Abstract
Patients with Pneumocystis pneumonia often develop respiratory failure after entry into medical care, and one mechanism for this deterioration may be increased alveolar epithelial cell injury. In vitro, we previously demonstrated that Pneumocystis is not cytotoxic for alveolar epithelial cells. In vivo, however, infection with Pneumocystis could increase susceptibility to injury by stressors that, alone, would be sublethal. We examined transient exposure to hyperoxia as a prototypical stress that does cause mortality in normal mice. Mice were depleted of CD4+ T cells and inoculated intratracheally with Pneumocystis. Control mice were depleted of CD4+ T cells but did not receive Pneumocystis. After 4 weeks, mice were maintained in normoxia, were exposed to hyperoxia for 4 days, or were exposed to hyperoxia for 4 days followed by return to normoxia. CD4-depleted mice with Pneumocystis pneumonia demonstrated significant mortality after transient exposure to hyperoxia, while all uninfected control mice survived this stress. We determined that organism burdens were not different. However, infected mice exposed to hyperoxia and then returned to normoxia demonstrated significant increases in inflammatory cell accumulation and lung cell apoptosis. We conclude that Pneumocystis pneumonia leads to increased mortality following a normally sublethal hyperoxic insult, accompanied by alveolar epithelial cell injury and increased pulmonary inflammation.
Pneumocystis pneumonia remains an important source of morbidity and mortality in individuals with AIDS or defects in host defense due to other causes, but the pathogenesis of this opportunistic pneumonia remains poorly understood (6). The frequency of Pneumocystis pneumonia in human immunodeficiency virus (HIV)-infected individuals has decreased significantly with the use of highly active antiretroviral therapy (19). However, many individuals who present with Pneumocystis pneumonia are unaware of their HIV infections, have no access to antiretroviral therapy, or cannot tolerate antiretroviral therapy (24). The mortality rate in HIV-infected individuals with Pneumocystis pneumonia has decreased to approximately 15%, but the mortality rate due to Pneumocystis pneumonia in other immunosuppressed individuals remains approximately 40% (31, 33).
It has long been appreciated that patients with Pneumocystis pneumonia may deteriorate clinically in the first several days after diagnosis and institution of therapy, even when appropriate antimicrobial therapy is selected (20). Individuals frequently are heavily infected yet may experience modest physiologic compromise initially. A significant fraction of patients with Pneumocystis pneumonia develop late respiratory failure after initiation of therapy. The goal of adjuvant therapy with corticosteroids is to prevent this deterioration. However, the mechanisms by which apparently stable infection progresses to respiratory failure have not been defined.
Similarly, the mechanisms by which Pneumocystis induces injury of alveolar epithelial cells require further investigation. Prior studies from our laboratories have demonstrated that Pneumocystis organisms are not directly toxic to alveolar epithelial cells in primary culture (5), although Pneumocystis trophic forms adhere tightly to alveolar epithelial cells. Instead, Pneumocystis organisms and alveolar epithelial cells coexist in short-term culture, with continued metabolic activity and viability of organisms and host cells. In vivo, however, it is apparent that both clinical and experimental Pneumocystis pneumonia result in acute lung injury (38), which would increase the severity of pneumonia.
Pneumocystis induces lung inflammation in experimental models (7), and an exuberant inflammatory response may also result in deterioration. For example, reconstitution of scid mice with splenocytes can result in clearance of organisms but can also produce a fatal hyperinflammatory response (28). Therefore, it is also possible that sublethal inflammatory stimuli, such as hyperoxia, could increase the severity of Pneumocystis pneumonia by exacerbating inflammation.
To better understand the roles of inflammatory responses and alveolar injury in the pathogenesis of Pneumocystis pneumonia, we investigated these mechanisms in immunocompromised mice. We hypothesized that infection with Pneumocystis would increase host susceptibility following additional insults that, alone, would be sublethal. Short-term exposure to hyperoxia is a clinically relevant stress that induces reversible lung injury without mortality in normal mice. However, transient exposure to hyperoxia led to significant mortality during Pneumocystis infection. To investigate the mechanisms by which the combination of two usually sublethal insults (Pneumocystis infection and sublethal hyperoxia) results in death, we examined the roles of increased organism burden, inflammatory responses, phagocytosis by alveolar macrophages, and induction of apoptosis.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free mice were obtained from Jackson Laboratory. Because Pneumocystis cannot be propagated in long-term culture, organisms for inoculation are passaged in the lungs of athymic (BALB/c background) or scid (C57BL/6 background) mice (7). These donor mice were used to inoculate BALB/c and C57BL/6 mice, respectively. Mice were housed in filter-topped cages under laminar-flow hoods and were provided with autoclaved food, water, and bedding. Routine health monitoring of cohoused sentinel mice did not demonstrate infections with pathogens other than Pneumocystis. All animal experimentation was approved by the Animal Care Subcommittee, Ann Arbor VA Medical Center.
Murine model of Pneumocystis pneumonia.
To deplete mice of CD4+ T cells, BALB/c and C57BL/6 mice received weekly intraperitoneal injections of monoclonal antibody GK1.5 (0.3 mg), continued for the duration of the experiments (7, 32). As in our previous work, flow cytometry demonstrated profound depletion of CD4+ T cells in lungs, blood, and spleen during this treatment.
CD4-depleted mice were inoculated intratracheally with murine Pneumocystis from the lungs of infected nude (BALB/c background) or scid (C57BL/6 background) mice as previously described (7). Infected donor mice were injected with a lethal dose of pentobarbital and were exsanguinated by aortic transection. The lungs were removed aseptically, placed in sterile phosphate-buffered saline (PBS), and frozen at −20°C for 2 h. Frozen lungs were then homogenized mechanically, filtered, and centrifuged at 500 × g for 10 min at 5°C. The pellet was resuspended in PBS, and smears were stained with modified Giemsa stain to count organisms.
Freshly prepared inoculum was always used for intratracheal inoculation to ensure the viability of organisms. Inoculation of mice was performed during pentobarbital anesthesia (7). After surgical exposure of the trachea, a blunted needle was passed through the mouth into the midtrachea under direct vision. A polyethylene catheter was passed through this needle until the catheter tip was just distal to the needle, and 0.1 ml (105 cysts) of the inoculum was injected. The inoculum was followed by injection of 0.4 ml of air to ensure adequate dispersion of the inoculum and clearance of the central airways. The neck incision was sutured, and the mice were placed prone for recovery.
Using this protocol, progressive infection with extensive inflammation and a heavy burden of organisms occurs by 4 weeks after inoculation. In contrast, we have not detected infections in CD4-depleted mice that were not inoculated with Pneumocystis. Thus, these CD4-depleted, uninfected mice served as controls.
