Abstract
Mice with targeted mutations of CD18, the common β2 subunit of CD11/CD18 integrins, have leukocytosis, impaired transendothelial neutrophil emigration, and reduced host defense to Streptococcus pneumoniae, a gram-positive extracellular bacterium. Previous studies using blocking monoclonal antibodies suggested roles for CD18 and CD11b in hepatic neutrophil recruitment and host innate response to Listeria monocytogenes, a gram-positive intracellular bacterium. We induced systemic listeriosis in CD18 knockout (CD18-ko) and wild-type (WT) mice by tail vein injection with Listeria. By 14 days postinjection (dpi), 8 of 10 WT mice died, compared with 2 of 10 CD18-ko mice (P < 0.01). Quantitative organ culture showed that numbers of Listeria organisms in livers and spleens were similar in both groups at 20 min postinfection. By 3, 5, and 7 dpi, however, numbers of Listeria organisms were significantly lower in livers and spleens of CD18-ko mice than in WT mice. Histopathology showed that following Listeria infection, CD18-ko mice had milder inflammatory and necrotizing lesions in both spleens and livers than did WT mice. Cytokine assays indicated that baseline interleukin-1β and granulocyte colony-stimulating factor (G-CSF) levels were higher in CD18-ko mice than in WT mice and that CD18-ko splenocytes produced higher levels of interleukin-1β and G-CSF than WT splenocytes under the same amount of Listeria stimulation. These findings show that CD18 is not an absolute requirement for antilisterial innate immunity or hepatic neutrophil recruitment. We propose that the absence of CD18 in the mice results in the priming of innate immunity, as evidenced by elevated cytokine expression, and neutrophilic leukocytosis, which augments antilisterial defense.
CD11/CD18 (β2) integrins are heterodimeric molecules expressed on the leukocyte surface and include CD11a/CD18 (leukocyte function-associated antigen 1 [LFA-1] or αLβ2), CD11b/CD18 (macrophage differentiation antigen 1 [Mac-1], complement receptor 3 [CR3], or αMβ2), CD11c/CD18 (p150,95, CR4, and αXβ2), and CD11d/CD18 (αdβ2), each with a separate α chain (CD11a, -b, -c, or -d) but a common β chain (CD18) (15, 23, 33). CD11/CD18 integrins have been shown to be involved in leukocyte adhesion and emigration (9, 15, 18, 24, 33). Leukocyte adhesion deficiency syndrome type I (LAD I), which results from mutation in CD18, leading to severe or total deficiency of all four CD11/CD18 integrins from the leukocyte surface, is characterized by severely reduced or completely deficient neutrophil adhesion and emigration. Clinically, LAD I is manifested by recurrent microbial infection, impaired wound healing, and leukocytosis (25).
Mice with genetic deficiency in CD18 (CD18-knockout [ko] mice) have features similar to humans with LAD I in that they have neutrophilic leukocytosis, spontaneous skin ulceration, splenomegaly, and impaired ex vivo T-cell proliferation in response to staphylococcal enterotoxin A and major histocompatibility complex alloantigens (30). CD18-ko neutrophils had reduced tumor necrosis factor α-induced emigration into a subcutaneous air pouch model of inflammation and also had reduced adhesion to purified intercellular adhesion molecule 1 (ICAM-1) and to ICAM-1 incorporated into lipid bilayers (9). Increased mortality compared with wild-type (WT) controls has been observed in CD18-ko mice (30) as well as in CD11a-ko and CD11b-ko mice (25) in a model of systemic infection with Streptococcus pneumoniae, a gram-positive extracellular pathogen.
Listeria monocytogenes is an enteroinvasive, gram-positive, facultative, intracellular bacterium responsible for disseminated infections in immunocompromised people and pregnant women (3, 4, 7). Because of the similarity of the pathogenesis in humans and rodents, a mouse model of listeriosis is widely used to study cell-mediated immunity, the main mechanism of a protective host response (7). However, a crucial role for neutrophils in the innate murine antilisterial host response was also shown following administration of the neutrophil-depleting monoclonal antibody RB6-8C5. Mice pretreated with RB6-8C5 had markedly increased mortality in response to challenge with Listeria (4). Antibody-blocking studies also suggested roles for CD18 and CD11b in hepatic neutrophil recruitment and in antilisterial host innate response. Pretreatment of mice with monoclonal antibodies specific for CD11b inhibited the accumulation of neutrophils in the liver and the elimination of Listeria organisms (14, 29). Mice deficient in CD18 lack CD11b and other CD11 integrins on leukocytes and have markedly impaired neutrophil adhesive emigration and increased susceptibility to infection with S. pneumonia (30). Mice deficient in CD18 have a markedly increased peripheral neutrophil count, and the efficiency of neutrophil extravasation to some stimuli that can occur by CD11/CD18-independent mechanisms varies with the tissue and stimulus (30). In these experiments, we have used mice with a targeted disruption in CD18 to determine host immune response to Listeria infection.
