Abstract
We examined the relationship between host survival and renal and splenic immune responses in a murine model of hematogenously disseminated candidiasis. Male BALB/c mice were infected via tail vein injection with wild-type C. albicans or with an isogenic, Δefg1/Δefg1 hypha-deficient mutant. Host survival, organ fungal burden, intracellular cytokine content of splenic and kidney lymphocytes, and whole-organ cytokine profiles were determined. Wild-type C. albicans induced type 2 splenocyte responses with both nonfatal and fatal inocula. In the kidney, conversely, wild-type inocula causing no or low mortality induced type 1 responses and 100% fatal inocula induced type 2 or interleukin-10 (IL-10)-dominant responses. Hypha-deficient mutant C. albicans caused no or low mortality while inducing type 1 responses in both the spleen and kidney. To our knowledge, this is the first demonstration that host survival during systemic infection correlates with the type of immune response engendered in a nonlymphoid, parenchymal organ and not with the response in the spleen. Furthermore, the results provide in vivo confirmation that hyphal formation by C. albicans induces type 2 or IL-10-dominant host responses in tissues.
Candida is the fourth most common bloodstream isolate in the nosocomial setting (31), and the cost associated with nosocomial candidemia in the United States approaches one billion dollars per year (18). Furthermore, disseminated candidiasis has an attributable mortality of nearly 40% overall (and >50% in myeloablated patients) even in the face of modern antifungal therapy (14, 21, 35). Strategies to potentiate host defense against the fungus are likely to be efficacious for preventing and/or treating this infection; hence, there has been intense interest in elucidating the precise nature of protective host defense mechanisms against Candida.
The paradigm of type 1 and/or type 2 immunity integrates cell-mediated and humoral host defense (32). T-helper 1 (Th1) cells, which secrete gamma interferon (IFN-γ) but not interleukin-4 (IL-4), stimulate type 1 immunity characterized by intense phagocytic activity. Conversely, Th2 cells, which secrete IL-4 but not IFN-γ, stimulate type 2 immunity characterized by induction of high antibody titers and suppression of phagocytic activity. It has been suggested that a type 1 immune response is protective against both disseminated and mucocutaneous candidiasis, while a dominant type 2 immune response results in increased susceptibility to disseminated disease (6, 10, 17, 28, 30). Furthermore, during Candida sepsis there is a down-regulation of the host response to the fungus (33), suggesting that T-regulatory (Treg) or Th3 cells, which predominantly secrete the immunosuppressive cytokines IL-10 and transforming growth factor beta (TGF-β) (8, 9, 11), regulate host immunity to high inoculum levels of Candida. This notion is supported by data from Romani's group, who recently reported that C. albicans induces IL-10+/TGF-β+ Treg cells that mediate oral tolerance following intragastric infection (19).
Recent observations indicate that the type of immune response generated in nonlymphoid organs may be different from the type of simultaneous immune response generated in secondary lymphoid organs, such as the spleen and lymph nodes (15, 36). However, whether the type of immune response in nonlymphoid or lymphoid organs determines the outcome of a systemic infection remains unclear. Seminal observations by Parish in the early 1970s revealed a significant effect of inoculum size on the generation of humoral versus cell-mediated immunity (22, 23). Furthermore, formation of hyphae by Candida induces antigen-presenting cells to secrete IL-4 (5) while inhibiting IL-12 secretion (3) in vitro, which may promote type 2 responses in vivo. Therefore, we modulated fungal inoculum and hyphal formation to study the simultaneous immune polarization in the kidney and spleen and to determine which response correlated with survival during murine disseminated candidiasis.
This work was presented in part at the 6th American Society for Microbiology Conference on Candida and Candidiasis, Orlando, Florida, January 2002.
MATERIALS AND METHODS
Reagents and antibodies.
All reagents were obtained from Sigma-Aldrich (St. Louis, Mo.) unless otherwise specified. RPMI 1640 medium and phosphate-buffered saline (PBS) were obtained from Irvine Scientific (Irvine, Calif.). Fetal bovine serum (FBS) was purchased from Invitrogen (Carlsbad, Calif.). The following reagents were obtained from Pharmingen (San Diego, Calif.): fluorescein isothiocyanate-conjugated anti-mouse CD4 (clone RM4-5) and an isotype control (clone R35-95), phycoerythrin-conjugated anti-mouse IFN-γ (clone XMG1.2) and an isotype control (clone R-34), allophycocyanin-conjugated anti-mouse IL-4 (clone 11B11) and an isotype control (clone R3-34), allophycocyanin-conjugated anti-mouse IL-10 (clone JES5-16E3) and an isotype control (clone A95-1), and unlabeled blocking antibodies directed against each of the above-named reagents.
Strains and culture conditions: Candida albicans.
SC5314, a well-characterized clinical isolate, was supplied by W. Fonzi (Georgetown), and CAN33, a Δefg1/Δefg1 isogenic mutant, was a gift from G. Fink (Whitehead Institute). This mutant (henceforth referred to as the hypha-deficient mutant) does not form hyphae under most growth conditions (12), and it has been confirmed by histopathology that the organism does not germinate in kidneys within the first week during disseminated infection in BALB/c mice (B. Spellberg and S. G. Filler, unpublished data). Both strains were grown overnight at room temperature on a rotating drum in yeast nitrogen base broth (Difco Laboratories, Kansas City, Mo.) containing 2% glucose, as previously described (34).
Mice and experimental protocol.
For the survival experiment, eight male BALB/c mice (weighing 25 g each) were infected via the tail vein with the appropriate inoculum of wild-type or hypha-deficient mutant C. albicans blastospores or with PBS alone (sham injection controls). For the immunology and organ fungal-burden experiments, three mice per group were similarly infected. At 12, 36, or 72 h or 1 week after infection, mice were sacrificed and their kidneys, livers, and spleens were processed for tissue fungal-burden determination as described below. Immunology and organ fungal-burden experiments were performed at least twice, and the data from all replicate experiments were pooled. All procedures involving mice were approved by the institutional animal use and care committee, according to the National Institutes of Health guidelines for animal housing and care.
Tissue fungal burden.
To determine the number of viable C. albicans cells at different time points, organs were weighed, homogenized, diluted with saline, and quantitatively cultured on Sabouraud dextrose agar at 37°C for 2 days. The results were expressed as log CFU/gram of tissue. Prior to processing, spleens were divided in half to allow simultaneous analysis of fungal colony counts and intracellular cytokine staining of splenocytes, as described below.
Intracellular cytokine staining.