Exposure to hyperoxia.
Mice were exposed to hyperoxia in shoebox-style cages within a 30- by 20- by 20-in. (width by depth by height) Plexiglas chamber (Reming Bioinstruments). This chamber was maintained at oxygen concentrations of >95% using a Proox Model 110 controller (Reming Bioinstruments). During the period of hyperoxia, mice remained unrestrained and had free access to water and food.
Grading of intensity of Pneumocystis pneumonia.
To determine the intensity of Pneumocystis infection, we performed histologic grading of lung sections using a method previously described and validated (29). Lungs were inflated with formalin, fixed overnight, paraffin embedded, and sectioned. Scoring for intensity of infection was performed on sections stained with the Gomori methenamine silver stain. This semiquantitative scale ranges from 0 (no Pneumocystis organisms detected) to 4+ (Pneumocystis cysts and foamy exudate present throughout alveoli in most regions).
Measurement of Pneumocystis infection by real-time PCR.
To confirm and extend the histologic grading, we performed real-time PCR on lung homogenates from uninfected mice, using a modification of the method originated by Zheng et al. (39). Total RNA was isolated from snap-frozen lungs by using Trizol (Gibco BRL), and individual reverse transcription reactions were performed. Real-time PCR was then performed using primer sets designed to amplify a portion of Pneumocystis rRNA and a fluorescently labeled reporter probe (39). Each set of reactions was performed using a cloned Pneumocystis rRNA standard and cDNA prepared from a mouse lung known to be heavily infected with Pneumocystis. The threshold cycle values were averaged from amplifications performed in triplicate on individual mouse lungs, and results were expressed as rRNA copy number based on a standard curve generated with the cloned rRNA.
Measurement of cell numbers and differentials in BAL fluid.
To quantify the accumulation of inflammatory cells in the lungs of mice, we measured cell numbers in bronchoalveolar lavage (BAL) fluid as previously described (4). After centrifugation of the BAL fluid at 500 × g for 10 min at 4°C, the pellets were washed twice in cold PBS. The cells were then suspended in PBS and counted in a hemocytometer. Lavaged cells were prepared for differential counting by gravity filtration (11). Briefly, 105 cells per specimen were washed onto nitrocellulose filters with 5-μm pores (Millipore). The filters were then mounted on glass slides, fixed overnight in 10% buffered formalin solution, and stained with Carazzi's hematoxylin-eosin (30). Blinded differential counts were performed on at least 300 cells per slide.
Alveolar macrophage phagocytosis assay.
To purify Pneumocystis organisms for phagocytosis assays, excised lungs from infected mice were minced in NaCaHEPES containing 0.5% glutathione (5, 18). Minced lungs were homogenized with a Stomacher lab blender (Tekmar) for 5 min at room temperature. The homogenate was passed through a sterile 60-mesh sieve and centrifuged at 925 × g for 10 min. The resultant pellet was lysed with 0.85% ammonium chloride and then diluted threefold. Sequential high (925 × g) and low (60 × g) centrifugations were used to isolate organisms from lung cells. The final pellet was then passed through a series of polycarbonate membranes (Poretics). The organism number in the final preparation was determined by counting trophic forms on slides stained with Diff-Quik (Dade). Enumerated organisms were suspended in 1% bovine serum albumin in PBS and then labeled with 2′,7′-bis-(2-carboxyethyl)-5(and-6)-carboxyfluorescein acetoxymethyl ester in dimethyl sulfoxide (Molecular Probes) (5).
Alveolar macrophages were obtained from normal C57BL/6 mice maintained in normoxia or from normal C57BL/6 mice exposed to >95% hyperoxia for 4 days. Mice underwent BAL, and alveolar macrophages were purified by adherence for 90 min. After the macrophages were washed, purified and labeled Pneumocystis organisms were added to the macrophages for 1 h. After the wells were washed vigorously with PBS, the preparations were fixed in formalin. Extracellular fluorescence was quenched with trypan, and internalized Pneumocystis were counted microscopically. Data are expressed as percentages of macrophages containing Pneumocystis, from experiments performed in triplicate.
Effect of Pneumocystis pneumonia on apoptosis following sublethal hyperoxia.
Paraffin-fixed lung sections were stained immunohistochemically for caspase-3 by using purified polyclonal rabbit cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology), biotin-conjugated goat anti-rabbit immunoglobulin G (Jackson ImmunoResearch), and detection with the proliferating cell nuclear antigen staining kit (Zymed). Semiquantitative scoring was then performed, by two observers blinded to the treatment conditions, on a scale of 1+ (<10% total membrane staining) to 5+ (76 to 100% total membrane staining). For each individual mouse, scores made by the two observers were averaged.
The extent of apoptosis was confirmed by analysis of lung homogenates with the Cell Death Detection ELISA Plus apparatus (Roche), which measures cytoplasmic histone-associated DNA fragments. This test uses an antibody directed against cleaved caspase-3 and therefore recognizes only the large fragment of activated caspase-3. Protein concentrations were measured in lung homogenates (Pierce) and adjusted to 200 μg/ml. Samples were then analyzed as specified by the manufacturer, and data were expressed as relative enzyme-linked immunosorbent assay (ELISA) absorbances.
Statistical analysis.
Statistics were performed using Prism for Macintosh Software (GraphPad, Inc.). Survival data were compared using Kaplan-Meier analysis, continuous data were compared using t-tests or ANOVA with Newman-Keuls follow-up testing, and ordinal data were compared using the Kruskal-Wallis test with Dunn follow-up testing. Statistical significance was accepted for P values <0.05.
RESULTS
Development of the sublethal-hyperoxia model.
To determine whether continuous hyperoxia altered mortality during Pneumocystis pneumonia, we first compared the survival of BALB/c mice during exposure to >95% hyperoxia. CD4-depleted BALB/c mice were inoculated with Pneumocystis and studied 4 weeks after inoculation. The 4-week interval was selected because CD4-depleted mice develop intense Pneumocystis infections but would still survive for several weeks under the usual circumstances. BALB/c mice that were CD4 depleted for equal lengths of time but were not infected with Pneumocystis served as the control group. CD4-depleted mice infected with Pneumocystis exhibited accelerated mortality, with deaths beginning after 72 h of hyperoxia (data not shown). However, CD4-depleted, uninfected mice also exhibited early mortality, which started after approximately 92 h of continuous hyperoxia. Since BALB/c mice are relatively intolerant of hyperoxia, it was apparent at the conclusion of these experiments that both groups of mice had unacceptably high mortality. Because a study of mechanistic events would be impossible using this model, we developed a sublethal model of hyperoxia in C57BL/6 mice. We found no mortality when mice were exposed to 4 days of >95% oxygen followed by return to normoxia, but longer durations of hyperoxia caused mortality. Therefore, we adopted a model of sublethal hyperoxia in which CD4-depleted C57BL/6 mice with Pneumocystis pneumonia or CD4-depleted, uninfected C57BL/6 mice were placed into >95% oxygen for 4 days and then returned to room air.
