3). Additionally, rBLS significantly (P < 0.05) induced the production of IFN-γ in cells from BLS-IFA-, BLS-MPA-, and BLS-Al-immunized mice whereas an increase of this cytokine that was not significant was observed in culture supernatants of splenocytes from BLS-PBS-immunized mice. Similarly, cells from BLS-IFA-, BLS-MPA-, and BLS-Al-immunized mice secreted IL-10 when stimulated with rBLS while cells from BLS-PBS-immunized mice did not. IL-4 production was detected in supernatants from BLS-Al- and BLS-MPA-immunized mice cells, whereas significant levels of this cytokine were not detected in culture supernatants of cells from BLS-PBS- or BLS-IFA-immunized animals (Fig. 3). In most cases, spontaneous cytokine production by unstimulated cells was below the detection limits. In spleen cells from PBS-immunized mice, the presence of rBLS did not induce gene transcription or cytokine production for any cytokine studied (Fig. 3). In response to the presence of ConA, cells from all of the animals studied produced IL-2, IL-4, IL-10, and IFN-γ, with no significant differences among the groups (data not shown). The levels of cytokines produced in vitro by splenocytes from immunized mice were similar to those reported by others (2, 29). Our results indicate that in spite of the intrinsic ability of BLS to induce a strong humoral response, this antigen is unable to elicit cell-mediated immune responses in the absence of adjuvants. They also show that once the cellular response is achieved, BLS induces a mixed Th1-Th2 response.
FIG. 3.
Determination of IL-2, IFN-γ, IL-10, and IL-4 levels in spleen cells from mice immunized with rBLS. Mice were immunized with BLS-PBS or with BLS-Al, BLS-MPA, or BLS-IFA. Mice immunized with PBS were used as controls. Spleen cells (4 × 106/ml) were stimulated with medium (RPMI 1640) or rBLS (5 μg/ml). Levels of IFN-γ (upper right panel), IL-10 (lower left panel), and IL-4 (lower right panel) in the cell supernatants were quantified (in picograms per milliliter) by antibody capture ELISA. Spontaneous (unstimulated) cytokine levels have been subtracted. The induced mRNA levels of IL-2 (upper left panel) were determined by RT-PCR as described in Materials and Methods. Each bar represents the geometric means ± standard errors of the means of the responses of spleen cells from five individual mouse experiments run in duplicate. Data are representative of two separate experiments. ★, significantly different from PBS-immunized group result (P < 0.05).
Different adjuvant formulations allow BLS to induce protection against Brucella infection.
Protection experiments were carried out by challenging vaccinated and control mice by i.p. injection of B. abortus 544, and the level of infection was evaluated by measuring CFU levels in spleens and livers. Mice immunized with BLS-Al, BLS-MPA, or BLS-IFA had a significant (P < 0.01) degree of protection compared with control mice receiving PBS. The reductions in bacterial burden were similar among all adjuvants used and comparable to that obtained with the plasmid carrying the BLS gene. No reduction in the number of CFU was seen in animals injected with BLS-PBS compared to that seen with control animals (Table 1). When we determined CFU levels in the liver, we observed that mice injected with BLS-Al or BLS-IFA had a significant (P < 0.01) degree of protection. In contrast, animals injected with BLS-MPA had numbers of CFU in the liver similar to those seen with controls (Table 1). These results indicate that immunization with BLS plus adjuvants afforded a significant degree of protection against Brucella infection.
TABLE 1.
Protection against B. abortus 544 provided to BALB/c mice by vaccination with rBLS and different adjuvant formulations
| Treatment group (n = 8) | Vaccine | Log10 brucellae CFU (mean ± SD) at:
|
Log protection at:
|
||
|---|---|---|---|---|---|
| Spleen | Liver | Spleen | Liver | ||
| 1 | PBS | 5.48 ± 0.13 | 2.45 ± 0.103 | 0 | 0 |
| 2 | B. melitensis H38 | 3.21 ± 0.13 | 1.44 ± 0.136 | 2.27a | 1.01a |
| 3 | BLS-PBS | 5.11 ± 0.41 | 2.31 ± 0.298 | 0.37 | 0.14 |
| 4 | BLS-IFA | 4.23 ± 0.37 | 1.75 ± 0.299 | 1.26a | 0.70a |
| 5 | BLS-MPA | 4.17 ± 0.22 | 1.89 ± 0.482 | 1.31a | 0.56 |
| 6 | BLS-Al | 4.07 ± 0.22 | 1.43 ± 0.088 | 1.40a | 1.02a |
| 7 | pcDNA-BLS | 3.83 ± 0.48 | 1.50 ± 0.398 | 1.61a | 0.95a |
Significantly different (P < 0.01) compared with value for control (PBS-inoculated) mice.