Intracellular cytokines in splenic and renal lymphocytes were detected by a modification of the method of Prussin and Metcalfe (25). Spleens were homogenized with a syringe plunger, while kidney homogenates were obtained by mincing the organ with a razor blade followed by incubation for 45 min at 37°C in RPMI 1640 medium containing collagenase at 2 μg/ml. To obtain splenic and renal lymphocytes, organ homogenates were passed though 70-μm-pore-size filters (Fisher), followed by red blood cell lysis with 0.15 M ammonium chloride. The cells were then rinsed with complete medium (RPMI 1640 medium supplemented with 50 U of penicillin/ml, 50 μg of streptomycin/ml, 2 mM l-glutamine, 10% FBS, and 5 μM 2-mercaptoethanol) and transferred to 6-well tissue culture plates (Falcon). Cells were stimulated for 4 h with 50 ng of phorbol myristate acetate/ml-1 μg of ionomycin/ml in the presence of 10 μg of brefeldin A/ml. The cells were transferred to a 96-well microtiter plate (Falcon), fixed with 2% paraformaldehyde, and permeabilized with 0.5% saponin in the presence of 10% normal mouse serum. Subsequently, the cells were stained for 30 min at room temperature with 10 μg of the appropriate combination of antibodies/ml in flow cytometry buffer (PBS containing 2% FBS and 0.1% sodium azide) plus 0.5% saponin. Wells containing excess unlabeled blocking antibody were included to confirm the specificity of antibody binding.
Flow cytometry.
Three-color flow cytometry was performed using FACSComp software per the manufacturer's recommendations on a FACScan instrument calibrated with CaliBRITE beads (Becton Dickinson, San Jose, Calif.). During data acquisition, CD4+ lymphocytes were gated by concatenation of forward and side scatter and fluorescein isothiocyanate-anti-CD4 antibody fluorescence properties. Data for each sample were acquired until 10,000 CD4+ lymphocytes had been analyzed. Th1 cells were defined as CD4+ IFNγ+ IL-4−, and Th2 cells were defined as CD4+ IFNγ− IL-4+. Results are presented as the medians ± 25th and 75th quartiles of the percentages of all gated lymphocytes that were Th1 or Th2 cells. Ratios of Th1/Th2 cells were generated to determine the presence of a polarized immune response. The immune response in uninfected mice was defined as unpolarized (4, 13, 16). Hence, ratios significantly higher than those measured in uninfected mice defined type 1 responses and ratios significantly lower than those measured in uninfected mice defined type 2 responses.
Whole-organ cytokines.
Following homogenization of each organ in 4 ml of PBS, aliquots of liver and kidney homogenates were centrifuged, filtered though 0.45-μm-pore-size syringe-tip filters, and frozen at −70°C. Subsequently, these homogenized organ supernatants were assayed by enzyme-linked immunosorbent assay (ELISA) for IL-4 and IL-10 (both from R&D Systems, Minneapolis, Minn.) and IFN-γ and TGF-β (both from BioSource International, Camarillo, Calif.). IFN-γ/IL-4 ratios were utilized to define immune polarization, and ratios significantly higher than or lower than those in uninfected mice defined type 1 or type 2 responses, respectively.
Statistical analysis.
Differences between the results from infected and control mice were compared using the Steel test for nonparametric multiple comparisons (27) or the Mann-Whitney U test for nonparametric unpaired comparisons, as appropriate. The log rank test was used to compare survival curves of mice infected with wild-type strains of C. albicans to those of mice infected with hypha-deficient mutant strains. Correlations were determined by the Spearman rank sum test. P values of ≤0.05 were considered significant.
RESULTS
The hypha-deficient mutant strain causes diminished mortality compared to wild-type C. albicans.
To establish a severity-of-infection reference point against which to compare immunologic parameters, survival curves were determined by infecting mice with various inocula of the wild-type or the hypha-deficient mutant strains of C. albicans. Mice inoculated with 5 × 105 or 105 organisms of wild-type C. albicans exhibited 100% mortality, with a median survival time of 6 or 10 days, respectively. The hypha-deficient mutant strain caused significantly less-severe infection, with median survival times of 9 and >42 days for inocula of 5 × 105 and 105 blastospores, respectively (P < 0.05 for both strains compared to the wild type). Mice infected with an inoculum of 104 wild-type C. albicans organisms had low but measurable mortality (13% at 42 days), whereas no mice infected with an inoculum of 103 organisms died. These results defined the inoculum threshold required for a lethal infection and confirmed that the hypha-deficient mutant strain is significantly less virulent in vivo than the wild-type strain (12).
Fungal colony counts in the kidney progressively increase during fatal infection and decrease during nonfatal infection.
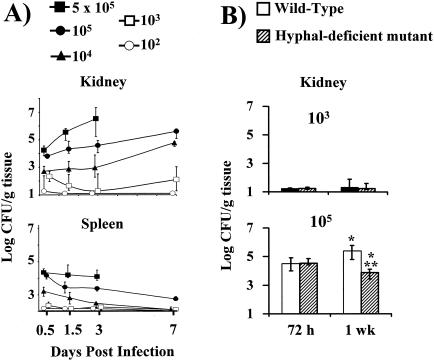
To further define the course of infection, organ fungal burden was measured in kidneys, spleens, and livers. Mice infected with the an inoculum of 104, 105, or 5 × 105 organisms of the wild-type strain had progressive increases in kidney colony counts over the first week following infection (Fig. 1A). Mice infected with an inoculum of 102 or 103 organisms of the nonfatal wild-type strain had nearly undetectable colony counts in their kidneys at all time points beyond 12 h. In contrast, splenic colony counts progressively declined in all mice except those infected with the highest-level, rapidly fatal wild-type inoculum (5 × 105 organisms). Similarly, liver colony counts were cleared within 72 h at all inoculum levels except that of the highest inoculum (5 × 105 organisms) (data not shown).
FIG. 1.
Tissue fungal burden of kidneys and spleens from mice infected via the tail vein with 1 × 102, 1 × 103, 1 × 104, 1 × 105, or 5 × 105 blastospores of wild-type C. albicans (A) or with 1 × 103 or 1 × 105 blastospores of wild-type or hypha-deficient mutant C. albicans (B). The lower boundary of the y axis on each graph represents the limit of detectability of quantitative colony counting for each organ (1 CFU/g of kidney versus 2 CFU/g of spleen). Data are displayed as medians ± interquartile ranges from two to five experiments with 6 to 15 mice per group. *, P ≤ 0.02 by the Mann-Whitney U test compared to mice infected with the same strain at 72 h; **, P = 0.0005 by the Mann-Whitney U test at 1 week for mutant versus wild-type strains.