Mortality from Pneumocystis pneumonia during sublethal hyperoxia.
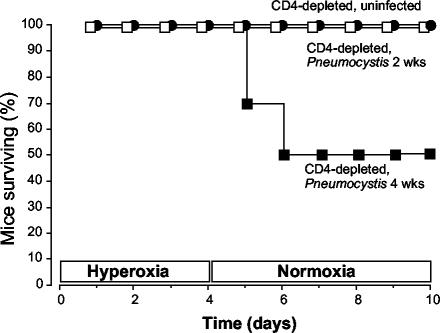
For these experiments, we exposed groups of mice to 4 days of continuous >95% hyperoxia followed by a return to room air. Mice were randomly selected from each group before hyperoxia exposure, and the median infection scores were 0 (for mice not inoculated), 2 (for mice 2 weeks after inoculation), and 4 (for mice 4 weeks after inoculation). All uninfected, CD4-depleted mice survived for the duration of the experiments (Fig. 1). Similarly, CD4-depleted mice with early Pneumocystis infection (exposed to hyperoxia only 2 weeks after inoculation) survived for the duration of the experiments. In contrast, CD4-depleted mice with advanced Pneumocystis infection (exposed to hyperoxia 4 weeks after inoculation) demonstrated mortality beginning on day 5, which was the first day of return to normoxia. Therefore, sublethal hyperoxia followed by return to room air results in increased mortality during advanced Pneumocystis infection.
FIG. 1.
Survival after sublethal hyperoxia. CD4-depleted, uninfected C57BL/6 mice were compared with CD4-depleted C57BL/6 mice at 2 and 4 weeks after Pneumocystis inoculation. All mice were exposed to 4 days of >95% hyperoxia and then returned to normoxia. Data are presented as percentages of mice surviving from initial groups of ≥12 mice. P < 0.0012 by Kaplan-Meier analysis.
Intensity of Pneumocystis infection burden during sublethal hyperoxia.
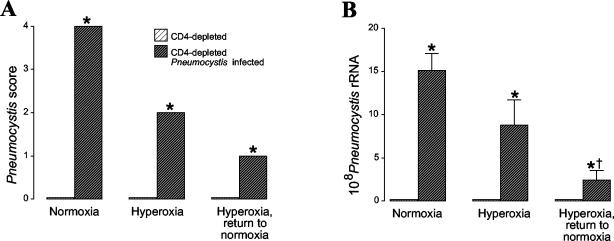
We questioned whether the increased mortality in Pneumocystis-infected mice exposed to sublethal hyperoxia could be caused by an increased organism burden, because only mice with advanced infection succumbed after exposure to hyperoxia. In these experiments, we compared mice maintained in normoxia and then killed; mice exposed to 95% hyperoxia for 4 days and then killed; and mice exposed to 95% hyperoxia for 4 days, returned to room air for 1 day, and then killed. The organism burden was first evaluated by scoring of histologic sections. CD4-depleted mice that were not inoculated with Pneumocystis demonstrated absence of organisms in their lungs at any time point (Fig. 2A, lighter bars). CD4-depleted mice with Pneumocystis infection maintained in normoxia demonstrated a uniformly high intensity of infection. CD4-depleted mice with Pneumocystis infection exposed to hyperoxia for 4 days demonstrated decreased intensity of infection compared with mice maintained in normoxia. Unexpectedly, CD4-depleted mice with Pneumocystis infection exposed to hyperoxia and then returned to normoxia demonstrated decreased intensity of infection compared with the other infected groups.
FIG. 2.
Intensity of Pneumocystis infection after sublethal hyperoxia. Mice were either maintained in normoxia, exposed to 4 days of >95% hyperoxia, or exposed to 4 days of >95% hyperoxia followed by 1 day of return to normoxia. (A) Histologic grading of stained lung sections was used to score the intensity of infection. Data represent medians from five or more mice per group. *, P < 0.01 for CD4-depleted Pneumocystis-infected mice compared with CD4-depleted uninfected mice by the Kruskal-Wallis test. (B) Real-time PCR measurement of Pneumocystis infection. PCR was performed on lung homogenates, and data were expressed as rRNA copy number, obtained from a standard curve of a cloned rRNA product. Data represent medians from five or more mice per group performed in triplicate. *, P < 0.01 for CD4-depleted Pneumocystis-infected mice compared with CD4-depleted uninfected mice; †, P < 0.01 for CD4-depleted Pneumocystis-infected mice exposed hyperoxia with return to normoxia compared with CD4-depleted Pneumocystis infected mice in the other two groups by analysis of variance.
To confirm the histologic grading of infection, we performed real-time PCR to quantitate Pneumocystis rRNA. The numbers of Pneumocystis rRNA copies per lung were quantitated in comparison to a standard curve generated with Pneumocystis-specific rRNA. The quantitative assay confirmed that the organism burden was reduced in mice exposed to hyperoxia compared with mice maintained in normoxia (Fig. 2B). Taken together, these data demonstrate that increased organism burden is not the cause of increased mortality in infected mice exposed to hyperoxia.
Intensity of inflammation during sublethal hyperoxia.
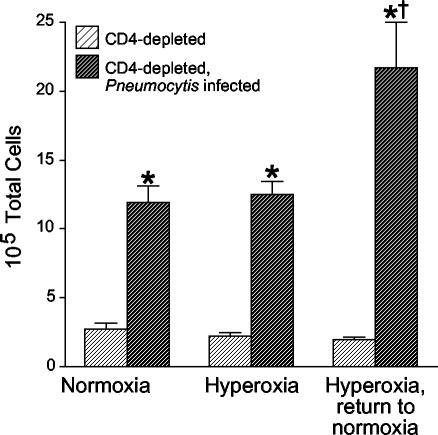
Because an increased organism burden did not explain the increased mortality in mice after sublethal hyperoxia, we reasoned that exaggerated inflammatory responses could contribute to deaths after return to normoxia. We measured the accumulation of inflammatory cells in BAL fluid in mice maintained in normoxia, in mice exposed to >95% hyperoxia for 4 days, and in mice exposed to >95% hyperoxia 4 for days and returned to room air for 1 day (Fig. 3). Uninfected, CD4-depleted mice did not show changes in total cell numbers in BAL fluid despite exposure to hyperoxia. Additionally, sublethal hyperoxia in CD4-depleted mice infected with Pneumocystis did not immediately increase the total cell numbers in BAL fluid. However, the CD4-depleted mice infected with Pneumocystis that were returned to normoxia demonstrated significant increases in total cell numbers in BAL fluid.
