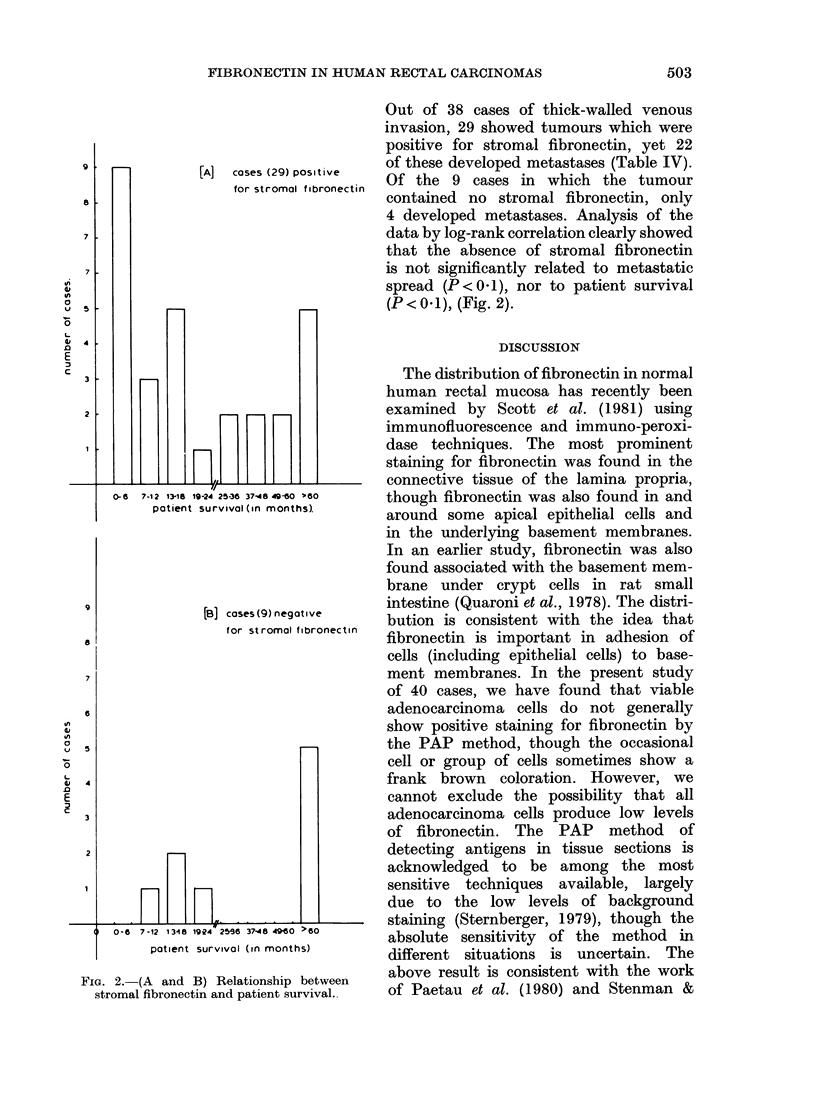
Abstract
In a retrospective study we have used an immunoperoxidase procedure to localize the glycoprotein fibronectin in human rectal carcinomas, concentrating on tumour invading thick-walled extramural veins. Fibronectin was present in 29 out of 38 cases, in connective tissue stroma, and was not in direct association with the tumour cells, except in areas of necrosis. We found no correlation between the presence or absence of stromal fibronectin and (1) the degree of cellular differentiation within the tumour, (2) tumour progression (Dukes' classification) (3) the subsequent development of metastases and (4) patient longevity. OUr results do not support the conclusions from in vitro studies (Smith et al., 1979) that the metastatic potential of carcinomas may be partly determined by the ability of tumour cells to synthesize pericellular fibronectin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., AMBROSE E. J. The surface properties of cancer cells: a review. Cancer Res. 1962 Jun;22:525–548. [PubMed] [Google Scholar]
- Brozman M. Immunohistochemical analysis of formaldehyde- and trypsin- or pepsin-treated material. Acta Histochem. 1978;63(2):251–260. doi: 10.1016/S0065-1281(78)80032-4. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis R. A., Sternberger L. A., Mann R. B., Banks P. M., Nakane P. K. Immunoperoxidase technics in diagnostic pathology. Report of a workshop sponsored by the National Cancer Institute. Am J Clin Pathol. 1979 May;71(5):483–488. doi: 10.1093/ajcp/71.5.483. [DOI] [PubMed] [Google Scholar]
- Der C. J., Stanbridge E. J. Alterations in the extracellular matrix organization associated with the reexpression of tumorigenicity in human cell hybrids. Int J Cancer. 1980 Oct 15;26(4):451–459. doi: 10.1002/ijc.2910260410. [DOI] [PubMed] [Google Scholar]
- Der C. J., Stanbridge E. J. Lack of correlation between the decreased expression of cell surface LETS protein and tumorigenicity in human cell hybrids. Cell. 1978 Dec;15(4):1241–1251. doi: 10.1016/0092-8674(78)90050-8. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., McDougall J. K., Chen L. B. Malignant behaviour of three adenovirus-2-transformed brain cell lines and their methyl cellulose-selected sub-clones. Int J Cancer. 1979 Oct 15;24(4):477–484. doi: 10.1002/ijc.2910240416. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Ali I. U., Destree A. T., Mautner V., Perkins M. E., Senger D. R., Wagner D. D., Smith K. K. A large glycoprotein lost from the surfaces of transformed cells. Ann N Y Acad Sci. 1978 Jun 20;312:317–342. doi: 10.1111/j.1749-6632.1978.tb16811.x. [DOI] [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani G. W., Parsons J. A., Erlandsen S. L. Versatility of Staphylococcus aureus protein A in immunocytochemistry. Use in unlabeled antibody enzyme system and fluorescent methods. J Histochem Cytochem. 1979 Nov;27(11):1438–1444. doi: 10.1177/27.11.390040. [DOI] [PubMed] [Google Scholar]