To confirm its utility as a marker for disease severity, the kidney fungal burden in mice infected with 103 or 105 organisms of wild-type C. albicans was compared to that in mice infected with hypha-deficient mutant C. albicans. In mice infected with a nonlethal inoculum of 103 blastospores of either strain (Fig. 1B), kidney colony counts were similar at both 72 h and 1 week. At an inoculum of 105 blastospores, the kidney fungal burdens were also similar in mice infected for 72 h with either strain. By 1 week postinfection, however, the kidney fungal burden was nearly 100-fold lower in mice infected with the mutant strain than that in mice infected with the wild-type strain. These results were concordant with the diminished lethality of the mutant versus the wild-type strain at this inoculum level.
Wild-type C. albicans induces type 2 splenic lymphocyte responses at all inoculum levels.
To determine the nature of the immune response in infected mice, the frequencies of Th1 (CD4+ IFNγ+ IL-4−) and Th2 (CD4+ IFNγ− IL-4+) cells in the spleen at selected time points and in response to specific inocula were determined by intracellular cytokine staining and flow cytometry. The Th1/Th2 cell ratio was used as an indicator of the relative polarization of the splenic immune response in each mouse, with the ratio seen in uninfected mice representing an unpolarized response (4, 13, 16). No significant differences were found when the splenic Th1/Th2 ratios of uninfected mice were compared to those of mice infected with any inoculum of wild-type C. albicans at 12, 36, or 72 h (Table 1). However, mice infected for 1 week with an inoculum of 102, 104, or 105 wild-type C. albicans organisms mounted significant type 2 splenic lymphocyte responses (low Th1/Th2 ratios) compared to uninfected mice (Table 1). The splenic Th1/Th2 ratio from mice infected with an inoculum of 103 organisms trended towards a type 2 response but was not significantly different from that of the control (P = 0.07). The immune responses of mice infected with an inoculum of 5 × 105 wild-type organisms could not be profiled at 1 week, because there was 100% mortality at this time point.
TABLE 1.
Splenic Th1/Th2 ratioslegend
| Inoculum (no. of organisms) | Median (25th quartile, 75th quartile) Th1/Th2 frequency ratio at indicated time postinfection
|
|||
|---|---|---|---|---|
| 12 h | 36 h | 72 h | 1 wk | |
| None | 11 (16, 8) | |||
| 102 | 10 (13, 8) | 8 (10, 6) | 8 (9, 6) | 6 (7, 5)b |
| 103 | 7 (8, 6) | 7 (9, 5) | 6 (8, 5)c | 7 (8, 6)c |
| 104 | 7 (11, 6) | 9 (32, 5) | 13 (19, 8) | 6 (7, 5)b |
| 105 | 9 (14, 8) | 11 (20, 9) | 11 (15, 10) | 5 (6, 4)b |
| 5 × 105 | 9 (20, 5) | 10 (11, 6) | 10 (12, 7) | |
aRatios of frequencies of Th1 to Th2 lymphocytes harvested from the spleens of male BALB/c mice inoculated with 1 × 102, 1 × 103, 1 × 104, 1 × 105, or 5 × 105 wild-type C. albicans organisms or with PBS (None).
P < 0.05 versus values for uninfected mice.
P = 0.07 versus values for uninfected mice.
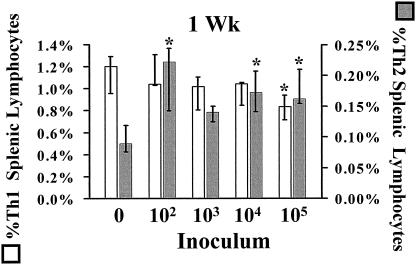
Analysis of individual splenic Th1 and Th2 frequencies was performed to determine whether polarized responses (altered Th1/Th2 ratios) were due to changes in Th1 or Th2 frequencies (individually or in combination). At 1 week postinfection, the type 2 responses (low Th1/Th2 ratio) seen in the splenic lymphocytes of mice infected with an inoculum of 102 or 104 wild-type C. albicans organisms were principally due to significant rises in Th2 cell levels rather than decreases in Th1 cell levels compared to those seen in uninfected mice (Fig. 2). In contrast, at the fatal inoculum of 105 organisms, the type 2 splenic lymphocyte response was a result of both a rise in Th2 and a fall in Th1 frequency.
FIG. 2.
Frequencies of Th1 and Th2 cells isolated from the spleens of mice 1 week after inoculation with 102, 103, 104, or 105 wild-type C. albicans organisms or with PBS (0). Data are displayed as medians ± interquartile ranges from three experiments with nine mice per group. *, P < 0.05 by the Steel test versus values for uninfected (0) mice.
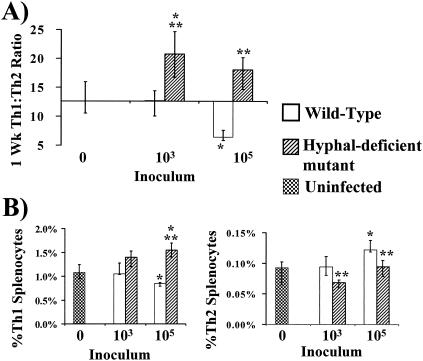
Hypha-deficient mutant C. albicans induces type 1 and unpolarized splenic lymphocyte responses at low and high inoculum levels, respectively.
Strikingly different immune responses were detected in mice infected with the hypha-deficient mutant compared to those detected in mice infected with the wild-type strain. As before, no significant polarization in splenic lymphocytes was noted at 72 h compared to that seen with uninfected mice (data not shown). However, after 1 week of infection an inoculum of 103 organisms of the hypha-deficient C. albicans mutant strain induced a significant type 1 splenic immune response (high Th1/Th2 ratio) compared to those of both uninfected mice and mice infected with wild-type C. albicans (Fig. 3A). At the higher inoculum of 105 organisms, the hypha-deficient mutant induced an unpolarized response which was not significantly different from that of the control but was significantly different from the type 2 response induced by the same inoculum of wild-type C. albicans organisms (Fig. 3A). Analysis of individual Th1 and Th2 frequencies was again performed to discern whether polarized responses were due to changes in Th1 or Th2 cells. After 1 week of infection, at both inoculum levels the hypha-deficient mutant strain induced higher frequencies of Th1 cells and lower frequencies of Th2 cells than did the wild-type C. albicans strain (Fig. 3B).
FIG. 3.
Th1 and Th2 splenocyte responses of mice 1 week after inoculation with 103 or 105 wild-type or hypha-deficient mutant C. albicans organisms or with PBS (Uninfected). (A) Th1/Th2 splenocyte ratios. (B) Frequencies of Th1 and Th2 splenocytes. Data are displayed as medians ± interquartile ranges from two experiments with six mice per group. *, P ≤ 0.05 versus values for uninfected mice; **, P < 0.05 for hypha-deficient mutant versus values for wild-type strains (both P values determined by the Steel test).