Experiments that use monoclonal antibodies that bind to leukocytes may be confounded if antibody-leukocyte binding has additional effects beyond specific inhibition of a receptor-ligand interaction. Binding of antibodies to leukocytes has been shown in some cases to lead to signal transduction events, removal of leukocytes from the circulation by the reticuloendothelial system, and capping and internalization of the antibody and cell membrane target protein with potential alterations in cellular function. Development of mice with a targeted mutation that have a deficiency in CD11/CD18 integrins allows another approach without the use of monoclonal antibodies to examine the role of CD11/CD18 integrins in host immunity to Listeria.
MATERIALS AND METHODS
Animals.
CD18-ko mice were developed as described previously (30, 37) and were backcrossed for eight generations with C57BL/6J purchased from the Jackson Laboratory (Bar Harbor, Maine). WT controls were C57BL/6J mice purchased from the Jackson Laboratory. For all experiments, 8- to 12-week-old, age- and sex-matched CD18-ko mice and WT mice were used. All mice were housed in autoclaved microisolator cages, with autoclaved feed and bedding. After inoculation, mice were housed individually (one mouse per cage) in a type 2B biosafety cabinet. All animal studies were approved by the Animal Protocol Review Committee of Baylor College of Medicine.
Bacteria and inoculation of mice.
To maintain virulence, L. monocytogenes (EGD strain) was passed through mice, and aliquots of Listeria were stored at −80°C. For each experiment, an aliquot was grown overnight in Trypticase soy broth. After being cultured, Listeria organisms were collected, washed twice with phosphate-buffered saline (PBS), and finally resuspended in saline and diluted to the concentration (estimated by optical density at 600 nm) needed for the injection. The actual concentration of Listeria organisms inoculated was determined by colony count. Mice were inoculated intravenously through the tail vein with 0.2 ml of Listeria suspension (3 × 104 to 30 × 104 CFU/mouse). Heat-killed L. monocytogenes (HKLM) bacteria were prepared by incubating bacteria at 60°C for 60 min. Then the killed bacteria were aliquoted and stored at −80°C until use.
Survival and leukocyte counts.
For this purpose, age- and sex-matched CD18-ko and WT mice were inoculated intravenously with Listeria organisms. At different time points, as described in Results, blood was obtained by tail vein nick for leukocyte counting. After inoculation, mice were observed on a daily basis to monitor survival and assess peripheral leukocyte counts. Leukocyte counting was performed with an electronic particle counter (Coulter Counter ZM, Coulter Corporation, Miami, Fla.) according to the manufacturer's instructions.
Quantitative organ culture.
At different time points following Listeria inoculation, mice were sacrificed with CO2. The liver and spleen were removed and weighed. Portions of livers and spleens were homogenized separately into PBS containing 0.05% Triton X-100 with a tissue grinder. After homogenization, aliquots of liver and spleen suspension were serially diluted in PBS and plated on Trypticase soy agar containing 5% sheep blood. Colonies were counted after 24 h of incubation at 37°C.
Histopathology.
At different time points after Listeria infection, mice were sacrificed via CO2 euthanasia, and complete necropsies were performed. Thirty tissues were collected for histopathology. Soft tissues were fixed in 10% neutral buffered formalin. Heads were fixed and decalcified in 23% formic acid (TBD-2; Shandon Liphaw, Pittsburgh, Pa.). After 24 to 48 h of fixation, tissues were trimmed and processed routinely in paraffin, sectioned at 3 to 5 μm, and stained with hematoxylin and eosin stain. Livers, spleens, and selected tissues were also stained with Brown and Hopp's tissue gram stain and Modified Steiner's silver stain to detect bacteria (2).
Spleen cell preparation and stimulation with HKLM.
After the mice were sacrificed with CO2, spleens were removed, minced with a sharp sterile blade, and teased to a single cell suspension. Erythrocytes were lysed in the lysing buffer (0.15 M NH4Cl, 10.0 mM KHCO3, 0.1 mM Na2EDTA [pH 7.4]). Spleen cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 10 μg of streptomycin/ml and stimulated at 5 × 106 cells/ml with HKLM (5 × 107/ml) at 37°C; cells without HKLM stimulation were used as a control. After different stimulation times, the cells were collected. The cell-free supernatants were stored at −80°C for enzyme-linked immunosorbent assay, and the cells (both adherent and suspended) were lysed in RNA STAT-60 (Tel-Test, Inc., Friendswood, Tex.) for RNA isolation.
RPA for cytokine expression.
To assess mRNA cytokine levels, total RNA was isolated from spleen cells stimulated with HKLM in vitro or from livers and spleens after intravenous infection with Listeria organisms. RNase protection assay (RPA) was performed by using RPA starter kit with mouse cytokine multiprobe set mCK2b from PharMingen (San Diego, Calif.). The [α-P32]UTP-labeled RNase-protected probes were resolved on CastAway 6% precast polyacrylamide sequencing gels (Stratagene, La Jolla, Calif.) and quantified by autoradiography. The quantity of each mRNA species was determined by the intensity of the appropriately sized, protected probe fragment relative to that of housekeeping genes for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or L32.
Determination of cytokine levels in serum and splenocyte culture supernatants.
Interleukin-1β (IL-1β) and granulocyte colony-stimulating factor (G-CSF) levels in mouse serum and spleen cell culture media were measured by using Quantikine enzyme-linked immunosorbent assay kits purchased from R & D Systems (Minneapolis, Minn.).
Statistical analysis.