FIG. 3.
Total-cell counts in BAL fluid. Cells were enumerated in BAL fluid from CD4-depleted uninfected C57BL/6 mice and CD4-depleted C57BL/6 mice with Pneumocystis infection. Mice were either maintained in normoxia, exposed to >95% hyperoxia, or exposed to >95% hyperoxia followed by return to normoxia. Data represent means and standard errors of the mean from eight or more mice per group. *, P < 0.01 for CD4-depleted Pneumocystis-infected mice compared with CD4-depleted uninfected mice; †, P < 0.01 for CD4-depleted Pneumocystis-infected mice exposed to hyperoxia with return to normoxia compared with CD4-depleted Pneumocystis-infected mice in the other two groups by analysis of variance.
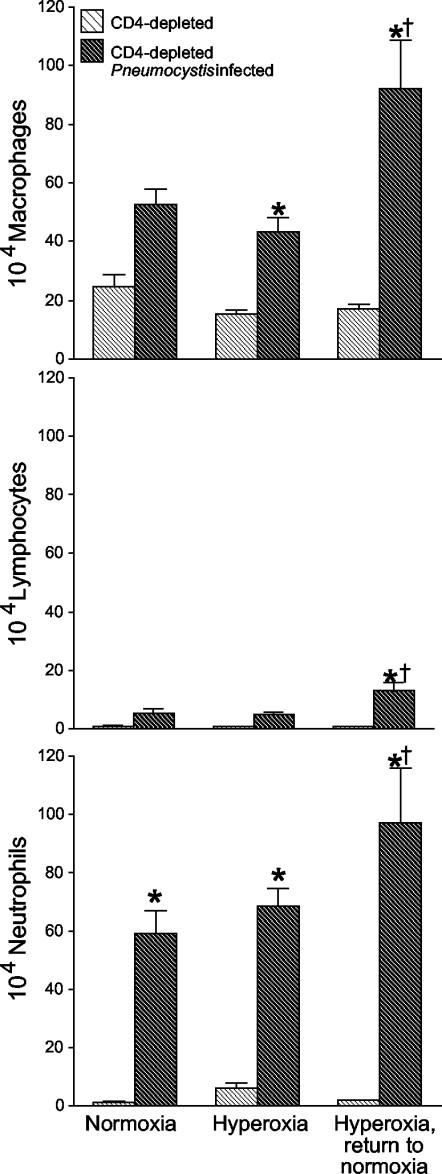
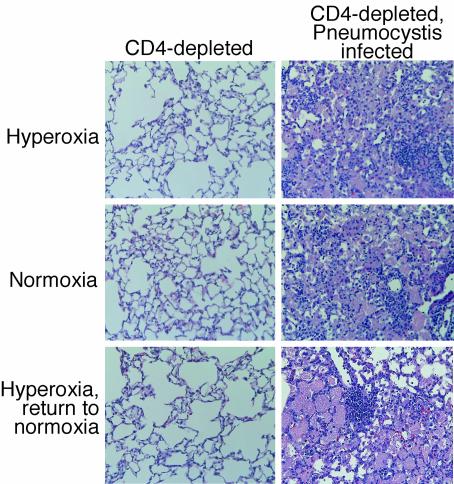
To determine which cells were responsible for the increased accumulation in BAL fluid, we examined differentials from the BAL fluid and expressed the data as total numbers of each cell type. The increased numbers of cells in BAL fluid from CD4-depleted mice infected with Pneumocystis reflected significant increases in numbers of alveolar macrophages, lymphocytes, and neutrophils (Fig. 4). Furthermore, histologic analysis of lung sections demonstrated exuberant inflammatory responses in the infected mice returned to normoxia, reflecting the cell count data (Fig. 5). These data demonstrate increased inflammatory responses in the lungs of CD4-depleted mice with Pneumocystis infection after exposure to sublethal hyperoxia and return to normoxia.
FIG. 4.
Cellular constituents of BAL fluid. Cells were enumerated in BAL fluid from CD4-depleted uninfected C57BL/6 mice and from CD4-depleted C57BL/6 mice with Pneumocystis infection. Mice were either maintained in normoxia, exposed to >95% hyperoxia, or exposed to >95% hyperoxia followed by return to normoxia. Data represent means and standard errors of the mean from eight or more mice per group. *, P < 0.01 for CD4-depleted Pneumocystis-infected mice compared with CD4-depleted uninfected mice; †, P < 0.01 for CD4-depleted Pneumocystis-infected mice exposed to hyperoxia with return to normoxia compared with CD4-depleted Pneumocystis-infected mice in the other two groups by analysis of variance.
FIG. 5.
Histology of Pneumocystis infection. Lung sections were obtained from CD4-depleted uninfected C57BL/6 mice and from CD4-depleted C57BL/6 mice with Pneumocystis infection. Mice were either maintained in normoxia, exposed to >95% hyperoxia, or exposed to >95% hyperoxia followed by return to normoxia. Lung sections were stained with hematoxylin-eosin. Magnification, ×20.
Alveolar macrophage phagocytosis of Pneumocystis.
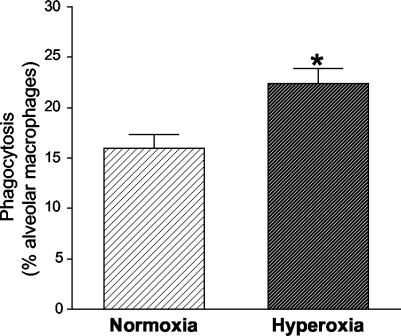
Given the decreased organism burdens and the increased number of alveolar macrophages present in the lungs of mice exposed to hyperoxia, we examined the ability of alveolar macrophages to phagocytose Pneumocystis after exposure to hyperoxia. To determine whether exposure to sublethal hyperoxia modulates the ability of alveolar macrophages to phagocytose Pneumocystis, we examined phagocytosis in vitro. Alveolar macrophages were isolated from C57/BL6 mice maintained in normoxia and from C57BL6 mice exposed to >95% hyperoxia for 4 days. Fluorescently labeled Pneumocystis organisms were added to the macrophages for 90 min, the fluorescence of extracellular organisms was quenched, and internalized organisms were enumerated by fluorescence microscopy. Alveolar macrophages from hyperoxia-exposed mice demonstrated enhanced phagocytosis compared with those from normoxia-maintained mice (Fig. 6). Sublethal hyperoxia does not impair alveolar macrophage phagocytosis but actually increases phagocytosis; this increase in phagocytosis probably contributes to the decreased organism burden we observed.