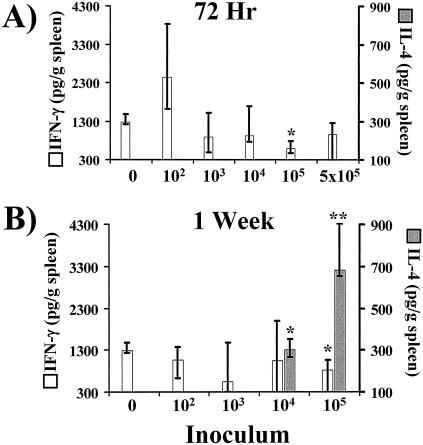
Splenic levels of IFN-γ and IL-4, but not IL-10, correspond to the frequency of intracellular cytokine detection in CD4+ lymphocytes.
Whole-spleen cytokines were measured by ELISA to determine whether splenic Th1 and Th2 frequencies accurately corresponded to the overall splenic immune polarization. Concordant with the results of splenic Th1 frequency analysis, splenic IFN-γ concentrations in mice infected with the wild-type strain were not significantly different from those in uninfected mice except at an inoculum of 105 organisms at 72 h and 1 week, when the concentrations of IFN-γ were decreased compared to those of the control (Fig. 4). The splenic IL-4 levels in mice infected at all inoculum levels were below the limit of detection at 72 h. However, after 1 week of infection there was detectable splenic IL-4 in mice infected with an inoculum of 104 or 105 organisms. The decreased IFN-γ and increased IL-4 levels collectively indicated that there was an increasingly potent type 2 response at 1 week in the spleens of mice infected with an inoculum of 104 or 105 organisms, consistent with the splenic Th1/Th2 lymphocyte ratios observed following infection with an inoculum of 104 or 105 organisms (Table 1 and Fig. 4). Because the splenic levels of IL-4 were below the limits of detection in both uninfected mice and in mice infected with an inoculum of 102 or 103 organisms, it was impossible to determine whether IL-4 was induced to levels higher than those of the control at these low inoculum levels. Therefore, for determinations of the nature of the splenic immune response in animals infected with an inoculum of 102 or 103 organisms, Th1/Th2 ratios were superior to IFN-γ/IL-4 ratios.
FIG. 4.
Whole-organ cytokine analysis by ELISA of spleens from mice inoculated with 1 × 102, 1 × 103, 1 × 104, 1 × 105, or 5 × 105 wild-type C. albicans organisms or with PBS (0). Whole-spleen IFN-γ and IL-4 concentrations at 72 h (A) and 1 week (B) after inoculation are indicated. The lowest points on the y axes represent the lower limits of detection of the ELISAs. Data are displayed as medians ± interquartile ranges from two experiments with six mice per group. *, P < 0.05 versus the uninfected mice; **, P < 0.05 versus values for uninfected mice and mice inoculated with 102, 103, or 104 organisms (both P values determined by the Steel test).
To discern the accuracy of measurements of intracellular CD4+ IL-10+ splenic lymphocyte frequencies as a reflection of splenic IL-10 production, the total splenic concentrations of IL-10 were also determined. Surprisingly, whereas CD4+ IL-10+ splenic lymphocyte frequencies did not change from those of the control at any time point or inoculum level during wild-type infection (data not shown), splenic IL-10 levels consistently decreased over time. In the spleens of uninfected control animals, the median (± 25th and 75th quartile) IL-10 level was 4 (± 2 and 2) ng/g of spleen, whereas at all wild-type inoculum levels for infected animals, IL-10 levels fell below the limit of detectability (<0.5 ng/g of spleen) at 12, 36, and 72 h after infection (P < 0.05 for all comparisons). Splenic IL-10 levels remained undetectable until 1 week, at which time the levels returned to near baseline in all groups. Thus, measuring CD4+ IL-10+ splenic lymphocyte frequencies did not account for the total production of IL-10 in the spleen.
Fatal inocula of wild-type C. albicans induce type 2 or IL-10-dominant responses in the kidney.
As the kidney fungal burden correlated with mortality, the kidney was chosen as the nonlymphoid organ in which to assess immune polarization to compare to that in the spleen. Given that lymphocytes comprise a much smaller fraction of the total cell population in the kidney than they do in the spleen, the kidney immune response was characterized by measuring whole-organ cytokine levels rather than intracellular cytokine frequencies.
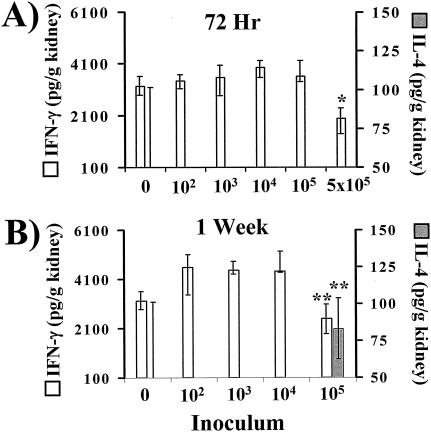
Surprisingly, the basal concentration of IFN-γ was markedly higher in the kidneys than the spleens of both uninfected mice and mice infected with wild-type C. albicans (Fig. 4 and 5). Infection with 5 × 105 wild-type organisms for 72 h significantly suppressed renal IFN-γ levels compared to those of uninfected mice (Fig. 5). As well, the renal IFN-γ concentration in mice infected for 1 week with an inoculum of 105 wild-type organisms was significantly less than in mice infected at lower inoculum levels. At no inoculum level or time point were renal IFN-γ levels significantly higher than those of the control mice, although the levels trended higher at 1 week at inocula of 102, 103, and 104 organisms.
FIG. 5.
Whole-organ IFN-γ and IL-4 analysis by ELISA of kidneys from mice inoculated with 1 × 102, 1 × 103, 1 × 104, 1 × 105, or 5 × 105 wild-type C. albicans organisms or with PBS (0). Kidneys were harvested at 72 h (A) or 1 week (B) after infection. The lowest points on the y axes represent the lower limits of detection of the ELISAs. Data are displayed as medians ± interquartile ranges from two experiments with six mice per group. *, P < 0.05 versus values for uninfected mice; **, P < 0.05 versus values for mice inoculated with 102, 103, or 104 organisms per mouse (both P values determined by the Steel test).
IL-4 levels remained below the limit of detection in the kidneys of mice infected with all wild-type inocula and at all time points except in the mice infected with 105 organisms (Fig. 5). In these mice, renal IL-4 levels increased to the detectable range after 1 week of infection. The decreased IFN-γ and increased IL-4 levels following 1 week of infection with 105 wild-type organisms indicated the presence of a type 2 response. In contrast, mice infected for 72 h with 5 × 105 organisms had a marked suppression of renal IFN-γ with no detectable IL-4, which was suggestive of either a Treg or Th3 response due to secretion of either IL-10 or TGF-β, respectively.