Differences in total mortality between WT and CD18-ko mice were determined by chi-square analysis. Kaplan-Meier curves were used to show survival over time. Daily leukocyte counts were analyzed by two-way analysis of variance and two-tailed unpaired Student's t test. Differences in quantitative organ culture and cytokine expression were analyzed by two-tailed unpaired Student's t test. Values are presented as means ± standard deviations. Differences are considered significant at a P value of <0.05.
RESULTS
Survival of CD18-ko mice after Listeria infection.
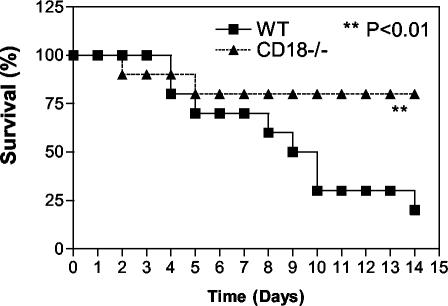
For the survival study, 10 mice in each group of WT and CD18-ko mice were inoculated with a single dose of Listeria through the tail vein and followed over a 14-day period. Eight of 10 WT mice died by 14 days postinjection (dpi). Among CD18-ko mice, however, after two early deaths (one at 2 dpi and one at 5 dpi), the remaining eight mice survived for 14 days after Listeria inoculation (Fig. 1) (P < 0.01). In addition, 10 age- and sex-matched mice deficient in CD11b were studied, and there was no mortality difference between these and WT mice (data not shown).
FIG. 1.
Survival curve after systemic infection with Listeria. WT and CD18-ko mice were inoculated with 3 × 105 CFU of Listeria organisms per mouse via tail vein injection. Significant decreased mortality was found for CD18-ko mice compared with WT mice (P < 0.01).
Peripheral leukocyte counts.
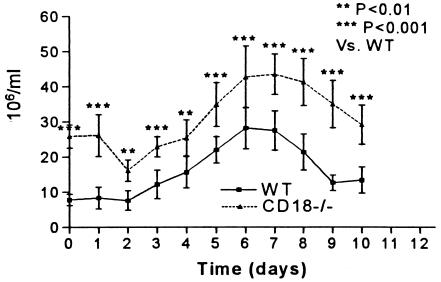
Mice deficient in CD18 had significantly higher peripheral leukocyte counts at baseline and all subsequent time points than WT mice, but both groups showed a similar pattern in the change in peripheral leukocyte counts over time (Fig. 2). At baseline, the marked leukocytosis in CD18-ko mice was primarily due to the elevated neutrophil numbers, as reported previously (30). The baseline percentage of neutrophils in peripheral leukocytes in CD18-ko mice was 59.4 ± 1.69, compared with 26.6 ± 1.56 in WT mice (P < 0.001). In CD18-ko and WT mice, total peripheral leukocyte counts were lower at 2 dpi than at baseline and then increased, with a peak at 7 dpi (Fig. 2), when the majority of leukocytes in both groups were neutrophils (74.4% ± 2.22% neutrophils in CD18-ko mice and 67.4% ± 2.01% in WT mice). After 7 dpi, leukocyte counts gradually declined.
FIG. 2.
Leukocyte counts in peripheral blood at baseline (time 0) and following systemic infection with Listeria. Peripheral leukocyte counts were significantly elevated in CD18-ko mice compared with WT controls both at baseline and at each time point following systemic Listeria infection (P < 0.01 or P < 0.001).
Bacterial burden in CD18-ko mouse livers and spleens after Listeria infection.
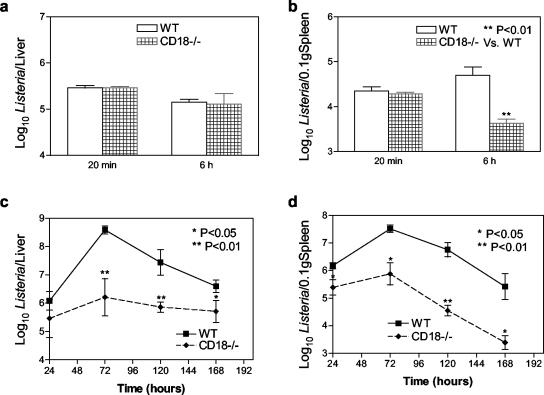
Bacterial burden in liver and spleen was assessed by colony culture at 20 min, 6 h, and 1, 3, 5, and 7 dpi. Due to the difference in spleen size between WT and CD18-ko mice (30), Listeria numbers in spleens were normalized to 0.1 g of spleens. By 20 min following inoculation, most (80 to 90%) of the Listeria organisms inoculated were trapped in livers and spleens, with the majority in livers, and no significant difference was observed between CD18-ko mice and WT mice in terms of numbers of Listeria organisms recovered from livers and spleens (Fig. 3A and B). By 6 h postinfection, Listeria numbers from livers were similar in both groups of mice (Fig. 3A), but CD18-ko spleens had lower Listeria numbers than WT spleens (Fig. 3B) (P < 0.01). Compared with numbers at 20 min postinfection, Listeria numbers at 6 h postinfection were decreased in CD18-ko spleens and were increased in WT spleens (Fig. 3B). By 3, 5, and 7 dpi, significantly fewer Listeria organisms were recovered from livers and spleens of CD18-ko mice than from WT mice (Fig. 3C and D).
