Abstract
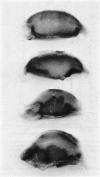
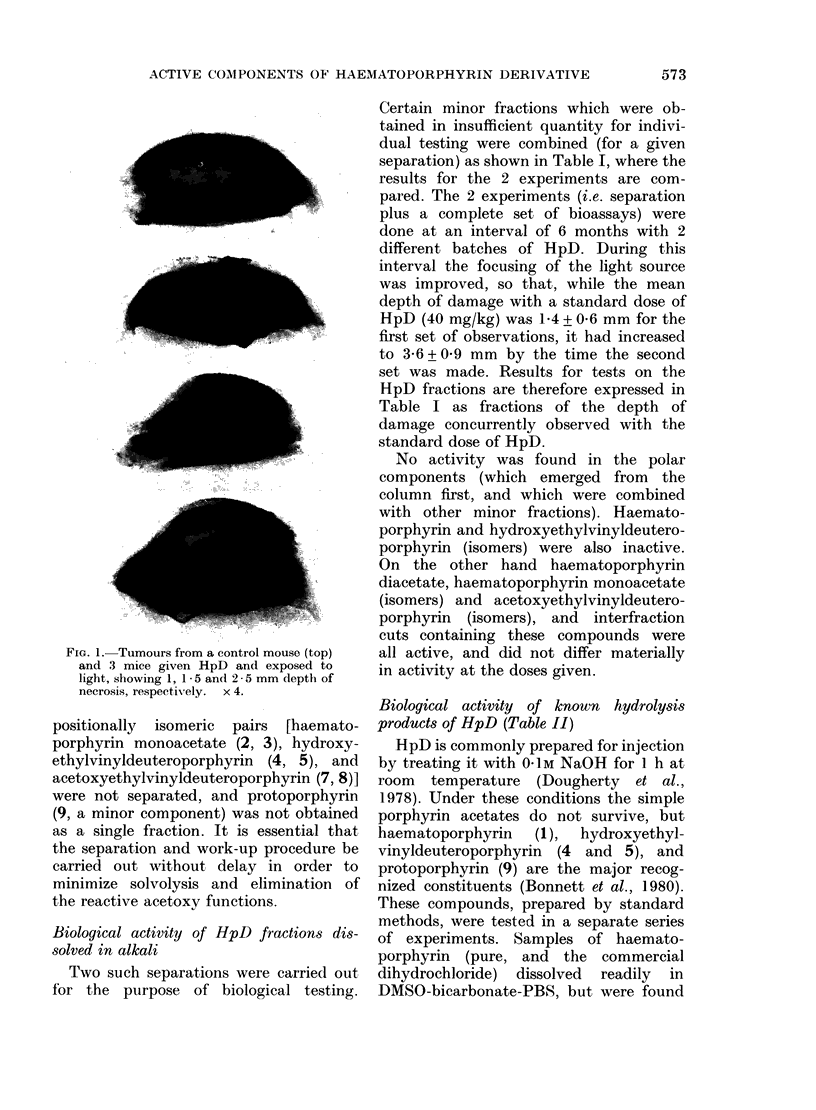
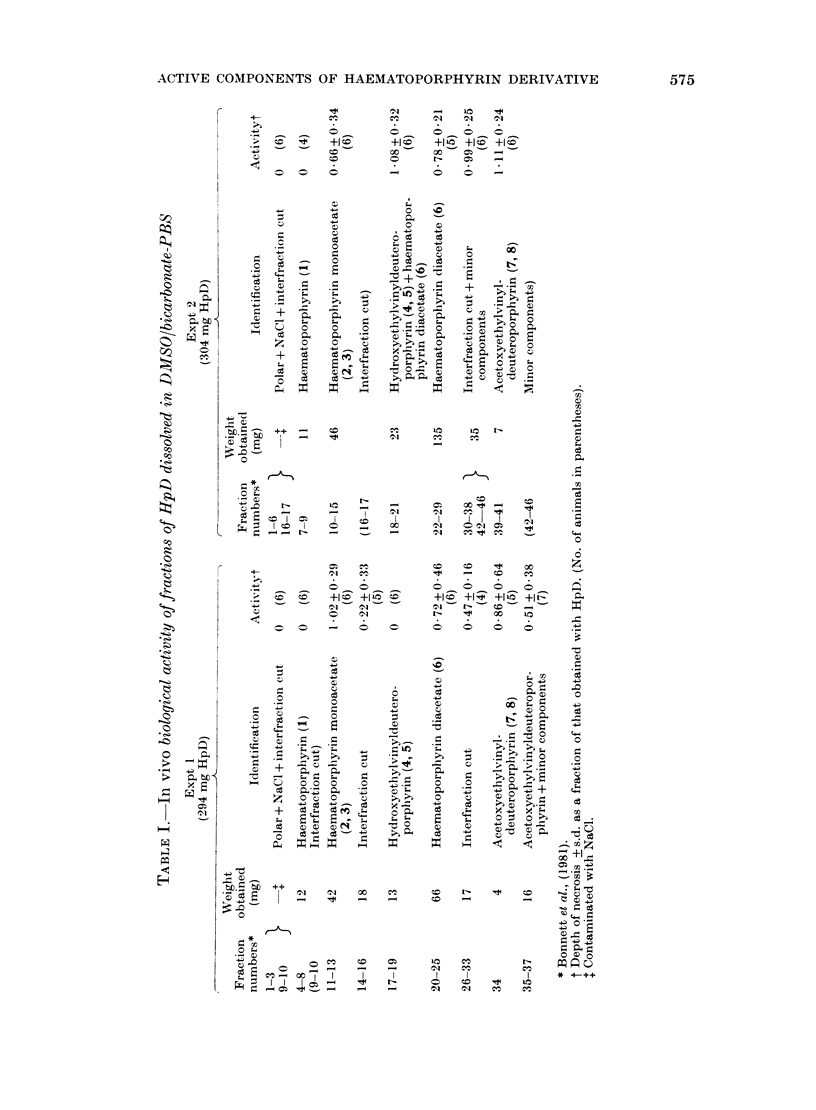
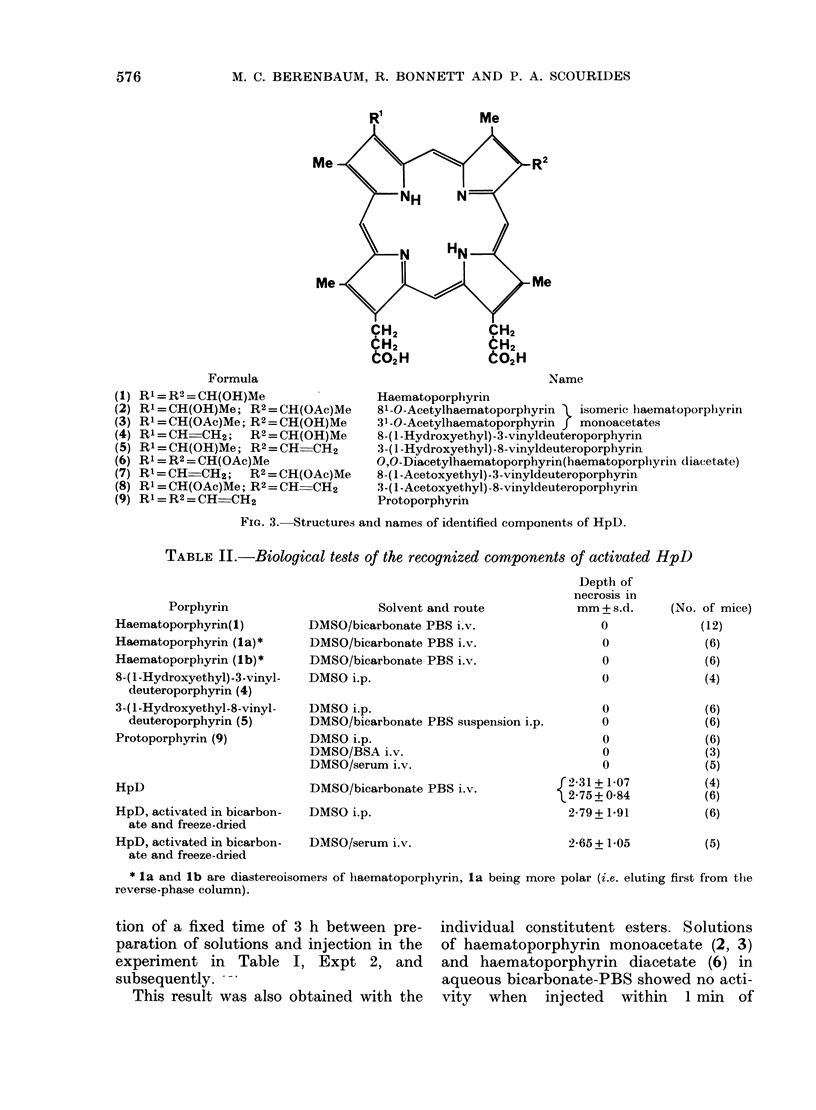
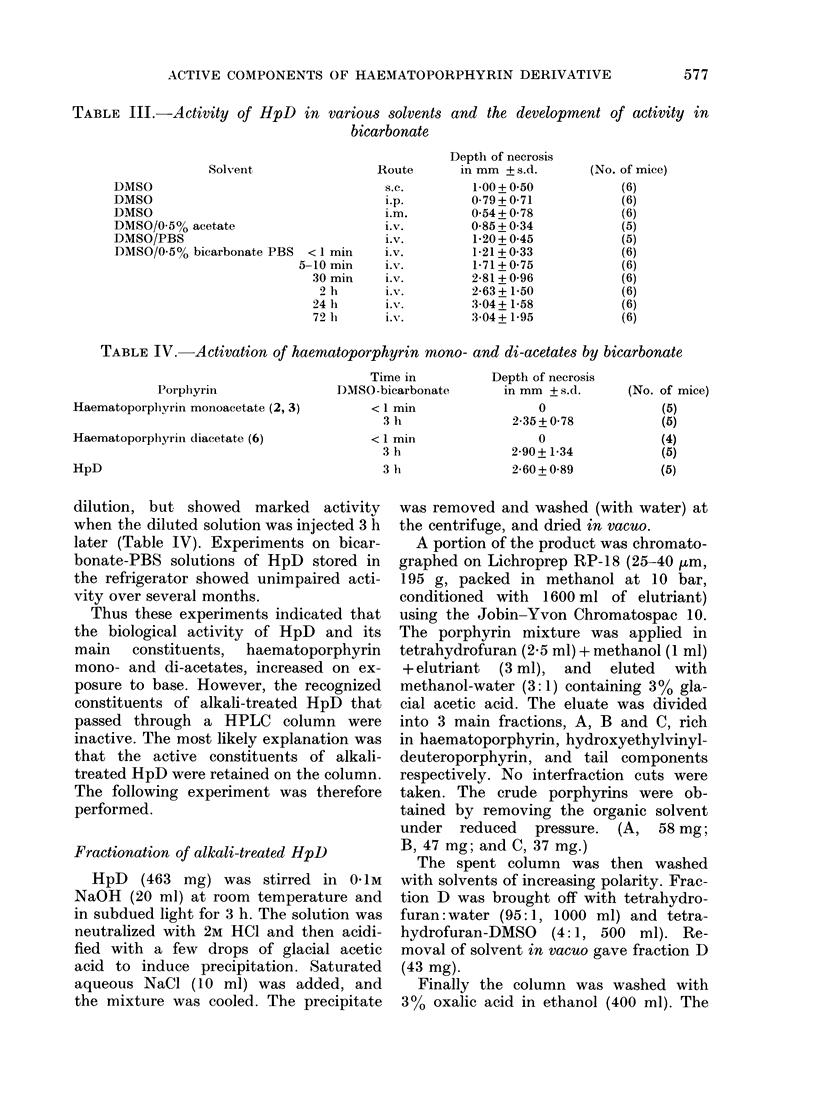
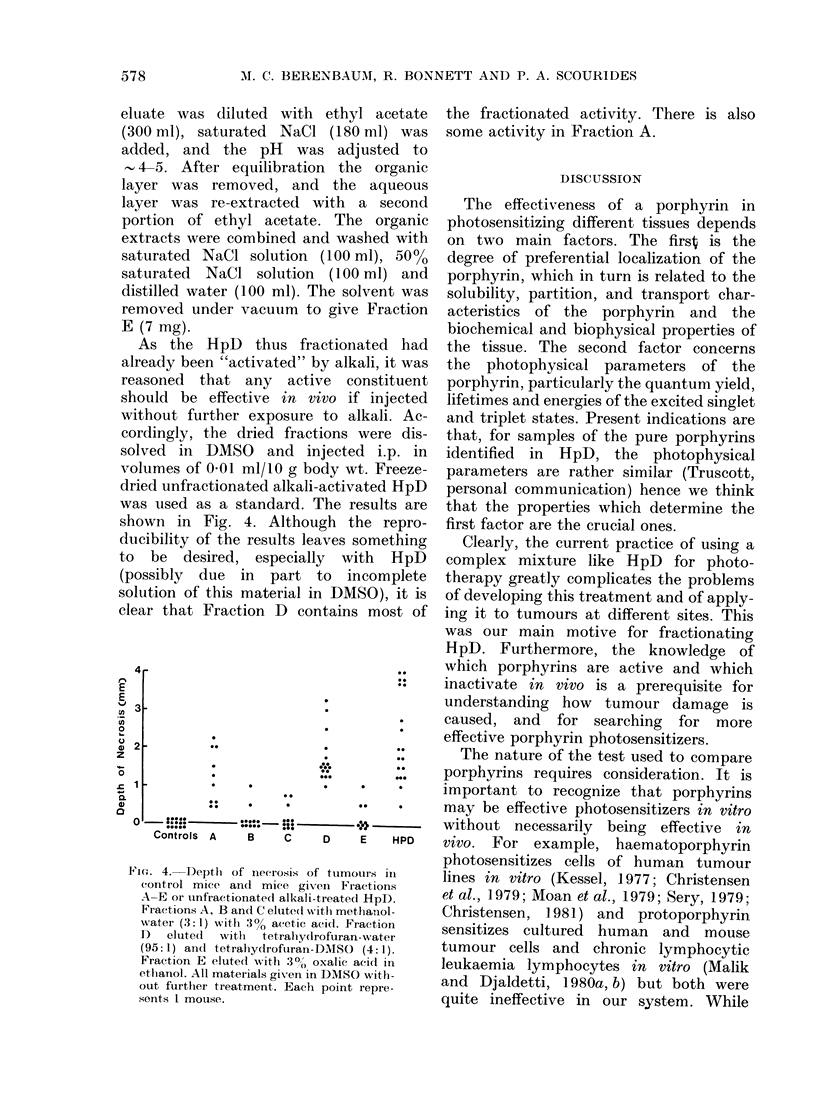
The in vivo biological activity of various fractions and components of haematoporphyrin derivative (HpD) have been determined by measuring the depth of necrosis of implanted tumours in mice exposed to light after the administration of standard doses of porphyrins dissolved in alkali. In this assay, haematoporphyrin, hydroxyethylvinyldeuteroporphyrin and protoporphyrin are inactive, but the mono- and di-acetates of haematoporphyrin (which are major components of HpD) and acetoxyethylvinyldeuteroporphyrin are active. However, the situation appears to be more complex than this. The normal method for preparing HpD for injection involves an alkali treatment which causes hydrolysis and elimination of the acetoxy functions, and the only recognized products (haematoporphyrin, hydroxyethylvinyldeuteroporphyrin and