DISCUSSION
New strategies are sought to prevent brucellosis while avoiding the disadvantages of the currently used live vaccines. An attractive approach is the development of subunit vaccines. When these formulations are used, the induction of protective immunity against intracellular pathogens depends on the identification of adjuvants or delivery systems that can augment cell-mediated immune responses to the target antigen. In this study, we investigated the effects of different adjuvants on the immunogenicity and the protective efficacy of BLS, a promising candidate for a subunit vaccine against brucellosis (34). We studied adjuvants such as Al and IFA, which induce a Th2-dominated response, and MPA, which generates a Th1-type response (36), that are widely used in commercial veterinary vaccines.
We detected antigen-specific production of Th1 cytokines (IL-2 and/or IFN-γ) and Th2 cytokines (IL-4 and/or IL-10) in spleen cells from BLS-IFA-, BLS-Al-, and BLS-MPA-immunized mice. This observation suggests that, independently of the adjuvant used, BLS is able to induce a mixed T-helper response. Likewise, while studying the influence of the antigenic form and the adjuvant type on the immune response, other investigators have obtained mixed Th1-Th2 responses (1, 16, 22). These studies employed polymeric antigens such as viruses or virus-like particles. Braden et al. have determined previously by X-ray crystallography that BLS is polymeric (10). This further supports the notion that polymeric antigens induce a mixed T-helper response that can be skewed towards a Th1 or Th2 response, depending on both the adjuvant and the immunization protocol employed.
Independently of the formulation employed, mice immunized with BLS and adjuvants were protected against a virulent Brucella challenge. Protection was observed at the spleen and also at the liver, an important site for the control of Brucella infection by T- and B-cell-mediated defenses (14, 20). From a practical point of view, an important conclusion can be drawn from our results. Hitherto, a wide variety of adjuvants have been used with the intention of boosting cell-mediated immunity responses elicited by different Brucella antigens (30). However, practical acceptance of these preparations has been very limited, mainly because of the unacceptable local inflammatory reactions elicited by the adjuvant. The fact that BLS induces significant protection against virulent Brucella challenge independently of the formulation employed makes this antigen suitable to be used with adjuvants (such as Al) that do not affect the quality of the meat when administered to livestock or that elicit minimal local reactions for humans.
All the adjuvants employed elicited BLS-specific IL-10-producing cells. Although IL-10 can down-regulate protective immunity during primary B. abortus infection (17), the level of protection achieved in each treatment was similar to that obtained by injecting plasmid DNA which does not induce antigen-specific IL-10 production (34). Thus, it seems that the induction of IL-10 did not diminish the degree of protection against Brucella challenge, which is in agreement with results reported by Pasquali et al. (29).
Another important feature of BLS per se is that it was able to induce a vigorous IgG response, with high titers of IgG1 as well as IgG2, in the absence of adjuvants. The structural characteristics of BLS could explain the strong B-cell response elicited when mice were immunized with rBLS. The polymeric nature of BLS (i.e., that of a repetitive and spatially ordered array of the same epitopes) would produce strong signal transduction mediated by B-cell receptors, as has been described previously for a study using haptens as model antigens (32). In this sense, the three-dimensional structure of BLS shows that any given epitope would be presented at five different points separated from each other at a distance of about 50 Å (10). This analysis is in agreement with the previous proposal that the immune system has evolved to respond strongly to antigens with an epitope spacing of 50 to 100 Å, as typically found on microbial surfaces (5, 12). In brucellosis, protection is dependent upon cell-mediated immunity (4, 13) and, under some conditions, upon the presence of antibodies specific to membrane proteins (9, 21). Since BLS is a cytoplasmic antigen, the strong antibody response elicited with rBLS is irrelevant from the point of view of protection, as evidenced in mice immunized with BLS in the absence of adjuvants. Yet the high immunogenicity of BLS (due to the repetitiveness and order of surface epitopes) can be exploited to improve the immunogenicity of foreign epitopes, as has been described elsewhere (27). Interestingly, X-ray structural studies of rBLS (6, 10) showed that is possible to insert foreign peptides at the amino terminus without disrupting its general folding. These peptides could be presented to the immune system in a polymeric way and in the context of a high specific immune response.
In conclusion, our results indicate that BLS could be a useful candidate for the development of subunit vaccines against brucellosis, since it elicits an antigen-specific cellular response, with production of IFN-γ and protection, independently of the adjuvant formulation used. The results also demonstrate that BLS is a strong B immunogen which, due to its structural characteristics (6, 10), could be used to engineer chimerical immunogens able to display predefined peptides on the same molecular scaffold.