FIG. 3.
Numbers of Listeria organisms recovered from livers and spleens following systemic Listeria infection (3 × 105 CFU/mouse). No significant difference was found between WT and CD18-ko mice in the numbers of Listeria organisms recovered from livers at 20 min and 6 h (a). Decreased numbers of Listeria organisms were found at 6 h in CD18-ko mouse spleens (b). At 72, 120, and 168 h, significantly reduced numbers of Listeria organisms were recovered from CD18-ko mouse livers (c) and spleens (d) compared with WT controls. Due to the difference in spleen size between WT and CD18-ko mice, numbers of Listeria organisms in spleens were normalized to 0.1 g of spleens.
mRNA levels of cytokines in CD18-ko mouse livers and spleens after Listeria inoculation in vivo.
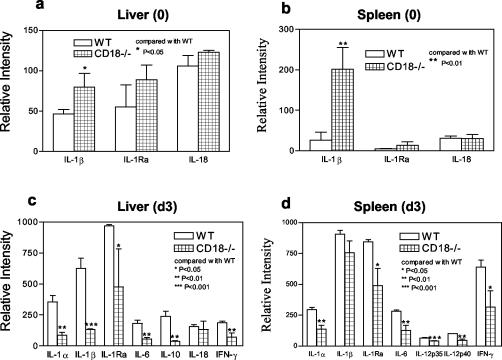
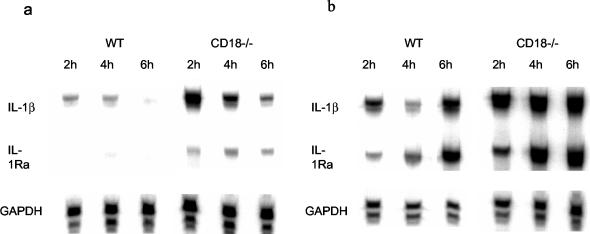
RPA was used to investigate cytokine responses to Listeria infection at the mRNA level in vivo. As shown in Fig. 4A and B, at baseline (before Listeria inoculation), CD18-ko mice expressed significantly higher levels of IL-1β mRNA in both livers and spleens than did WT mice. After Listeria challenge, both CD18-ko and WT mice showed increases in cytokine mRNA levels compared with baseline. However, at 3 dpi, CD18-ko mice had significantly lower mRNA levels of IL-1α, IL-1β, IL-1 receptor antagonist, IL-6, IL-10, and gamma interferon in livers than WT mice (Fig. 4C). At 3 dpi, the mRNA levels of IL-1α, IL-1 receptor antagonist, IL-6, IL-12p35, IL-12p40, and gamma interferon were significantly lower in spleens of CD18-ko mice than in spleens of WT mice (Fig. 4D).
FIG. 4.
Cytokine mRNA levels in livers and spleens at baseline (0) and day 3 following Listeria infection (d3) (3 × 105 CFU/mouse). Cytokine mRNA levels were detected by using RPA and quantitated as described in Materials and Methods. At baseline, CD18-ko mice showed elevated IL-1β mRNA level both in liver (0) (a) and spleen (0) (b). After Listeria infection, however, CD18-ko mice displayed reduced cytokine mRNA levels in both organs (d3) in comparison with WT.
Cytokine responses at mRNA level in CD18-ko splenocytes upon HKLM stimulation in vitro.
As described above, following Listeria inoculation, CD18-ko mice had lower bacterial burdens in livers and spleens and lower cytokine mRNA levels than did WT mice. To examine whether the lower mRNA cytokine levels in CD18-ko mice were due to a reduced response of splenocytes to Listeria, spleen cells were prepared and identically stimulated with HKLM in vitro. Unstimulated splenocytes from CD18-ko mice had higher levels of IL-1β mRNA than did unstimulated splenocytes from WT mice (Fig. 5A), consistent with the whole-organ mRNA levels (Fig. 4). After HKLM stimulation, both CD18-ko and WT mice showed increased mRNA levels of cytokines by 2, 4, and 6 h relative to levels of unstimulated splenocytes. At all time points, HKLM-stimulated splenocytes from CD18-ko mice showed higher mRNA levels for cytokines than did HKLM-stimulated splenocytes from WT mice (Fig. 5B).
FIG. 5.
Cytokine mRNA levels in splenocytes cultured in vitro. Splenocytes were cultured in vitro without (a) or with (b) HKLM stimulation for 2, 4, and 6 h. Cytokine mRNA levels were assayed as described in Materials and Methods. (a) Without stimulation, CD18-ko splenocytes displayed higher IL-1β mRNA level than WT splenocytes. The in vivo-derived increased RNA degraded quickly in vitro, as reflected by the decreasing levels of IL-1β from 2 through 6 h after culture. (b) Under the same amount of HKLM stimulation, CD18-ko splenocytes showed normal—or even increased—cytokine mRNA levels compared with WT splenocytes.
Cytokine production: protein assessment.