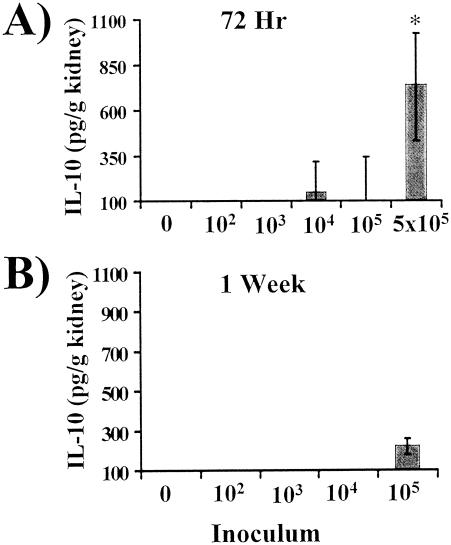
To determine whether an IL-10/Treg response or a TGF-β/Th3 response was present in the kidneys of mice infected for 72 h with 5 × 105 wild-type organisms, renal IL-10 and TGF-β concentrations were measured. Kidney IL-10 levels were undetectable in uninfected mice and only consistently increased above the limit of detectability in mice infected with a 100% fatal inoculum (Fig. 6A). In mice infected for 72 h with 5 × 105 wild-type organisms, IL-10 levels increased dramatically. As well, following 1 week of infection with an inoculum of 105 organisms, IL-10 also increased above the level of detection (Fig. 6B). The marked increase in IL-10 at 72 h at an inoculum of 5 × 105 organisms was consistent with the presence of an IL-10-dominant-Treg response in the kidney, as IFN-γ was suppressed and IL-4 was absent. Conversely, the smaller increase in IL-10 at an inoculum of 105 organisms at 1 week paralleled an increase in IL-4 and suppression of IFN-γ levels, indicating the presence of a classic type 2 response. TGF-β did not play a role in the suppression of IFN-γ, as kidney TGF-β levels were similar in both control and uninfected animals at all time points (data not shown).
FIG. 6.
Whole-organ IL-10 analysis by ELISA of kidneys from mice inoculated with 1 × 102, 1 × 103, 1 × 104, 1 × 105, or 5 × 105 wild-type C. albicans organisms or with PBS (0). The lowest points on the y axes represent the lower limits of detection of the ELISA. Data are displayed as medians ± interquartile ranges from two experiments with six mice per group. *, P < 0.05 by the Steel test versus values for uninfected mice.
Induction of type 2 immunity in the kidney is specifically associated with fatal host outcome and not just with high inocula.
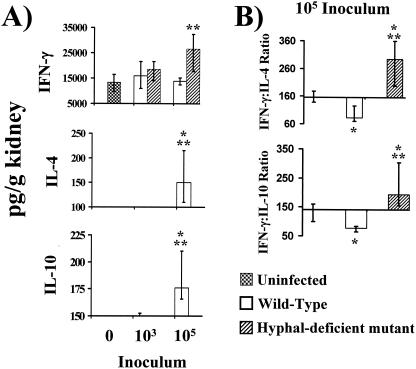
Pathogens at high inocula have been suggested to induce type 2 immune responses (32), and the levels of fatal wild-type C. albicans inocula were all higher than those of nonfatal inocula. Therefore, to eliminate the possibility that high inoculum levels induced type 2 renal immunity regardless of host outcome, mice were infected with wild-type or hypha-deficient mutant organisms at identical inocula. The renal immune responses in mice infected with an inoculum of 103 organisms of either strain were indistinguishable, with similar levels of IFN-γ seen, and neither IL-4 nor IL-10 was detected (Fig. 7A). Conversely, at an inoculum of 105 organisms, mice infected with hypha-deficient mutant C. albicans produced higher levels of IFN-γ in their kidneys than did uninfected mice or mice infected with wild-type C. albicans. IL-4 was not detectable in the kidneys at any inoculum of the hypha-deficient mutant but was detectable at markedly increased levels in mice infected with the fatal inoculum of 105 wild-type C. albicans bacteria.
FIG. 7.
(A) Whole-organ IFN-γ, IL-4, and IL-10 analysis by ELISA of kidneys from mice inoculated 1 week earlier with 103 or 105 wild-type or hypha-deficient mutant C. albicans organisms or with PBS (0). The lowest points on the y axes represent the lower limits of detection of the ELISAs. (B) Ratio of total IFN-γ to total IL-4 and IL-10. For organs in which IL-4 or IL-10 levels were undetectable, ratios were calculated using the lower limits of the IL-4 and IL-10 ELISA standard curves. Data are displayed as medians ± interquartile ranges from two experiments with six mice per group. *, P < 0.05 versus values for uninfected mice; **, P < 0.05 for values for the hypha-deficient mutant versus values for wild-type stains (both P values determined by the Steel test).
To assess the overall type 1-type 2 response to wild-type and hypha-deficient mutant strains at an inoculum level of 105 organisms, whole-kidney IFN-γ/IL-4 ratios were determined. Infection with the hypha-deficient mutant strain caused a significant type 1 response (high IFN-γ/IL-4 ratio) relative to those seen with mice infected with the wild-type strain and with uninfected mice (Fig. 7B). In contrast, the wild-type strain caused a type 2 response (low IFN-γ/IL-4 ratio) relative to that seen with the uninfected mice. Thus, at the same inoculum of 105 organisms, the mutant and wild-type strains induced disparate renal immune responses.
IL-10 was undetectable in the kidneys of mice infected with the hypha-deficient mutant at either inoculum level (Fig. 7). In contrast, wild-type C. albicans again induced a marked stimulation of IL-10 production at the fatal inoculum level of 105 organisms compared to the results seen with uninfected mice and mice infected with the hypha-deficient mutant. The IFN-γ/IL-10 ratios seen in the kidneys of mice infected for 1 week with 105 organisms paralleled the IFN-γ/IL-4 ratios, indicating the presence of a type 2 response (low IFN-γ and high IL-4 and IL-10 levels).
The nature of the immune response in the kidney, but not the spleen, correlates with survival.