Acknowledgments
This work was supported by grant PICT 05-06324 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) and SECYT (UNICEN). C.A.V. and J.C. are recipients of a fellowship from CONICET.
S.E. is a member of C.I.C. (Buenos Aires). G.H.G., F.A.G., and C.A.F. are members of the Research Career of CONICET. C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
Editor: D. L. Burns
REFERENCES
- 1.Aberle, J. H., S. W. Aberle, S. L. Allison, K. Stiasny, M. Ecker, C. W. Mandl, R. Berger, and F. X. Heinz. 1999. A DNA immunization model study with constructs expressing the tick-borne encephalitis virus envelope protein E in different physical forms. J. Immunol. 163:6756-6761. [PubMed] [Google Scholar]
- 2.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godfroid, K. Walravens, and J.-J. Letesson. 2001. Induction of immune response in BALB/c mice with a DNA vaccine encoding bacterioferritin or P39 of Brucella spp. Infect. Immun. 69:6264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araya, L. N., P. H. Elzer, G. H. Rowe, F. M. Enright, and A. J. Winter. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330-3337. [PubMed] [Google Scholar]
- 4.Araya, L. N., and A. J. Winter. 1990. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect. Immun. 58:254-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., and R. M. Zinkernagel. 1996. The influence of virus structure on antibody responses and virus serotype formation. Immunol. Today 17:553-558. [DOI] [PubMed] [Google Scholar]
- 6.Baldi, P. C., C. A. Velikovsky, B. C. Braden, G. H. Giambartolomei, C. A. Fossati, and F. A. Goldbaum. 2000. Structural, functional and immunological studies on a polymeric bacterial protein. Braz. J. Med. Biol. Res. 33:741-747. [DOI] [PubMed] [Google Scholar]
- 7.Baldi, P. C., G. H. Giambartolomei, F. A. Goldbaum, L. F. Abdón, C. A. Velikovsky, R. Kittelberger, and C. A. Fossati. 1996. Humoral immune response against lipopolysaccharide and cytoplasmic proteins of Brucella abortus in cattle vaccinated with Brucella abortus S19 or experimentally infected with Yersinia enterocolitica serotype 0:9. Clin. Diagn. Lab. Immunol. 3:472-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldi, P. C., M. M. Wanke, M. E. Loza, N. Monachesi, and C. A. Fossati. 1997. Diagnosis of canine brucellosis by detection of IgG antibodies against an 18 kDa cytoplasmic protein of Brucella spp. Vet. Microbiol. 57:273-281. [DOI] [PubMed] [Google Scholar]
- 9.Bowden, R. A., A. Cloeckaert, M. Zygmunt, and G. Dubray. 1995. Outer membrane protein and rough lipopolysaccharide-specific monoclonal antibodies protect mice against Brucella ovis. J. Med. Microbiol. 43:344-347. [DOI] [PubMed] [Google Scholar]
- 10.Braden, B. C., C. A. Velikovsky, A. A. Cauerhff, I. Polikarpov, and F. A. Goldbaum. 2000. Divergence in macromolecular assembly: X-ray crystallographic structure analysis of lumazine synthase from Brucella abortus. J. Mol. Biol. 297:1031-1036. [DOI] [PubMed] [Google Scholar]
- 11.Cespedes, S., E. Andrews, H. Folch, and A. Onate. 2000. Identification and partial characterisation of a new protective antigen of Brucella abortus. J. Med. Microbiol. 49:165-170. [DOI] [PubMed] [Google Scholar]
- 12.Chackerian, B., P. Lenz, D. R. Lowy, and J. T. Schiller. 2002. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J. Immunol. 169:6120-6126. [DOI] [PubMed] [Google Scholar]
- 13.Cheers, C. 1984. Pathogenesis and cellular immunity in experimental murine brucellosis. Dev. Biol. Stand. 56:237-246. [PubMed] [Google Scholar]
- 14.Cheville, N. F., R. A. Kunkle, A. E. Jensen, and M. V. Palmer. 1995. Persistence of Brucella abortus in the livers of T cell-deficient nude mice. Lab. Investig. 73:96-102. [PubMed] [Google Scholar]
- 15.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falchetti, R., P. Di Francesco, G. Lanzilli, R. Gaziano, I. A. Casalinuovo, A. T. Palamara, G. Ravagnan, and E. Garaci. 1998. Determination of cytokine co-expression in individual splenic CD4+ and CD8+ T cells from influenza virus-immune mice. Immunology 95:346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes, D., and C. Baldwin. 1995. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect. Immun. 63:1130-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldbaum, F. A., C. A. Velikovsky, P. C. Baldi, S. Mortl, A. Bacher, and C. A. Fossati. 1999. The 18-kDa cytoplasmic protein of Brucella spp.—an antigen useful for diagnosis—is a lumazine synthase. J. Med. Microbiol. 48:833-839. [DOI] [PubMed] [Google Scholar]