As shown in Table 1, consistent with cytokine mRNA expression, baseline serum IL-1β protein level was higher in CD18-ko mice (mean, 210 pg/ml; range, 95 to 410 pg/ml) than in WT mice (<8 pg/ml). After Listeria inoculation, both WT and CD18-ko mice showed an increase in IL-1β production, with a much greater increase in WT mice relative to the baseline level. The in vitro study showed that without stimulation, neither WT nor CD18-ko splenocytes produced high levels of IL-1β. With HKLM stimulation, however, both WT and CD18-ko mice showed a significant production of IL-1β, with the supernatant from CD18-ko splenocytes having a higher level of IL-1β (Table 1).
TABLE 1.
Mouse IL-1β and G-CSF levels in vivo (serum) and in vitro (cell supernatant)
| Cytokine | Mouse type | Level (pg/ml) (mean ± SD) in:
|
|||
|---|---|---|---|---|---|
| Serum at:
|
Supernatant from:
|
||||
| Baseline | Day 3 | Control cells | HKLM- stimulated cells | ||
| IL-1β | WT | <8.0 | 358 ± 84 | 15 ± 2 | 75 ± 7 |
| CD18−/− | 210 ± 100a | 291 ± 105 | 23 ± 2 | 112 ± 11b | |
| G-CSF | WT | 160 ± 44 | 3,316 ± 274 | <15 | 480 ± 56 |
| CD18−/− | 1,180 ± 145a | 2,216 ± 389 | <15 | 1,383 ± 76c | |
P < 0.01 versus WT.
P < 0.05 versus WT.
P < 0.001 versus WT.
CD18-ko mice also had higher baseline serum G-CSF (1,180 versus 160 pg/ml for WT mice). After Listeria infection, both WT and CD18-ko mice showed an increase in serum G-CSF, with a much greater (more than 20-fold) increase in WT mice relative to the baseline level. Unstimulated splenocytes did not produce detectable levels of G-CSF in either WT or CD18-ko mice. With HKLM stimulation, both WT and CD18-ko splenocytes showed a significant production of G-CSF, with CD18-ko splenocytes showing a higher level of secretion (Table 1).
Histopathology of CD18-deficient and WT mice.
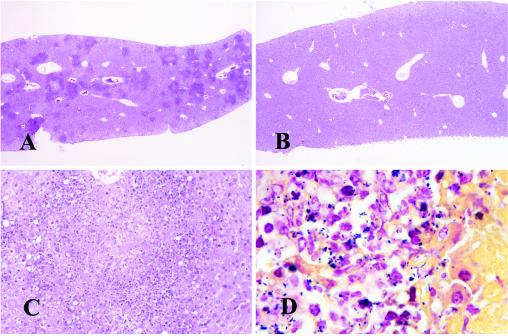
Necropsies of both CD18-ko and WT mice were performed 1, 2, 3, 5, and 7 dpi. WT mice inoculated with Listeria had multifocal, moderate to marked necrosis in splenic white pulp from day 1 to day 5 following inoculation, with marked splenic lymphoid depletion by day 3, while CD18-ko mice had much milder lesions. At 3 and 5 dpi, WT mice showed mild to moderate necrotizing hepatitis, while CD18-ko mice had much milder lesions (Fig. 6). In addition, WT mice had marked gallbladder involvement and peritonitis by 5 dpi and had thymic depletion and relative myeloid hyperplasia starting from 3 dpi. At day 7, two out of three infected WT mice died, and the only survivor had meningitis, necrotizing myocarditis, adrenalitis, lymphadenitis, and peritonitis in addition to necrotizing and inflammatory lesions in the liver and spleen. Gram stain and Modified Steiner stain demonstrated abundant intralesional Listeria in livers (Fig. 6D) and spleens (data not shown) in WT mice, and Listeria organisms were also found in gallbladders at day 5 and in heart lesions and peritonitis at day 7 (data not shown).
FIG. 6.
Mouse livers at 3 dpi with hematoxylin and eosin stain (A, B, and C) or Brown and Hopps Gram stain (D). (A) WT liver shows multifocal moderate necrotizing hepatitis. (B) CD18-ko liver is essentially normal. (C) WT liver shows focally marked necrosis and loss of hepatocytes with mild suppurative inflammation. (D) A Gram stain of WT liver shows many Listeria organisms (gram-positive bacilli) in and around an area of necrosis; many intralesional bacteria were identified by Gram and silver stain in WT livers and spleens (not shown) and were more difficult to find in livers or spleens from CD18-ko mice (not shown). Original magnifications, ×6.6 (A and B), ×66 (C), and ×40 (D).
Control CD18-ko mice inoculated with sterile saline differed from control WT mice in that CD18-ko mice consistently had neutrophilic leukocytosis and myeloid hyperplasia in bone marrow and spleen with splenomegaly, as reported previously (30). The splenomegaly in CD18-ko mice appears to be due to massive expansion of red pulp by myeloid hyperplasia, with many more neutrophils and neutrophil precursors in CD18-ko spleens than in WT spleens. After Listeria inoculation, CD18-ko mice did not have significant lesions in spleens at day 1 compared with the necrotic lesions in WT spleens. At subsequent time points from days 2 to 5, CD18-ko mice had milder necrosis and inflammation in spleens and livers than did WT mice (Fig. 6). At day 7, all three infected CD18-ko mice had only minimal to mild lesions in livers and spleens.