protoporphyrin) are inactive in the in vivo assay. It is concluded that the active component here is a porphyrin, possibly a dimer or oligomer, which is retained on the column during the normal separation by HPLC. This conclusion is supported by the observations that (i) the crude material obtained from the spent column is active without further alkali treatment, and (ii) activity develops over 30 min, when HpD or the mono- or diacetates of haematoporphyrin are treated with sodium bicarbonate in aqueous DMSO. The advantages of working with a pure substance (e.g. haematoporphyrin diacetate) rather than a mixture (HpD) are stressed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnett R., Charalambides A. A., Jones K., Magnus I. A., Ridge R. J. The direct determination of porphyrin carboxylic acids. High-pressure liquid chromatography with solvent systems containing phase-transfer agents. Biochem J. 1978 Aug 1;173(2):693–696. doi: 10.1042/bj1730693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T., Moan J. Photodynamic inactivation of synchronized human cells in vitro in the presence of hematoporphyrin. Cancer Res. 1979 Sep;39(9):3735–3737. [PubMed] [Google Scholar]
- Christensen T., Moan J., Wibe E., Oftebro R. Photodynamic effect of haematoporphyrin throughout the cell cycle of the human cell line NHIK 3025 cultivated in vitro. Br J Cancer. 1979 Jan;39(1):64–68. doi: 10.1038/bjc.1979.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T. Multiplication of human NHIK 3025 cells exposed to porphyrins in combination with light. Br J Cancer. 1981 Sep;44(3):433–439. doi: 10.1038/bjc.1981.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I., Granelli S. G., McDonagh A. F., Nielsen S., Wilson C. B., Jaenicke R. Photodynamic therapy of malignant tumours. Lancet. 1972 Dec 2;2(7788):1175–1177. doi: 10.1016/s0140-6736(72)92596-2. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Kaufman J. E., Goldfarb A., Weishaupt K. R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978 Aug;38(8):2628–2635. [PubMed] [Google Scholar]