- 19.Goldbaum, F. A., J. Leoni, J. C. Wallach, and C. A. Fossati. 1993. Characterization of an 18-kilodalton Brucella cytoplasmic protein which appears to be a serological marker of active infection of both human and bovine brucellosis. J. Clin. Microbiol. 31:2141-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izadjoo, M. J., Y. Polotsky, M. G. Mense, A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2000. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 68:5314-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacques, I., A. Cloeckaert, J. N. Linet, and G. Dubray. 1992. Protection conferred on mice by combinations of monoclonal antibodies directed against outer-membrane proteins or smooth lipopolysaccharide of Brucella. J. Med. Microbiol. 37:100-103. [DOI] [PubMed] [Google Scholar]
- 22.Katayama, S., K. Oda, T. Ohgitani, T. Hirahara, and Y. Shimizu. 1999. Influence of antigenic forms and adjuvants on the IgG subclass antibody response to Aujeszky's disease virus in mice. Vaccine 17:2733-2739. [DOI] [PubMed] [Google Scholar]
- 23.Miller, M. J., R. A. Wrightsman, and J. E. Manning. 1996. Trypanosoma cruzi: protective immunity in mice immunized with paraflagellar rod proteins is associated with a T-helper type 1 response. Exp. Parasitol. 84:156-167. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, E. A., J. Sathiyaseelan, M. A. Parent, B. Zou, and C. L. Baldwin. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuzil, K. M., J. E. Johnson, Y. W. Tang, J. P. Prieels, M. Slaoui, N. Gar, and B. S. Graham. 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 15:525-532. [DOI] [PubMed] [Google Scholar]
- 26.Nicoletti, P. 1990. Vaccination, p. 284-299. In K. Nielsen, and J. R. Duncan (ed.), Animal brucellosis. CRC Press, Boca Raton, Fla.
- 27.Nieba, L., and M. F. Bachmann. 2000. A new generation of vaccines. Mod. Asp. Immunobiol. 1:36-39. [Google Scholar]
- 28.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 29.Pasquali, P., R. Adone, L. C. Gasbarre, C. Pistoia, and F. Ciuchini. 2001. Mouse cytokine profiles associated with Brucella abortus RB51 vaccination or B. abortus 2308 infection. Infect. Immun. 69:6541-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schurig, G. G., N. Sriranganathan, and M. J. Corbel. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90:479-496. [DOI] [PubMed] [Google Scholar]
- 31.Schurig, G. G., R. M. Roop II, T. Bagchi, S. Boyle, D. Buhrman, and N. Sriranganathan. 1991. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28:171-188. [DOI] [PubMed] [Google Scholar]
- 32.Sulzer, B., and A. S. Perelson. 1997. Immunons revisited: binding of multivalent antigens to B cells. Mol. Immunol. 34:63-74. [DOI] [PubMed] [Google Scholar]
- 33.Tabatabai, L. B., and G. W. Pugh, Jr. 1992. Modulation of immune responses in BALB/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919-924. [DOI] [PubMed] [Google Scholar]
- 34.Velikovsky, C. A., J. Cassataro, G. H. Giambartolomei, F. A. Goldbaum, S. Estein, R. A. Bowden, L. Bruno, C. A. Fossati, and M. Spitz. 2002. A DNA vaccine encoding lumazine synthase from Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 70:2507-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Victoratos, P., M. Yiangou, N. Avramidis, and l. Hadjipetrou. 1997. Regulation of cytokine gene expression by adjuvants in vivo. Clin. Exp. Immunol. 109:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yip, H. C., A. Y. Karulin, M. Tary-Lehmann, M. D. Hesse, H. Radeke, P. S. Heeger, R. P. Trezza, F. P. Heinzel, T. Forsthuber, and P. V. Lehmann. 1999. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J. Immunol. 162:3942-3949. [PubMed] [Google Scholar]
- 37.Young, E. J. 1983. Human brucellosis. Rev. Infect. Dis. 5:821-842. [DOI] [PubMed] [Google Scholar]
- 38.Zhan, Y., and C. Cheers. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]