Abstract
Malignant effusions and tumour tissue obtained at surgery provided material for a study of the prognostic value of the various inflammatory cells in the prognosis of human ovarian cancer. Ascitic fluids predominantly contained inflammatory cells; tumour cells, both singly and in clusters, were a minor component. Tumour cells were usually in excess in dispersed solid material. Some patients had significant proportions of lymphocytes and macrophages in their solid tumour, and these patients invariably responded to therapy. Sedimentation-velocity separation at unit gravity provided tow populations of inflammatory cells. One consisted of mononuclear cells similar in size to those in the patients blood: the other consisted of one or more large macrophage populations, distinct in morphology and enzymatic markers from both blood monocytes and each other. T lymphocytes were enriched in ascites fractions (78%) and in the tumour-derived mononuclear fraction (71%) compared to patient blood (60%). The T-cell subset characterized by ANAE reactivity was markedly depleted in the tumour-infiltrating fraction (17%) compared to patient blood (62%) or patient blood (51%). Esterase-positive monocyte-like cells were more frequent in the tumour-infiltrating fraction (17%) than ascites (7%) or blood (12%). B lymphocytes were infrequent in solid tumours and difficult to assess in ascites. Histiocyte-like macrophages were present in the higher-velocity tumour-cell containing fractions of both solid and ascitic material. The variation in infiltrating cells between patients and between tumour and ascites of the same individual was marked.
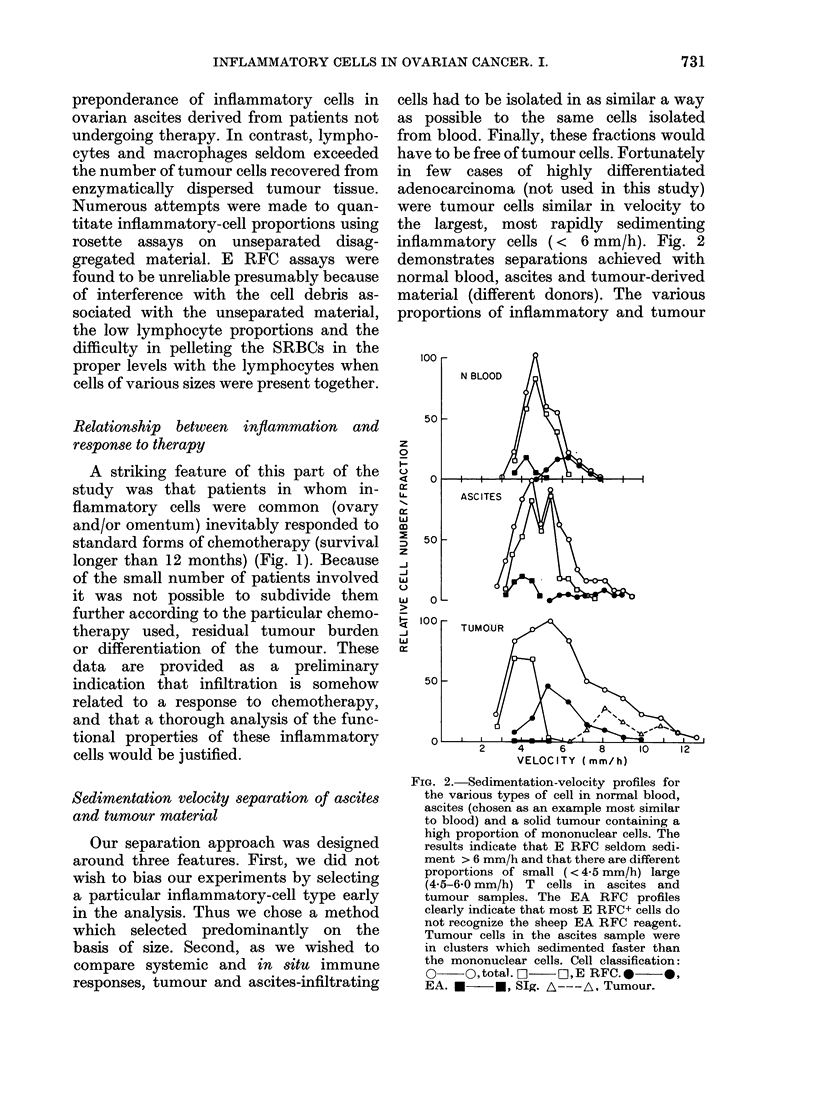
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTONE M. S. Histochemical demonstration of acid phosphatase activity in osteoclasts. J Histochem Cytochem. 1959 Jan;7(1):39–41. doi: 10.1177/7.1.39. [DOI] [PubMed] [Google Scholar]
- Becker S., Haskill S. Characterization of the presumptive sarcoma cells in primary MSV tumors. Int J Cancer. 1980 Apr 15;25(4):543–550. doi: 10.1002/ijc.2910250417. [DOI] [PubMed] [Google Scholar]
- Becker S., Haskill S. Non-T-cell-mediated cytotoxicity in MSV tumor-bearing mice. III. Macrophage-mediated cytotoxicity against autochthonous MSV tumor-isolated target cells. Int J Cancer. 1980 Apr 15;25(4):535–541. doi: 10.1002/ijc.2910250416. [DOI] [PubMed] [Google Scholar]
- Bevan A., Burns G. F., Gray L., Cawley J. C. Cytochemistry of human T-cell subpopulations. Scand J Immunol. 1980;11(2):223–233. doi: 10.1111/j.1365-3083.1980.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Haskill J. S., Key M., Radov L. A., Parthenais E., Korn J. H., Fett J. W., Yamamura Y., DeLustro F., Vesley J., Gant G. The importance of antibody and macrophages in spontaneous and drug-induced regression of the T1699 mammary adenocarcinoma. J Reticuloendothel Soc. 1979 Oct;26(4):417–425. [PubMed] [Google Scholar]