FIG. 6.
Alveolar macrophage phagocytosis of Pneumocystis. Alveolar macrophages were isolated from C57BL/6 mice maintained in normoxia or from C57BL/6 mice exposed to >95% hyperoxia for 4 days. After isolation, alveolar macrophages were exposed to fluorescently labeled Pneumocystis organisms for 90 min. After washing and quenching of unbound organisms, phagocytosed organisms were counted under microscopy. Data represent the percentages of alveolar macrophages containing organisms, expressed as means and standard errors of the mean from experiments performed in triplicate. *, P < 0.01 by the t test.
Lung cell apoptosis during Pneumocystis infection and sublethal hyperoxia.
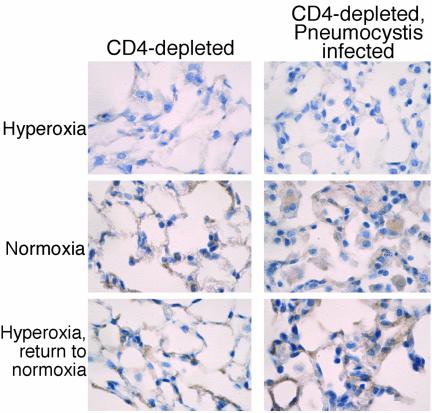
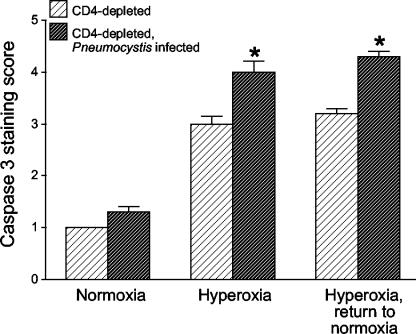
We reasoned that during increased lung inflammation, sublethal hyperoxia could induce increased lung cell apoptosis in the context of Pneumocystis infection, thereby increasing mortality. To measure the extent of apoptosis, mice were again exposed to sublethal >95% hyperoxia followed by a return to normoxia. Lung sections were stained immunohistochemically for caspase-3 and scored semiquantitatively. The immunohistochemical stains demonstrated no increase in apoptosis attributable to Pneumocystis infection in the mice maintained in normoxia (Fig. 7, top panels). Increased apoptosis occurred in all CD4-depleted mice exposed to hyperoxia, but the extent of apoptosis was significantly greater in mice infected with Pneumocystis than in uninfected mice (Fig. 7 and 8). Histologically, both alveolar macrophages and alveolar epithelial cells demonstrated increased apoptosis. Therefore, sublethal hyperoxia in the setting of Pneumocystis infection results in increased lung cell apoptosis compared with the effect of hyperoxia alone.
FIG. 7.
Immunohistochemical staining for caspase-3. Lung sections were obtained from CD4-depleted uninfected C57BL/6 mice and from CD4-depleted C57BL/6 mice with Pneumocystis infection. Mice were either maintained in normoxia, exposed to >95% hyperoxia, or exposed to >95% hyperoxia followed by return to normoxia. Lung sections were stained for caspase-3, and scoring was performed as described in the text. Magnification, ×40.
FIG. 8.
Scoring of immunohistochemical staining for caspase-3. Lung sections were obtained from CD4-depleted uninfected C57BL/6 mice and from CD4-depleted C57BL/6 mice with Pneumocystis infection. Mice were either maintained in normoxia, exposed to >95% hyperoxia, or exposed to >95% hyperoxia followed by return to normoxia. Lung sections were stained for caspase-3 as described in the text. Data represent medians from six mice per group. *, P < 0.05 for CD4-depleted Pneumocystis-infected mice compared with CD4-depleted uninfected mice by the Kruskal-Wallis test.
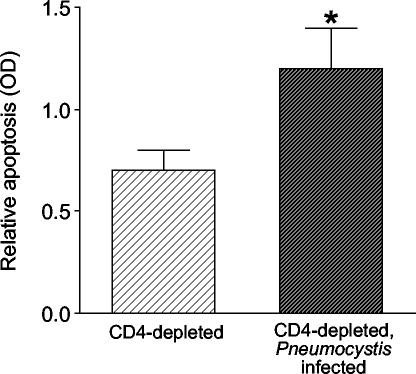
To confirm these data using an additional method to measure apoptosis, we measured histone-associated DNA in lung homogenates by ELISA. We compared uninfected CD4-depleted mice and CD4-depleted mice infected with Pneumocystis after sublethal hyperoxia and return to normoxia. The infected mice demonstrated significantly more lung cell apoptosis by this method than did the uninfected mice (Fig. 9), confirming that sublethal hyperoxia and return to normoxia during Pneumocystis infection results in increased lung cell apoptosis. Therefore, Pneumocystis pneumonia is associated with increased lung cell apoptosis following hyperoxic stress.
FIG. 9.
Immunoassay for apoptosis. Lung homogenates were obtained from CD4-depleted uninfected C57BL/6 mice and from CD4-depleted C57BL/6 mice with Pneumocystis infection. Mice were exposed to >95% hyperoxia followed by return to normoxia. Individual mouse samples were matched for protein content. Data represent means and standard errors of the mean from eight or more mice per group. *, P < 0.05 by the t test.
DISCUSSION
Clinically, it has long been appreciated that significant numbers of patients with Pneumocystis pneumonia deteriorate clinically after entry into the medical care system. However, the mechanisms of this deterioration remain unknown and require further investigation. We reasoned that Pneumocystis infection increases lung susceptibility to additional insults that, alone, would not lead to respiratory failure. Patients with Pneumocystis pneumonia may be exposed to a variety of respiratory insults, including oxygen therapy, bacterial infections, drug effects, and release of fungal products from dead and dying Pneumocystis organisms. We chose to investigate hyperoxia as a clinically relevant prototypic insult because hyperoxia is known to have detrimental effects on the lungs. It is noteworthy that hyperoxia has been associated with respiratory compromise in combination with other insults such as bleomycin exposure (9, 16).