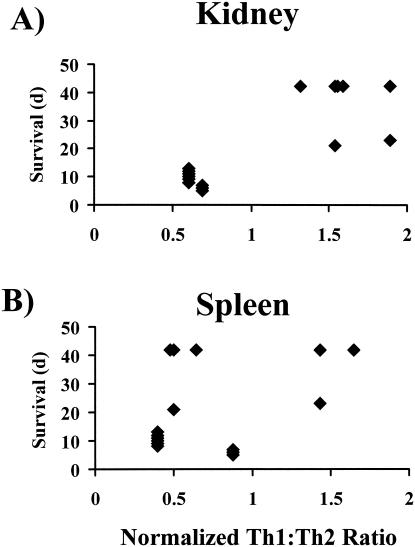
To correlate organ-specific immune responses with outcome from infection, splenic Th1/Th2 and renal IFN-γ/IL-4 ratios for infected animals were normalized to the unpolarized ratios measured in uninfected control animals, generating a quantitative assessment of the degree of organ-specific immune polarization. Normalization of the ratios for infected animals to those measured for the control animals from each experiment allowed comparison of the ratios across different experiments. Normalized ratios of >1 indicated the presence of a type 1 response in infected animals, while normalized ratios of <1 indicated the presence of a type 2 response. The normalized ratios were compared to the survival results for mice infected at each inoculum level. The presence of a type 1 response in the kidney (normalized Th1/Th2 ratio of >1) significantly correlated with prolonged survival (P < 0.001) (Fig. 8) compared to survival with the presence of a type 2 renal response. In contrast, the nature of the splenic immune response did not correlate with survival (P > 0.1) (Fig. 8).
FIG. 8.
Normalized kidney IFN-γ/IL-4 (A) and splenic Th1/Th2 (B) ratios compared to survival durations of 42 infected mice (8 per group). Normalized ratios of <1 indicate the presence of a type 2 response, and ratios of >1 indicate the presence of a type 1 response. The Th1/Th2 ratios were measured at 72 h postinfection for inocula of 5 × 105 organisms and at 1 week postinfection for other inoculum levels. (A) P < 0.001 and rho = 0.7 (determined by Spearman rank sum test). (B) P > 0.1 and rho = 0.2 (determined by Spearman rank sum test).
Renal cytokine levels do not correspond to the frequency of renal lymphocytes expressing the cytokines.
To assess the possibility that CD4+ lymphocytes recruited into the kidney produced the detected renal cytokines, mice were infected with 105 wild-type or hypha-deficient mutant C. albicans organisms and the frequencies of renal Th1 and Th2 cells were determined. Contrary to the results from whole-organ cytokine analysis, the frequency of Th1 cells in the kidneys of mice infected with wild-type C. albicans trended higher than the frequency in the kidneys of uninfected mice (median [± 25th and 75th quartile] = 15% [± 3 and 3%] versus 7% [± 1 and 2%]; P = 0.053) and was not significantly different from the frequency in mice infected with the hypha-deficient mutant (20% [± 4 and 6%]). Furthermore, the frequencies of Th2 cells in the kidneys of mice infected with both wild-type (0.7% [± 0.1 and 0.1%]) and hypha-deficient mutant (0.6% [± 0.2 and 0.4%]) C. albicans strains were little changed from the frequency in uninfected mice (0.6% [± 0.1 and 0.3%]). As a result, the renal Th1/Th2 ratio measured during wild-type infection trended higher than the ratio for uninfected mice (25 [± 8 and 7] versus 12 [± 1 and 2]; P = 0.07) and was not significantly different from the ratio measured during infection with the hypha-deficient mutant strain (41 [± 11 and 28]). As well, the frequencies of CD4+ IL-10+ lymphocytes harvested from the kidneys were not significantly different between mice infected with wild-type or hypha-deficient mutant C. albicans (4% [± 1 and 2%] versus 4% [± 1 and 1%]). Hence, the frequencies of CD4+ IFN-γ+, CD4+ IL-4+, and CD4+ IL-10+ lymphocytes in the kidney were not reflective of the overall renal levels of these cytokines.
DISCUSSION
We report a striking difference between the profiles of immune responses in the spleen and kidney during murine disseminated candidiasis. Infection with wild-type C. albicans induced type 2 immunity in the spleen at both a low inoculum that resulted in 100% survival and a high inoculum that caused 100% mortality. Hence, the splenic immune response did not vary with survival of the host. In contrast, the nature of the kidney immune response correlated with the outcome of infection. Wild-type inocula that caused 100% fatal infection with short median survival times induced either a type 2 or an IL-10-dominant, generalized suppressive response in the kidney. Conversely, inocula causing low or no mortality induced high renal IFN-γ levels and undetectable IL-4 levels indicative of type 1 responses.
It has been suggested that infection with inocula at high levels preferentially induces type 2 immune polarization (32). A possible confounding correlation of renal type 2 immune response with high fungal inoculum levels was excluded by comparing the renal immune responses during infection with inocula of the wild-type and hypha-deficient mutant strains at the same high level (105 organisms). After 1 week the mutant strain, which caused low mortality, induced a significant type 1 response (high IFN-γ/IL-4 ratio) in the kidney relative to the results seen for mice with wild-type infections and for uninfected mice. In contrast, the wild-type strain, which caused 100% fatal infection, induced a type 2 response (low IFN-γ/IL-4 ratio) in the kidney. Thus, infections with mutant and wild-type strains at identical inoculum levels of 105 organisms induced disparate renal immune responses, indicating that a type 2 immune response in the kidney, rather than a higher inoculum level, correlated with higher mortality. We believe that this is the first report indicating that it is the nature of the immune response in a nonlymphoid, parenchymal organ, and not that in the spleen, that predicts survival during systemic infection. These results suggest that examination of the host response during systemic infection should focus on the local immune responses in target organs rather than the responses in the spleen.
In similarity to the findings of Lo et al. (12), the isogenic, hypha-deficient mutant strain of C. albicans was attenuated in virulence compared to its parent wild-type strain, as evidenced by a lower renal fungal burden at 1 week and significantly reduced mortality. Homogenization of tissues containing extensive hyphae may kill the fungus by rupturing long hyphal strands, possibly leading to underestimation of the organ fungal burden of hyphal-forming wild-type C. albicans. Nevertheless, even with this possible underestimation, the organ fungal burdens of mice infected with wild-type C. albicans were significantly higher than those of mice infected with the hypha-deficient mutant. Hence, any underestimation of the wild-type organ fungal burden would further increase the detected difference, underscoring the higher virulence of the wild-type strain than that of the mutant strain.
The hypha-deficient mutant strain induced type 1 immune responses in the spleen and kidney for both uninfected mice and mice infected with wild-type C. albicans. These results reinforce the concept that type 1 immunity, which stimulates phagocytic activity, enhances host defense against C. albicans (28, 32). As well, the results suggest that one mechanism by which hypha formation acts as a virulence factor is the suppression of endogenous type 1 immunity and induction of a nonprotective type 2 host response. As thoroughly reviewed by Romani et al. (29), seminal work by her group has established that in vitro, C. albicans hyphae induce antigen-presenting cells to secrete a profile of cytokines favoring type 2 immune induction (5). Our study provides in vivo confirmation of this finding. The exact mechanism by which hyphae induce type 2 immunity is not clear but may be due to induction of type 2 cytokines (such as IL-4 and IL-10) during damage to host cells, to induction of “frustrated phagocytosis” in host leukocytes, or to recognition of hyphal constituents by a theoretical host receptor that induces a type 2 response (33).