- Haskill S., Koren H., Becker S., Fowler W., Walton L. Mononuclear-cell infiltration in ovarian cancer. II. Immune function of tumour and ascites-derived inflammatory cells. Br J Cancer. 1982 May;45(5):737–746. doi: 10.1038/bjc.1982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häyry P., Tötterman T. H. Cytological and functional analysis of inflammatory infiltrates in human malignant tumors. I. Composition of the inflammatory infiltrates. Eur J Immunol. 1978 Dec;8(12):866–871. doi: 10.1002/eji.1830081208. [DOI] [PubMed] [Google Scholar]
- Ioachim H. L. The stromal reaction of tumors: an expression of immune surveillance. J Natl Cancer Inst. 1976 Sep;57(3):465–475. doi: 10.1093/jnci/57.3.465. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Korn J. H., Haskill J. S., Holden H. T., Radov L. A., Ritter F. L. In situ Fc receptor-bearing cells in two murine tumors. I. Isolation and identification. J Natl Cancer Inst. 1978 Jun;60(6):1387–1390. doi: 10.1093/jnci/60.6.1387. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sessa C., Bolis G., Mangioni C. Natural killer activity of lymphoid cells isolated from human ascitic ovarian tumors. Int J Cancer. 1980 May 15;25(5):573–582. doi: 10.1002/ijc.2910250505. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Peri G., Polentarutti N., Bolis G., Mangioni C., Spreafico F. Effects on in vitro tumor growth of macrophages isolated from human ascitic ovarian tumors. Int J Cancer. 1979 Feb;23(2):157–164. doi: 10.1002/ijc.2910230204. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Polentarutti N., Peri G., Shavit Z. B., Vecchi A., Bolis G., Mangioni C. Cytotoxicity on tumor cells of peripheral blood monocytes and tumor-associated macrophages in patients with ascites ovarian tumors. J Natl Cancer Inst. 1980 Jun;64(6):1307–1315. doi: 10.1093/jnci/64.6.1307. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Moretta A., Cooper M. D. Human T lymphocyte subpopulations: studies of the mechanism by which T cells bearing Fc receptors for IgG suppress T-dependent B cell differentiation induced by pokeweed mitogen. J Immunol. 1979 Mar;122(3):984–990. [PubMed] [Google Scholar]
- Tötterman T. H., Parthenais E., Häyry P., Timonen T., Saksela E. Cytological and functional analysis of inflammatory infiltrates in human malignant tumors. III. Further functional investigations using cultured autochthonous tumor cell lines and freeze-thawed infiltrating inflammatory cells. Cell Immunol. 1980 Sep 15;55(1):219–226. doi: 10.1016/0008-8749(80)90153-7. [DOI] [PubMed] [Google Scholar]
- Underwood J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974 Dec;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkmeister J. A., Pihl E., Nind A. P., Flannery G. R., Nairn R. C. Immunoreactivity by intrinsic lymphoid cells in colorectal carcinoma. Br J Cancer. 1979 Dec;40(6):839–847. doi: 10.1038/bjc.1979.274. [DOI] [PMC free article] [PubMed] [Google Scholar]