DISCUSSION
CD18-ko mice have a phenotype similar to that of humans with LAD I (30), and these mice have been a useful model to study LAD I and the roles of CD11/CD18 integrins in vivo. Following systemic infection with S. pneumonia, a gram-positive extracellular pathogen, increased early mortality was observed in CD18-ko mice (30) as well as in CD11a-ko and CD11b-ko mice compared with WT controls (25), indicating the important roles of CD18 and CD11/CD18 integrins in host resistance against this bacterium.
L. monocytogenes is an important natural pathogen of humans and also a useful research tool for examining protective immune responses during systemic infection of mice (3, 4, 6, 19). Previously, antibody-blocking studies suggested roles for CD18 and CD11b in hepatic neutrophil recruitment and in antilisterial host innate response (14, 29). Results seen in these studies, which used intact monoclonal antibodies, may have been confounded by monoclonal antibody receptor-induced signaling, removal of leukocytes by the reticuloendothelial system, and capping and internalization of CD11/CD18 and possible associated cell surface receptors. CD18-ko mice have neutrophilic leukocytosis, and the efficiency of neutrophil extravasation that can take place by CD18-independent mechanisms varies in different tissues and with different stimuli (1, 5, 30). In the present study, we used the systemic murine listeriosis model to investigate the roles of CD18 (and CD11b) in the innate immune response to this bacterium. Following intravenous inoculation of Listeria, decreased mortality was observed in CD18-ko mice compared with WT mice, and no mortality difference was found between CD11b-ko mice and WT mice (data not shown).
After intravenous injection, Listeria organisms are cleared rapidly from the bloodstream, and most bacteria are trapped in the liver and spleen by 10 to 20 min after inoculation (13, 38). In our study, at 20 min postinfection, no significant difference was found between CD18-ko mice and WT mice in terms of numbers of Listeria organisms recovered from livers and spleens. By 6 h, however, fewer bacteria were recovered from CD18-ko spleens than from WT spleens, and at days 3, 5, and 7 after Listeria infection, markedly fewer Listeria organisms were detected in both livers and spleens of CD18-ko mice than in those of WT mice, suggesting enhanced killing of Listeria in CD18-ko mice.
These data suggest that CD18 is not absolutely required for either clearance or killing of Listeria organisms. Resident macrophages such as Kupffer cells are postulated to play an important role in the immediate clearance of Listeria, whereas neutrophils are required to kill the trapped Listeria organisms (13, 14). The most critical of the early defenses to Listeria infection is mediated by neutrophils that rapidly accumulate in large numbers at foci of Listeria infection in livers and spleens (4, 6, 10, 11, 13, 26, 28). Both at baseline and following Listeria infection, CD18-ko mice have markedly increased peripheral leukocyte counts, which are largely due to elevated neutrophil numbers (30). Previous studies have shown that neutrophil depletion worsened mortality, so the markedly increased neutrophil number may have promoted enhanced killing of Listeria organisms in CD18-ko mice. Supporting this possibility is the recently published paper showing that CD11a-deficient mice, which have marked neutrophilia and increased levels of G-CSF, are resistant to listeriosis (22). Additionally, G-CSF contributes to host resistance against Listeria infection by both the induction of granulopoiesis, resulting in elevated neutrophil numbers, and neutrophil activation (16, 17, 31, 32). Neutrophils primed with G-CSF released more superoxide anions when stimulated with phorbol myristate acetate (31). The elevated level of G-CSF in CD18-ko mice may have primed neutrophils, thereby enhancing their ability to kill Listeria organisms (12).
In addition to neutrophils, a number of cell types are involved in the control and resolution of Listeria infection. These cells communicate by producing and responding to cytokines (2). Serum levels of IL-3 and IL-6 were previously shown to be highly elevated in CD18-ko mice (30). We presently show that the IL-1β level is also significantly increased in CD18-ko mice at baseline. The increase in these cytokine levels may prime the immune system in CD18-ko mice. Supporting this possibility is the finding that CD18-ko splenocytes produced higher levels of IL-1β and G-CSF than the splenocytes of WT mice stimulated with the same number of HKLM in vitro. The primed state of the immune system in CD18-ko mice should contribute to the reduced number of Listeria organisms after infection. Furthermore, both IL-6 and IL-1β are important for host resistance to Listeria infection (8, 27, 35). Basal elevations of IL-6 and IL-1β in CD18-ko mice may both promote enhanced innate immunity to Listeria.
After stimulation with identical amounts of HKLM in vitro, splenocytes from CD18-ko mice expressed higher cytokine levels than WT splenocytes. Thus CD18 is not essential for the cytokine response of splenocytes to Listeria stimulation. The lower cytokine mRNA levels in CD18-ko mice at day 3 after Listeria inoculation in vivo was consistent with the reduced bacterial burden and decreased inflammation observed on histopathology in CD18-deficient tissues compared with WT tissues.