Mice infected with inocula at the rapidly fatal level of 5 × 105 organisms exhibited a generalized regulatory (or suppressive) response indicated by the presence in kidneys of high levels of IL-10, decreased IFN-γ levels, and an absence of IL-4. Because Treg cells secreting IL-10 and TGF-β have been reported to regulate immunity during intragastric murine candidiasis (19), we explored the possibility that the rapidly fatal intravenous inoculum of 5 × 105 C. albicans organisms induced a Treg or Th3 response. However, we found no evidence that differences in recruitment of IL-10+ CD4+ lymphocytes accounted for the increase in total IL-10 levels seen in the kidneys of mice infected with wild-type C. albicans. Furthermore, TGF-β levels in the kidney and spleen were not significantly altered by C. albicans infection. Hence, we found no evidence of a Treg or Th3 response in this model. Differing routes of infection (intragastric versus intravenous) likely account for the finding of Treg cells in the study by Montagnoli et al. (19) and for the lack of detection of such cells in the model used in the present study.
Given the reports describing IL-10 production by renal epithelial cells (1, 20, 26) and the absence of leukocytes in the kidney during the first 12 h of disseminated candidiasis (2), it is possible that renal parenchymal cells, and not influxing leukocytes, produced the bulk of the IL-10 during fatal wild-type infection. This renal IL-10 might be induced by the tissue damage caused by wild-type C. albicans (7, 20). The hypha-deficient mutant strain is defective in its ability to injure host cells in vitro (12, 24), and it is therefore likely that this mutant strain causes less damage to the renal parenchyma and is a weaker stimulus for IL-10 production than the wild-type strain. For two reasons, it is unlikely that the cytokines detected in the kidney were of serum origin. First, if the majority of the cytokines detected in the whole-organ analysis were of serum origin, similar profiles would have been detected in the kidney and in the highly vascularized spleen. Furthermore, investigators have found that serum levels of inflammatory cytokines are low during lethal disseminated candidiasis (2, 33).
The lack of concordance between whole-kidney cytokine levels of IFN-γ and IL-4 and the frequencies of Th1 and Th2 cells isolated from the kidney suggested that cells other than CD4+ lymphocytes were producing the bulk of the cytokines measured in the kidneys. In support of this possibility, the frequency of Th2 cells did not increase in the kidneys of mice infected with 105 wild-type C. albicans organisms and the level of IL-4 in the kidney increased significantly. As well, extremely low absolute numbers of Th1 lymphocytes were harvested from the whole kidneys of uninfected mice (median, 20 cells/kidney; interquartile range, 16 to 25 cells/kidney). These few lymphocytes were unlikely to be the source of the high basal level of IFN-γ present in these kidneys. Finally, the frequencies of CD4+ IL-10+ lymphocytes were not significantly different in the kidneys of mice infected with wild-type versus hypha-deficient mutant C. albicans. These results are in contrast to the marked increase in IL-10 levels seen in whole-kidney cytokine analysis of mice infected with the wild-type strain but not with the mutant strain. In aggregate, these data support the notion that cells other than CD4+ lymphocytes synthesize the majority of the IFN-γ, IL-4, and IL-10 in the kidney.
As both the spleen and kidney are infected in this model of murine disseminated candidiasis, it is not clear why such disparate immune responses are mounted in these two organs. The possibility that Th1 cells are selectively depleted from the spleen by recruitment to the site of infection in the kidney is not supported by the increase in Th1 cells seen in both the spleens and kidneys of mice infected with 105 organisms of the hypha-deficient mutant strain. Alternatively, it may be that renal microenvironments are more prone than splenic microenvironments to the induction of type 1 responses. The presence of a level of IFN-γ in the kidneys of both uninfected and infected mice higher than that detected in the spleens supports this notion. Regardless, clearance of C. albicans from the kidney correlated with the presence of a type 1 or unpolarized local immune response. In contrast, clearance of the organism from the spleen occurred even in the presence of a type 2 response, likely due to the extraordinary density of phagocytes in the spleen. However, in the presence of an IL-10-dominant, generalized suppressive response, clearance of the organism was inhibited despite this high density of phagocytes.
In conclusion, an isogenic, hypha-deficient mutant strain of C. albicans is significantly less virulent than its wild-type parent strain. During in vivo infection, the mutant strain induces type 1 immunity in the spleen and kidney relative to the wild-type strain, suggesting that hypha formation contributes to C. albicans virulence by inducing nonprotective type 2 immunity that results in an ineffectual host response to the organism. The wild-type organism induced type 2 immunity in the spleen irrespective of host survival, indicating that the type of immune polarization in the spleen did not correlate with host survival. Conversely, the nature of immune polarization in the kidney did correlate with host survival, with IFN-γ-dominant, type 1 responses seen during infection causing no or low mortality and type 2 or IL-10-dominant responses seen during 100% fatal infection. Thus, in this model of systemic infection, the nature of immune polarization in a key, parenchymal target organ correlated with survival, while the nature of the immune polarization in the spleen did not.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases grants R29 AI040636, R01 DEI3874, R01 AI19990, and P01 AI37194. S. G. Filler and A. S. Ibrahim were also supported by Burroughs Wellcome Fund New Investigator Awards in Molecular Pathogenic Mycology, and J. E. Edwards, Jr., was supported by an unrestricted Infectious Diseases Research Award from Bristol-Myers Squibb. The flow cytometry facility is supported by grant RR-13004 from the National Institutes of Health.