We found that CD4-depleted mice with advanced Pneumocystis pneumonia exhibited significantly increased mortality after exposure to sublethal hyperoxia compared with CD4-depleted mice without pneumonia. This excess mortality did not occur with mild Pneumocystis infections. The increase in mortality was not caused by increased organism burden, because the lungs of mice exposed to hyperoxia contained decreased numbers of organisms. The combination of Pneumocystis infection and transient hyperoxia resulted in increased accumulation of inflammatory cells in the lungs compared to either stress alone. Alveolar macrophages exposed to hyperoxia demonstrated enhanced ex vivo phagocytosis of Pneumocystis organisms compared to control alveolar macrophages. However, following transient hyperoxia, there was increased apoptosis of alveolar cells in mice infected with Pneumocystis. This finding indicates that in the setting of Pneumocystis pneumonia, the lungs are more susceptible to acute injury due to insults, such as hyperoxic stress, that are well tolerated in the absence of infection.
Several important features of the model we developed deserve emphasis. First, we rendered mice susceptible to Pneumocystis pneumonia by depletion of CD4+ T cells. This approach mimics many of the characteristics of humans who have been immunosuppressed by infection with HIV. It avoids the more complex interactions in models using scid mice or mice immunosuppressed with corticosteroids. While these CD4-depleted mice become heavily infected with Pneumocystis, at the 4-week time point used in these studies the mice are active and do not appear seriously ill (7). Untreated, they would survive for several more weeks before succumbing to overwhelming infection. Second, hyperoxia provides a well-characterized model of acute lung injury, with variability in susceptibility depending on the particular strain of mouse (17). When C57BL/6 mice are returned to normoxia after 4 days in hyperoxia, lung injury is limited and the mice survive. Therefore, we developed a system in which mice that have Pneumocystis pneumonia (but would normally survive for several weeks) are stressed by transient exposure to hyperoxia (for a period that normally results in no mortality) and found that the combination of these two stresses leads to significant mortality.
Increased organism burden in the mice exposed to hyperoxia cannot account for the increased mortality we observed. As in our previous work, the intensity of Pneumocystis infection increases with time after inoculation, becoming heavy after 4 weeks (7). Mice with less intense infection were not susceptible to sublethal hyperoxia, but mice with advanced infections showed increased mortality after hyperoxia. These data suggest that a threshold of infection intensity is required before the interactive effect with hyperoxia becomes significant. Additionally, the lungs of mice exposed to hyperoxia had decreased organism burdens compared to the lungs of mice maintained in normoxia. We confirmed the histologic grading by using quantitative PCR (39), and the two methods agreed closely. Interestingly, we found that increased mortality occurred only in mice with advanced Pneumocystis infections, because mice with moderate, early infections exhibited no mortality following transient exposure to hyperoxia. Whether it is the duration of the infection or its intensity that determines susceptibility to secondary insults is the subject of ongoing studies.
The combined stress of Pneumocystis infection and exposure to hyperoxia resulted in significantly increased apoptosis of alveolar cells compared to either stress alone. Death of cells of the alveolar wall is a consistent feature in hyperoxic lung injury (14). Although the relative contributions of apoptosis and necrosis are not totally clear, a number of studies have focused on the specific role of apoptosis in lung injury due to hyperoxia (2, 3). Overexpression in the lungs of molecules that block alveolar epithelial cell apoptosis, including interleukin-11 (34), an activated form of the transcription factor Akt (23), and granulocyte-macrophage colony-stimulating factor (R. Paine III, S. E. Wilcoxen, S. B. Morris, C. Sartori, C. E. O. Bakeiro, M. A. Matthay, and P. J. Christensen, submitted for publication), prevents mortality secondary to hyperoxia. Therefore, it is likely that the increased apoptosis of cells of the alveolar wall found in Pneumocystis-infected mice exposed to hyperoxia is the cause of increased mortality in these mice.
There are several potential mechanisms by which the combination of two stresses that individually lead to modest lung cell apoptosis may result in sufficient apoptosis to cause mortality. We have shown previously that direct interaction of Pneumocystis organisms with alveolar epithelial cells in primary culture did not lead to epithelial cell injury (5). However, Pneumocystis trophic forms live in very close association with type I alveolar epithelial cells and presumably receive nutritional support from these host cells (26). It is possible that this “parasitic” interaction increases the vulnerability of epithelial cells to death due to an additional insult, such as hyperoxia. A second possibility is that inflammatory factors are the proximate cause of alveolar epithelial cell apoptosis. We have found that the numbers of inflammatory cells are increased in the lungs of mice infected with Pneumocystis after hyperoxic stress. Furthermore, these inflammatory cells appear to be activated, as demonstrated by the increased phagocytic activity of alveolar macrophages for Pneumocystis ex vivo. It is reported that exposure to high level tumor necrosis factor (TNF) can induce apoptosis of rat alveolar epithelial cells (8). However, we found no more TNF in BAL fluid from Pneumocystis-infected mice exposed to hyperoxia than in similarly infected mice in room air (data not shown). Alternatively, alveolar epithelial cells are subject to apoptosis due to binding of Fas on T cells to its ligand (12, 13). Therefore, it is quite plausible that the increased numbers of inflammatory cells, including lymphocytes, in the alveolar space of Pneumocystis-infected mice exposed to hyperoxia may trigger epithelial cell apoptosis and subsequent respiratory failure. If this hypothesis is correct, it might explain the beneficial effect of adjuvant corticosteroid therapy in AIDS patients with moderately severe Pneumocystis pneumonia.
We also found that hyperoxia did not impair the ability of alveolar macrophages to phagocytose Pneumocystis. Instead, alveolar macrophages exposed to hyperoxia demonstrated enhanced phagocytosis for Pneumocystis organisms. Few studies have examined phagocytosis by alveolar macrophages after in vivo hyperoxia, but the published literature suggests that in vitro hyperoxia impairs phagocytosis. For example, murine pulmonary macrophages demonstrate impaired Fc-gamma receptor-mediated phagocytosis after in vitro hyperoxic challenge (10). Additionally, human alveolar macrophages demonstrate reduced phagocytosis of Saccharomyces cerevisiae after exposure to hyperoxia in vitro (35). Alveolar macrophages are essential for clearance of Pneumocystis, as shown in experiments in which rats transiently depleted of alveolar macrophages cannot clear an infectious challenge with organism (21). Previous work from our laboratory correlated the failure of in vivo clearance of Pneumocystis in mice deficient in granulocyte-macrophage colony-stimulating factor with defective in vitro phagocytosis by alveolar macrophages (25). It is likely that cytokine products of alveolar macrophages released during clearance of Pneumocystis, such as TNF (25), contributed to additional lung injury and alveolar damage.