- Dougherty T. J., Thoma R. E., Boyle D. G., Weishaupt K. R. Interstitial photoradiation therapy for primary solid tumors in pet cats and dogs. Cancer Res. 1981 Feb;41(2):401–404. [PubMed] [Google Scholar]
- Eichler J., Knof J., Lenz H. Measurements on the depth of penetration of light (0.35--1.0 microgram) in tissue. Radiat Environ Biophys. 1977 Oct 12;14(3):239–242. doi: 10.1007/BF01323942. [DOI] [PubMed] [Google Scholar]
- Granelli S. G., Diamond I., McDonagh A. F., Wilson C. B., Nielsen S. L. Photochemotherapy of glioma cells by visible light and hematoporphyrin. Cancer Res. 1975 Sep;35(9):2567–2570. [PubMed] [Google Scholar]
- Henderson R. W., Christie G. S., Clezy P. S., Lineham J. Haematoporphyrin diacetate: a probe to distinguish malignant from normal tissue by selective fluorescence. Br J Exp Pathol. 1980 Aug;61(4):345–350. [PMC free article] [PubMed] [Google Scholar]
- Kelly J. F., Snell M. E., Berenbaum M. C. Photodynamic destruction of human bladder carcinoma. Br J Cancer. 1975 Feb;31(2):237–244. doi: 10.1038/bjc.1975.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Effects of photoactivated porphyrins at the cell surface of leukemia L1210 cells. Biochemistry. 1977 Jul 26;16(15):3443–3449. doi: 10.1021/bi00634a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J. The photodynamic properties of a particular hematoporphyrin derivative. Arch Dermatol. 1960 Oct;82:508–516. doi: 10.1001/archderm.1960.01580040026005. [DOI] [PubMed] [Google Scholar]
- Malik Z., Djaldetti M. Cytotoxic effect of hemin and protoporphyrin on chronic lymphocytic leukemia lymphocytes. Exp Hematol. 1980 Aug;8(7):867–879. [PubMed] [Google Scholar]
- Malik Z., Djaldetti M. Destruction of erythroleukemia, myelocytic leukemia and Burkitt lymphoma cells by photoactivated protoporphyrin. Int J Cancer. 1980 Oct 15;26(4):495–500. doi: 10.1002/ijc.2910260415. [DOI] [PubMed] [Google Scholar]
- Moan J., Pettersen E. O., Christensen T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br J Cancer. 1979 Apr;39(4):398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sery T. W. Photodynamic killing of retinoblastoma cells with hematoporphyrin and light. Cancer Res. 1979 Jan;39(1):96–100. [PubMed] [Google Scholar]