We thank Angela Sanchez and Lucio Loza for their assistance with the animal studies and Michael Yeaman and Eric Brass for their critical review of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Brauner, A., M. Soderhall, S. H. Jacobson, J. Lundahl, U. Andersson, and J. Andersson. 2001. Escherichia coli-induced expression of IL-1 alpha, IL-1 beta, IL-6 and IL-8 in normal human renal tubular epithelial cells. Clin. Exp. Immunol. 124:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannom, R. M., S. W. French, D. Johnston, J. E. J. Edwards, and S. G. Filler. 2002. Candida albicans stimulates local expression of leukocyte adhesion molecules and cytokines in vivo. J. Infect. Dis. 186: 389-396. [DOI] [PubMed] [Google Scholar]
- 3.Chiani, P., C. Bromuro, and A. Torosantucci. 2000. Defective induction of interleukin-12 in human monocytes by germ-tube forms of Candida albicans. Infect. Immun. 68:5628-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman, R. L., K. Varkila, P. Scott, and R. Chatelain. 1991. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol. Rev. 123:189-207. [DOI] [PubMed] [Google Scholar]
- 5.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidel, P. L., Jr. 2002. Immunity to Candida. Oral Dis. 8(Suppl. 2):69-75. [DOI] [PubMed] [Google Scholar]
- 7.Goes, N., J. Urmson, V. Ramassar, and P. F. Halloran. 1995. Ischemic acute tubular necrosis induces an extensive local cytokine response. Evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-10. Transplantation 59:565-572. [PubMed] [Google Scholar]
- 8.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J. E. de Vries, and M. G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389:737-742. [DOI] [PubMed] [Google Scholar]
- 9.Groux, H., and F. Powrie. 1999. Regulatory T cells and inflammatory bowel disease. Immunol. Today 20:442-445. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi, M., H. Kobayashi, D. N. Herndon, R. B. Pollard, and F. Suzuki. 1998. Burn-associated Candida albicans infection caused by CD30+ type 2 T cells. J. Leukoc. Biol. 63:723-731. [DOI] [PubMed] [Google Scholar]
- 11.Levings, M. K., and M. G. Roncarolo. 2000. T-regulatory 1 cells: a novel subset of CD4 T cells with immunoregulatory properties. J. Allergy Clin. Immunol. 106:S109-S112. [DOI] [PubMed] [Google Scholar]
- 12.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939-949. [DOI] [PubMed] [Google Scholar]
- 13.Maggi, E., M. Mazzetti, A. Ravina, F. Annunziato, M. de Carli, M. P. Piccinni, R. Manetti, M. Carbonari, A. M. Pesce, G. del Prete, et al. 1994. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science 265:244-248. [DOI] [PubMed] [Google Scholar]
- 14.Martino, P., C. Girmenia, A. Micozzi, R. Raccah, G. Gentile, M. Venditti, and F. Mandelli. 1993. Fungemia in patients with leukemia. Am. J. Med. Sci. 306:225-232. [DOI] [PubMed] [Google Scholar]
- 15.Marzo, A. L., V. Vezys, K. Williams, D. F. Tough, and L. Lefrancois. 2002. Tissue-level regulation of Th1 and Th2 primary and memory CD4 T cells in response to Listeria infection. J. Immunol. 168:4504-4510. [DOI] [PubMed] [Google Scholar]
- 16.McDyer, J. F., M. N. Hackley, T. E. Walsh, J. L. Cook, and R. A. Seder. 1997. Patients with multidrug-resistant tuberculosis with low CD4+ T cell counts have impaired Th1 responses. J. Immunol. 158:492-500. [PubMed] [Google Scholar]
- 17.Mencacci, A., R. Spaccapelo, G. Del Sero, K. H. Enssle, A. Cassone, F. Bistoni, and L. Romani. 1996. CD4+ T-helper-cell responses in mice with low-level Candida albicans infection. Infect. Immun. 64:4907-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, L. G., R. A. Hajjeh, and J. E. Edwards, Jr. 2001. Estimating the cost of nosocomial candidemia in the United States. Clin. Infect. Dis. 32:1110. [DOI] [PubMed] [Google Scholar]
- 19.Montagnoli, C., A. Bacci, S. Bozza, R. Gaziano, P. Mosci, A. H. Sharpe, and L. Romani. 2002. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 169:6298-6308. [DOI] [PubMed] [Google Scholar]
- 20.Niemir, Z. I., M. Ondracek, G. Dworacki, H. Stein, R. Waldherr, E. Ritz, and H. F. Otto. 1998. In situ upregulation of IL-10 reflects the activity of human glomerulonephritides. Am. J. Kidney Dis. 32:80-92. [DOI] [PubMed] [Google Scholar]
- 21.Nucci, M., W. Pulcheri, N. Spector, A. P. Bueno, P. C. Bacha, M. J. Caiuby, A. Derossi, R. Costa, J. C. Morais, and H. P. de Oliveira. 1995. Fungal infections in neutropenic patients. A 8-year prospective study. Rev. Inst. Med. Trop. Sao Paulo 37:397-406. [DOI] [PubMed] [Google Scholar]
- 22.Parish, C. R. 1971. Immune response to chemically modified flagellin. I. Induction of antibody tolerance to flagellin by acetoacetylated derivatives of the protein. J. Exp. Med. 134:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parish, C. R. 1971. Immune response to chemically modified flagellin. II. Evidence for a fundamental relationship between humoral and cell-mediated immunity. J. Exp. Med. 134:21-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan, Q. T., P. H. Belanger, and S. G. Filler. 2000. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 68:3485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prussin, C., and D. D. Metcalfe. 1995. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J. Immunol. Methods 188:117-128. [DOI] [PubMed] [Google Scholar]
- 26.Rangan, G. K., Y. Wang, Y. C. Tay, and D. C. Harris. 1999. Inhibition of NFκB activation with antioxidants is correlated with reduced cytokine transcription in PTC. Am. J. Physiol. 277:F779-F789. [DOI] [PubMed] [Google Scholar]
- 27.Rhyne, A. L., and R. G. Steel. 1967. A multiple comparisons sign test: all pairs of treatments. Biometrics 23:539-549. [PubMed] [Google Scholar]
- 28.Romani, L. 2000. Innate and adaptive immunity in Candida albicans infections and saprophytism. J. Leukoc. Biol. 68:175-179. [PubMed] [Google Scholar]
- 29.Romani, L., F. Bistoni, and P. Puccetti. 2002. Fungi, dendritic cells and receptors: a host perspective of fungal virulence. Trends Microbiol. 10:508-514. [DOI] [PubMed] [Google Scholar]
- 30.Romani, L., A. Mencacci, L. Tonnetti, R. Spaccapelo, E. Cenci, P. Puccetti, S. F. Wolf, and F. Bistoni. 1994. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J. Immunol. 153:5167-5175. [PubMed] [Google Scholar]
- 31.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91:72S-75S. [DOI] [PubMed] [Google Scholar]
- 32.Spellberg, B., and J. E. Edwards, Jr. 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 33.Spellberg, B. J., and J. E. Edwards, Jr. 2002. The pathophysiology and treatment of Candida sepsis. Curr. Infect. Dis. Rep. 4:387-399. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchimori, N., L. L. Sharkey, W. A. Fonzi, S. W. French, J. E. Edwards, Jr., and S. G. Filler. 2000. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect. Immun. 68:1997-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, L., E. F. Lizzio, E. Gubina, T. Chen, H. Mostowski, and S. Kozlowski. 2002. Organ-specific cytokine polarization induced by adoptive transfer of transgenic T cells. J. Immunol. 169:5514-5521. [DOI] [PubMed] [Google Scholar]