Although it is difficult to establish a causal relationship between the extent of increased inflammation and mortality, our data are quite compatible with the published literature linking exuberant inflammatory responses to Pneumocystis with mortality. In humans, the number of neutrophils recovered by BAL is a strong predictor of patient survival, while the number of Pneumocystis organisms recovered does not predict mortality (22). Several groups have used murine models of Pneumocystis infection to demonstrate that inflammatory responses may clear infection but result in the death of the host (15). CD4+ T cells cause fatal hyperinflammatory reactions after reconstitution of scid mice in some models (27). Recent work may help explain why inflammatory responses to Pneumocystis have been beneficial in some systems but detrimental in others. A subset of CD4+ T cells that constitutively express the interleukin-2 receptor α chain (CD25) have been shown to prevent autoimmunity (15). Transfer of CD25− cells into RAG-2-deficient mice infected with Pneumocystis resulted in lethal pneumonia. However, transfer of CD25+ cells did not produce fatal pneumonitis, and it prevented the inflammation caused by CD4+ CD25− cells (15).
Much more clearly appreciated is the detrimental role of CD8+ T cells in causing unwanted lung inflammation during Pneumocystis infection. Although we did not perform phenotyping in these experiments, our previous work demonstrates that CD8+ T cells account for the vast majority of T-cell accumulation in these infected CD4-depleted mice (7). Data from our laboratory demonstrate that CD8+ T cells contribute to defense against Pneumocystis during states of CD4+ T-cell depletion (4). However, several investigations implicate CD8+ T cells in induction of undesired, and detrimental, lung inflammation. Mice depleted of CD4+ T cells developed Pneumocystis pneumonia with intense inflammation, decreased oxygenation, and decreased lung compliance (36). In contrast, mice depleted of both CD4+ and CD8+ T cells developed pneumonia, but did not develop physiologic derangements. In this system, then, the CD8+ T cells recruited in response to Pneumocystis induced lung damage. Additionally, the same investigators determined that CD8+ T cells, and the lung inflammation they produced, altered lung compliance and oxygenation. CD8+ cells have also been implicated in derangements of surfactant function that occur during Pneumocystis pneumonia. Pneumocystis reduces levels of the hydrophobic surfactant proteins B and C, leading to increased alveolar surface tension, atelectasis, and hypoxia (1). When infected scid mice are reconstituted with splenocytes, the inflammatory response decreases the biophysical activity of surfactant (37). Importantly, CD8+ T cells drive this response, as shown by the fact that reconstitution with CD8-depleted splenocytes abrogates this dysfunction.
In summary, we have demonstrated that CD4-depleted mice with advanced Pneumocystis pneumonia exhibit significantly increased mortality after exposure to sublethal hyperoxia compared with CD4-depleted mice without pneumonia. The increase in mortality was not caused by increased organism burden, because the lungs of mice exposed to hyperoxia contained decreased numbers of organisms. Additionally, alveolar macrophages exposed to hyperoxia demonstrated enhanced phagocytosis of Pneumocystis organisms, thereby decreasing lung burdens. Sublethal hyperoxia, in the setting of established Pneumocystis pneumonia, caused significantly increased apoptosis of alveolar cells and induced exuberant inflammatory responses. Taken together, our data demonstrate that during Pneumocystis pneumonia, the lungs are more susceptible to acute injury due to insults, such as hyperoxic stress, that are well tolerated in the absence of infection. Future investigations will be directed toward modulation of injury to improve survival, including therapy with corticosteroids.
Acknowledgments
We appreciate the assistance of Jay Kolls and Chad Steele, Louisiana State University in New Orleans, and Antonello Punturieri, Ann Arbor VA Medical Center, for assistance in establishing the real-time PCR assay in the laboratory.
These studies were supported by National Heart, Lung, and Blood Institute grants HL570111 and HL59823 (J.M.B.) and HL50496 (R.P.) and by the Medical Research Service, Department of Veterans Affairs.
Editor: T. R. Kozel
REFERENCES
- 1.Atochina, E. N., M. F. Beers, S. T. Scanlon, A. M. Preston, and J. M. Beck. 2000. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L599-L609. [DOI] [PubMed] [Google Scholar]
- 2.Barazzone, C., S. Horowitz, V. R. Donati, I. Rodriguez, and P. F. Piguet. 1998. Oxygen toxicity in mouse lung: pathways to cell death. Am. J. Respir. Cell Mol. Biol. 19:573-581. [DOI] [PubMed] [Google Scholar]
- 3.Barazzone, C., and C. W. White. 2000. Mechanisms of cell injury and death in hyperoxia: role of cytokines and Bcl-2 family proteins. Am. J. Respir. Cell Mol. Biol. 22:517-519. [DOI] [PubMed] [Google Scholar]
- 4.Beck, J. M., R. L. Newbury, B. E. Palmer, M. L. Warnock, P. K. Byrd, and H. B. Kaltreider. 1996. Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J. Lab. Clin. Med. 128:477-487. [DOI] [PubMed] [Google Scholar]
- 5.Beck, J. M., A. M. Preston, J. G. Wagner, S. E. Wilcoxen, P. Hossler, S. R. Meshnick, and R. Paine III. 1998. Interaction of rat Pneumocystis carinii and rat alveolar epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 275:L118-L125. [DOI] [PubMed] [Google Scholar]
- 6.Beck, J. M., M. J. Rosen, and H. H. Peavy. 2001. Pulmonary complications of HIV infection: report of the fourth NHLBI workshop. Am. J. Respir. Crit. Care Med. 164:2120-2126. [DOI] [PubMed] [Google Scholar]
- 7.Beck, J. M., M. L. Warnock, J. L. Curtis, M. J. Sniezek, S. M. Arraj-Peffer, H. B. Kaltreider, and J. E. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5:186-197. [DOI] [PubMed] [Google Scholar]
- 8.Brown, L. A., F. L. Harris, and D. M. Guidot. 2001. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L377-L386. [DOI] [PubMed] [Google Scholar]
- 9.Comis, R. L. 1992. Bleomycin pulmonary toxicity: current status and future directions. Semin. Oncol. 19:64-70. [PubMed] [Google Scholar]
- 10.Crowell, R. E., G. Hallin, E. Heaphy, and C. Mold. 1995. Hyperoxic suppression of Fc-gamma receptor-mediated phagocytosis by isolated murine pulmonary macrophages. Am. J. Respir. Cell Mol. Biol. 12:190-195. [DOI] [PubMed] [Google Scholar]
- 11.Curtis, J. L., and H. B. Kaltreider. 1989. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am. Rev. Respir. Dis. 139:393-400. [DOI] [PubMed] [Google Scholar]
- 12.Fine, A., N. L. Anderson, T. L. Rothstein, M. C. Williams, and B. R. Gochuico. 1997. Fas expression in pulmonary alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 273:L64-L71. [DOI] [PubMed] [Google Scholar]
- 13.Fine, A., Y. Janssen-Heininger, R. P. Soultanakis, S. G. Swisher, and B. D. Uhal. 2000. Apoptosis in lung pathophysiology. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L423-L427. [DOI] [PubMed] [Google Scholar]
- 14.Folz, R., C. Piantadosi, and J. Crapo. 1997. Oxygen toxicity, p. 2713-2722. In R. Crystal, J. West, P. Barnes, and E. Weibel (ed.), The lung: scientific foundations, 2nd ed. Lippincott-Raven, Philadelphia, Pa.