- Paetau A., Mellström K., Vaheri A., Haltia M. Distribution of a major connective tissue protein, fibronectin, in normal and neoplastic human nervous tissue. Acta Neuropathol. 1980;51(1):47–51. doi: 10.1007/BF00688849. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I., Schlessinger J., Yamada K. M. Fibronectin delays the fusion of L6 myoblasts. Exp Cell Res. 1979 Sep;122(2):317–326. doi: 10.1016/0014-4827(79)90308-2. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J., Ruoslahti E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5548–5552. doi: 10.1073/pnas.75.11.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Gregory T. J., Blumenstock F. A. Circulating immunoreactive and bioassayable opsonic plasma fibronectin during experimental tumour growth. Br J Cancer. 1980 Jun;41(6):956–965. doi: 10.1038/bjc.1980.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. L., Morris C. J., Blake A. E., Low-Beer T. S., Walton K. W. Distribution of fibronectin in the rectal mucosa. J Clin Pathol. 1981 Jul;34(7):749–758. doi: 10.1136/jcp.34.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Riggs J. L., Mosesson M. W. Production of fibronectin by human epithelial cells in culture. Cancer Res. 1979 Oct;39(10):4138–4144. [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Fibronectin in human solid tumors. Int J Cancer. 1981;27(4):427–435. doi: 10.1002/ijc.2910270403. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Talbot I. C., Ritchie S., Leighton M. H., Hughes A. O., Bussey H. J., Morson B. C. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980 Jun;67(6):439–442. doi: 10.1002/bjs.1800670619. [DOI] [PubMed] [Google Scholar]
- Talbot I. C., Ritchie S., Leighton M., Hughes A. O., Bussey H. J., Morson B. C. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981 Mar;5(2):141–163. doi: 10.1111/j.1365-2559.1981.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Gospodarowicz D. Respective roles of laminin and fibronectin in adhesion of human carcinoma and sarcoma cells. Nature. 1981 Jan 22;289(5795):304–306. doi: 10.1038/289304a0. [DOI] [PubMed] [Google Scholar]
- West C. M., Lanza R., Rosenbloom J., Lowe M., Holtzer H., Avdalovic N. Fibronectin alters the phenotypic properties of cultured chick embryo chondroblasts. Cell. 1979 Jul;17(3):491–501. doi: 10.1016/0092-8674(79)90257-5. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Immunological characterization of a major transformation-sensitive fibroblast cell surface glycoprotein. Localization, redistribution, and role in cell shape. J Cell Biol. 1978 Aug;78(2):520–541. doi: 10.1083/jcb.78.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]