The most significant histopathological changes were necrosis and inflammation in livers and spleens with splenic lymphoid depletion, which is consistent with previous reports for a murine listeriosis model (6, 20, 21). Mice deficient in CD18 showed markedly reduced lesions in both livers and spleens. Although CD18-ko mice had less inflammation and necrosis than WT mice, neutrophil infiltration and inflammation clearly occurred in the absence of CD11/CD18 integrins. Neutrophil extravasation into the peritoneal cavity induced by Listeria has been previously shown to be CD18 independent, in contrast to that induced by Salmonella enterica serovar Typhimurium, which was dependent on CD18 (5). Mice deficient in ICAM-1, one of the ligands for CD11b, showed no defect in host resistance to Listeria (34). A reduction in inflammation, in contrast to the absence of neutrophilic inflammation, may have had beneficial effects for survival, as noted previously in improved survival in mice deficient in ICAM-1 and infected with Haemophilus influenzae type b (36).
A limitation of this study is that these results do not rule out the possibility that CD11b or CD11/CD18 integrins play an important role in the innate immune response of WT mice to infection with Listeria. In addition, the CD18-deficient mice are not suitable to test the possibility that the results of previous studies that used monoclonal antibodies to CD18 or CD11b may have been confounded by effects of the antibody on leukocyte function beyond specific inhibition of CD11b or CD18. Mice deficient in CD18 do not express CD11b or CD18 on the cell surface of leukocytes, and thus monoclonal antibodies to CD11b or CD18 would not bind to leukocytes and would therefore not cause secondary effects such as signaling events, removal of leukocytes from the circulation, or capping and internalization. However, the results of these studies do show that CD11/CD18 integrins, including CD11b, are not absolutely required for an effective early host response to L. monocytogenes and that neutrophil extravasation may occur via a CD18-independent pathway after Listeria infection. We propose that the decreased mortality and reduced organ bacterial burden in CD18-ko mice may result from the compensatory changes with priming of innate immunity, as evidenced by increased levels of cytokines (G-CSF, IL-6, and IL-1β) and neutrophilic leukocytosis, which augment antilisterial defense.
Acknowledgments
We acknowledge the technical assistance of Elizabeth Priest, the helpful discussion of Yasunori Abe, and the editorial assistance of Kerrie Jara.
This work was supported by National Institutes of Health grants HL-42550 (to C.M.B. and C.W.S.) and RO1-HL62243-01 (to C.M.B.), an American Heart Association Established Investigator Award (to C.M.B.), and National Aeronautics and Space Administration grant NAG9-1253 (to C.W.S.).
Editor: F. C. Fang
REFERENCES
- 1.Bowden, R. A., Z. M. Ding, E. M. Donnachie, T. K. Petersen, L. H. Michael, C. M. Ballantyne, and A. R. Burns. 2002. Role of α4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ. Res. 90:562-569. [DOI] [PubMed] [Google Scholar]
- 2.Carson, F. 1997. Histotechnology: a self-instructional text, 2nd ed., p. 96, 189, 202. ASCP Press, Chicago, Ill.
- 3.Cellin, B. G., and C. V. Broome.1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 4.Conlan, J. W. 1999. Early host-pathogen interactions in the liver and spleen during systemic murine listeriosis: an overview. Immunobiology 201:178-187. [DOI] [PubMed] [Google Scholar]
- 5.Conlan, J. W., and R. J. North. 1994. Listeria monocytogenes, but not Salmonella typhimurium, elicits a CD18-independent mechanism of neutrophil extravasation into the murine peritoneal cavity. Infect. Immun. 62:2702-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousens, L. P., and E. J. Wing. 2000. Innate defenses in the liver during Listeria infection. Immunol. Rev. 174:150-159. [DOI] [PubMed] [Google Scholar]
- 7.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-γ receptor-deficient mice. J. Immunol. 158:5297-5304. [PubMed] [Google Scholar]
- 8.Dalrymple, S. A., L. A. Lucian, R. Slattery, T. McNeil, D. M. Aud, S. Fuchino, F. Lee, and R. Murray. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 63:2262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding, Z. M., J. E. Babensee, S. I. Simon, H. F. Lu, J. L. Perrard, D. C. Bullard, X. Y. Dai, S. K. Bromley, M. L. Dustin, M. L. Entman, C. W. Smith, and C. M. Ballantyne. 1999. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 163:5029-5038. [PubMed] [Google Scholar]
- 10.Drevets, D. A. 1997. Listeria monocytogenes infection of cultured endothelial cells stimulates neutrophil adhesion and adhesion molecule expression. J. Immunol. 158:5305-5313. [PubMed] [Google Scholar]
- 11.Ehlers, S., M. E. A. Mielke, T. Blankenstein, and H. Hahn. 1992. Kinetic analysis of cytokine gene expression in the livers of naïve and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J. Immunol. 149:3016-3022. [PubMed] [Google Scholar]
- 12.Forlow, S. B., J. R. Schurr, J. K. Kolls, G. J. Bagby, P. O. Schwarzenberger, and K. Ley. 2001. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood 98:3309-3314. [DOI] [PubMed] [Google Scholar]