- 15.Hori, S., T. L. Carvalho, and J. Demengent. 2002. CD25+ CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282-1291. [DOI] [PubMed] [Google Scholar]
- 16.Ingrassia, T. S., J. H. Ryu, V. F. Trastek, and E. C. Rosenow. 1991. Oxygen-exacerbated bleomycin pulmonary toxicity. Mayo Clin. Proc. 66:173-178. [DOI] [PubMed] [Google Scholar]
- 17.Johnston, C. J., B. Stripp, R., B. Piedbeouf, T. W. Wright, G. W. Mango, C. K. Reed, and J. N. Finkelstein. 1998. Inflammatory and epithelial responses in mouse strains that differ in sensitivity to hyperoxic injury. Exp. Lung Res. 24:189-202. [DOI] [PubMed] [Google Scholar]
- 18.Kaneshiro, E. S., M. A. Wyder, L. H. Zhou, J. E. Ellis, D. R. Voelker, and S. G. Langreth. 1993. Characterization of Pneumocystis carinii preparations developed for lipid analysis. J. Eukaryot. Microbiol. 40:805-815. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, J. E., H. Masur, K. K. Holmes, USPHS, and Infectious Disease Society of America. 2002. Guidelines for preventing opportunistic infections among HIV-infected persons—2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Morb. Mortal. Wkly. Rep. 51:1-52. [PubMed] [Google Scholar]
- 20.Limper, A. H. 1991. Parasitic adherence and host responses in the development of Pneumocystis carinii pneumonia. Semin. Respir. Infect. 6:19-26. [PubMed] [Google Scholar]
- 21.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limper, A. H., K. P. Offord, T. F. Smith, and W. J. Martin, Jr. 1989. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 140:1204-1209. [DOI] [PubMed] [Google Scholar]
- 23.Lu, Y., L. Parkyn, L. E. Otterbein, Y. Kureishi, K. Walsh, A. Ray, and P. Ray. 2001. Activated Akt protects the lung from oxidant-induced injury and delays death of mice. J. Exp. Med. 193:545-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masur, H. 2002. Acquired immunodeficiency syndrome in the intensive care unit: will human immunodeficiency virus-related admissions continue to decline? Am. J. Respir. Crit. Care Med. 166:258-261. [DOI] [PubMed] [Google Scholar]
- 25.Paine, R., III., A. M. Preston, S. Wilcoxen, H. Jin, B. B. Siu, S. B. Morris, J. A. Reed, G. Ross, J. A. Whitsett, and J. M. Beck. 2000. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J. Immunol. 164:2602-2609. [DOI] [PubMed] [Google Scholar]
- 26.Pesanti, E. L. 1994. Host defense effector mechanisms and Pneumocystis carinii, p. 289-315. In P. D. Walzer (ed.), Pneumocystis carinii pneumonia, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 27.Roths, J. B., and C. L. Sidman. 1992. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J. Clin. Investig. 90:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roths, J. B., A. L. Smith, and C. L. Sidman. 1993. Lethal exacerbation of Pneumocystis carinii pneumonia in severe combined immunodeficiency mice after infection by pneumonia virus of mice. J. Exp. Med. 177:1193-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudmann, D. G., A. M. Preston, M. W. Moore, and J. M. Beck. 1998. Susceptibility to Pneumocystis carinii in mice depends on simultaneous depletion of interferon-gamma and type 1 and 2 tumor necrosis factor receptor genes. J. Immunol. 161:360-366. [PubMed] [Google Scholar]
- 30.Saltini, C., A. J. Hance, V. J. Ferrans, F. Basset, P. B. Bitterman, and R. G. Crystal. 1984. Accurate quantification of cells recovered by bronchoalveolar lavage. Am. Rev. Respir. Dis. 130:650-658. [DOI] [PubMed] [Google Scholar]
- 31.Sepkowitz, K. A., A. E. Brown, and D. Armstrong. 1995. Pneumocystis carinii pneumonia without acquired immunodeficiency syndrome. More patients, same risk. Arch. Intern. Med. 155:1125-1128. [PubMed] [Google Scholar]
- 32.Shellito, J., V. V. Suzara, W. Blumenfeld, J. M. Beck, H. J. Steger, and T. H. Ermak. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, C. F., Jr., and A. H. Limper. 1998. Pneumocystis pneumonia: clinical presentation and diagnosis in patients with and without acquired immune deficiency syndrome. Semin. Respir. Infect. 13:289-295. [PubMed] [Google Scholar]
- 34.Waxman, A. B., O. Einarsson, T. Seres, R. G. Knickelbein, J. B. Warshaw, R. Johnston, R. J. Homer, and J. A. Elias. 1998. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J. Clin. Investig. 101:1970-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesselius, L. J., W. L. Williams, K. Bailey, S. Vamos, A. R. O'Brien-Ladner, and T. Wiegmann. 1999. Iron uptake promotes hyperoxic injury to alveolar macrophages. Am. J. Respir. Crit. Care Med. 159:100-106. [DOI] [PubMed] [Google Scholar]
- 36.Wright, T. W., F. Gigliotti, J. N. Finkelstein, J. T. McBride, C. L. An, and A. G. Harmsen. 1999. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J. Clin. Investig. 104:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright, T. W., R. H. Notter, Z. Wang, A. G. Harmsen, and F. Gigliotti. 2001. Pulmonary inflammation disrupts surfactant function during Pneumocystis carinii pneumonia. Infect. Immun. 69:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoneda, K., and P. D. Walzer. 1981. Mechanism of pulmonary alveolar injury in experimental Pneumocystis carinii pneumonia in the rat. Br. J. Exp. Pathol. 62:339-346. [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, M. Q., J. E. Shellito, L. Marrero, Q. Zhong, S. Julian, P. Ye, V. Wallace, P. Schwarzenberger, and J. K. Kolls. 2001. CD4+ T cell-independent vaccination against Pneumocystis carinii in mice. J. Clin. Investig. 108:1469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]