- 13.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157:2514-2520. [PubMed] [Google Scholar]
- 14.Gregory, S. H., L. P. Cousens, N. van Rooijen, E. A. Dopp, T. M. Carlos, and E. J. Wing. 2002. Complementary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J. Immunol. 168:308-315. [DOI] [PubMed] [Google Scholar]
- 15.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 16.Kayashima, S., S. Tsuru, N. Hata, and M. Rokutanda. 1993. Therapeutic effect of granulocyte colony-stimulating factor (G-CSF) on the protection against Listeria infection in SCID mice. Immunology. 80:471-476. [PMC free article] [PubMed] [Google Scholar]
- 17.Lieschke, G. J., D. Grail, G. Hodgson, D. Metcalf, E. Stanley, C. Cheers, K. J. Fowler, S. Basu, Y. F. Zhan, and A. R. Dunn. 1994. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84:1737-1746. [PubMed] [Google Scholar]
- 18.Lu, H. F., C. W. Smith, J. Perrard, D. Bullard, L. P. Tang, S. B. Shappell, M. L. Entman, A. L. Beaudet, and C. M. Ballantyne. 1997. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J. Clin. Investig. 99:1340-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 20.Mandel, T. E., and C. Cheers. 1980. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect. Immun. 30:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marco, A. J., M. Domingo, M. Prates, V. Briones, M. Pumarola, and L. Dominguez. 1991. Pathogenesis of lymphoid lesions in murine experimental listeriosis. J. Comp. Pathol. 105:1-15. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto, M., M. Emoto, Y. Emoto, V. Brinkmann, I. Yoshizawa, P. Seiler, P. Aichele, E. Kita, and S. H. Kaufmann. 2003. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J. Immunol. 170:5228-5234. [DOI] [PubMed] [Google Scholar]
- 23.Noti, J. D., A. K. Johnson, and J. D. Dillon. 2000. Structural and functional characterization of the leukocyte integrin gene CD11d. Essential role of Sp1 and Sp3. J. Biol. Chem. 275:8959-8969. [DOI] [PubMed] [Google Scholar]
- 24.Prince, J. E., and C. M. Ballantyne. 1999. Adhesion molecules in cardiovascular disease. Emerg. Ther. Targets 3:263-277. [Google Scholar]
- 25.Prince, J. E., C. F. Brayton, M. C. Fossett, J. A. Durand, S. L. Kaplan, C. W. Smith, and C. M. Ballantyne. 2001. The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J. Immunol. 166:7362-7369. [DOI] [PubMed] [Google Scholar]
- 26.Rogers, H. W., and E. R. Unanue. 1993. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect. Immun. 61:5090-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers, H. W., C. S. Tripp, R. D. Schreiber, and E. R. Unanue. 1994. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J. Immunol. 153:2093-2101. [PubMed] [Google Scholar]
- 28.Rogers, H. W., M. P. Callery, B. Deck, and E. R. Unanue. 1996. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 156:679-684. [PubMed] [Google Scholar]
- 29.Rosen, H., S. Gordon, and R. J. North. 1989. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J. Exp. Med. 170:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharffetter-Kochanek, K., H. F. Lu, K. Norman, N. V. Nood, F. Munoz, S. Grabbe, M. McArthur, I. Lorenzo, S. Kaplan, K. Ley, C. W. Smith, C. A. Montgomery, S. Rich, and A. L. Beaudet. 1998. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 188:119-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serushago, B. A., Y. Yoshikai, T. Handa, M. Mitsuyama, K. Muramori, and K. Nomoto. 1992. Effect of recombinant human granulocyte colony-stimulating factor (rh G-CSF) on murine resistance against Listeria monocytogenes. Immunology 75:475-480. [PMC free article] [PubMed] [Google Scholar]
- 32.Shinomiya, N., S. Tsuru, Y. Katsura, S. Kayashima, and K. Nomoto. 1991. Enhanced resistance against Listeria monocytogenes achieved by pretreatment with granulocyte colony-stimulating factor. Infect. Immun. 59:4740-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Springer, T. A. 1990. Adhesion receptors of the immune system. Nature 346:425-434. [DOI] [PubMed] [Google Scholar]
- 34.Steinhoff, U., U. Klemm, M. Greiner, K. Bordasch, and S. H. E. Kaufmann. 1998. Altered intestinal immune system but normal antibacterial resistance in the absence of P-selectin and ICAM-1. J. Immunol. 160:6112-6120. [PubMed] [Google Scholar]
- 35.Sullivan, G. W., H. T. Carper, J. A. Sullivan, T. Murata, and G. L. Mandell. 1989. Both recombinant interleukin-1β and purified human monocyte interleukin-1 prime human neutrophils for increased oxidative activity and promote neutrophil spreading. J. Leukoc. Biol. 45:389-395. [DOI] [PubMed] [Google Scholar]
- 36.Tan, T. Q., C. W. Smith, E. P. Hawkins, E. O. Mason, Jr., and S. L. Kaplan. 1995. Hematogenous bacterial meningitis in an intercellular adhesion molecule-1-deficient infant mouse model. J. Infect. Dis. 171:342-349. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, R. W., C. M. Ballantyne, C. W. Smith, C. Montgomery, A. Bradley, W. E. O'Brien, and A. L. Beaudet. 1993. Gene targeting yields a CD18-mutant mouse for study of inflammation. J. Immunol. 151:1571-1578. [PubMed] [Google Scholar]
- 38.Wing, E. J., A. Waheed, and R. K. Shadduck. 1984. Changes in serum colony stimulating factor (CSF) and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect. Immun. 45:180-184. [DOI] [PMC free article] [PubMed] [Google Scholar]