Abstract
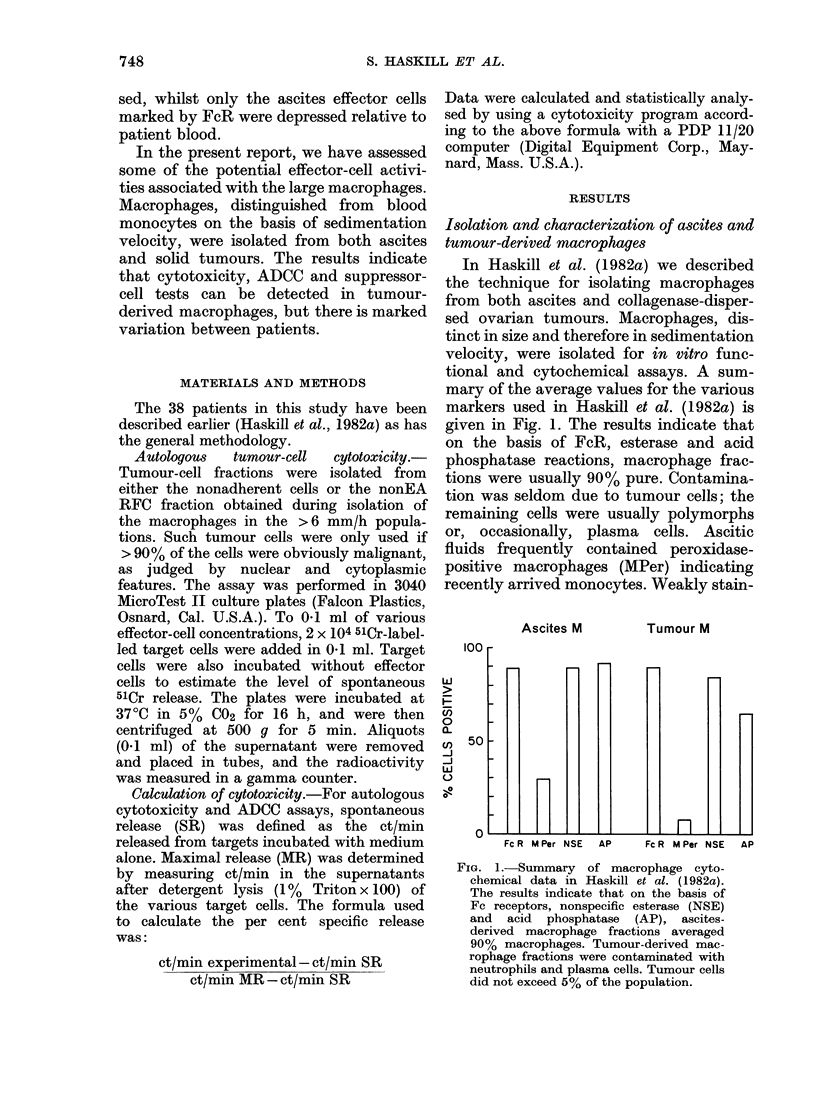
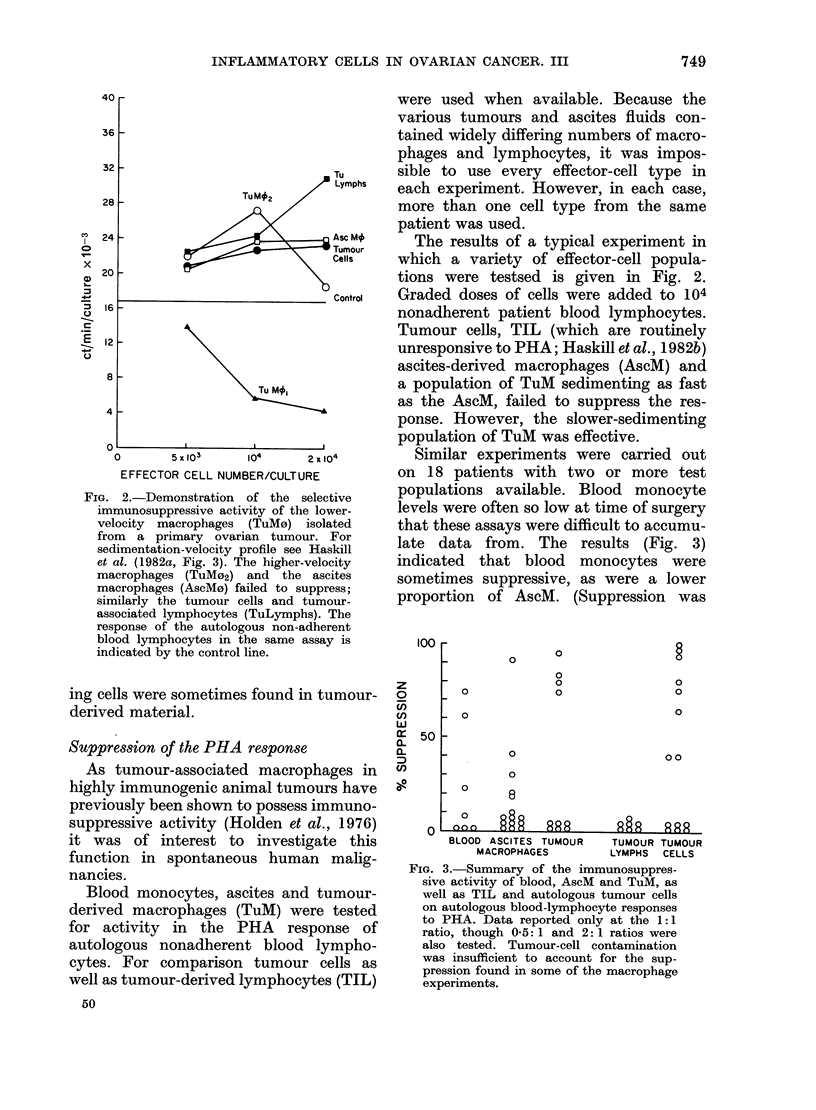
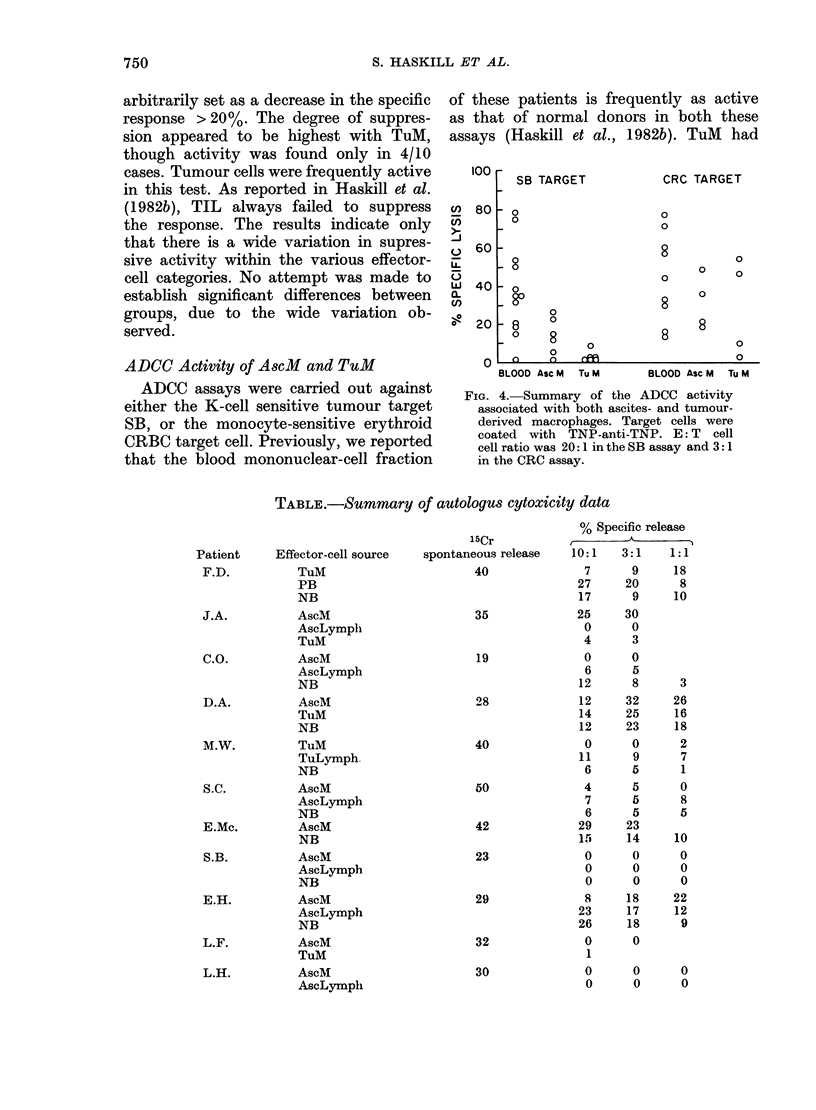
Macrophages have been isolated from ascitic and collagenase-dispersed tumours from patients undergoing surgery for ovarian cancer. Macrophages were present in varying proportions in both sites, though the ration of macrophages to tumour cells was higher in ascites. Marked variation in size (as detected by sedimentation velocity) and cytochemical markers in the macrophages was noted. Highly enriched macrophage fractions were isolated from the ascites and collagenase-dispersed solid tumours by a combination of sedimentation velocity and selective EA RFC or adherence techniques. Suppressor activity in the PHA assay was detected in tumour macrophages (4/10 giving less than 50% inhibition), ascitic macrophages (1/15) and blood monocytes (2/7). Lymphocyte fractions from tumours were unresponsive to PHA and failed to suppress the blood response. Suppressor activity was also present in the purified tumour-cell fraction of 6/14 patients. ADCC activity was tested in a few patients. When the activity was determined against the SB target cells, tumour-derived macrophages were inactive, whereas the ascitic fraction showed low but significant activity which averaged much lower than patients blood values. The ADCC assays carried out with the CRC target cell indicated activity within the range of patient blood values in 4/4 ascites and 2/4 tumour macrophage fractions. Cytotoxicity was also assessed against co-purified autologous tumour cells. Although activity was detected in many of the tests, the results seemed to reflect target cell sensitivity. There appeared to be a correlation between cytotoxicity with test macrophages and normal blood mononuclear cells. The results indicate that the cytochemical heterogeneity and the variation in size between macrophage fractions is associated with a spectrum of activities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker S., Haskill S. Non-T-cell-mediated cytotoxicity in MSV tumor-bearing mice. III. Macrophage-mediated cytotoxicity against autochthonous MSV tumor-isolated target cells. Int J Cancer. 1980 Apr 15;25(4):535–541. doi: 10.1002/ijc.2910250416. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Elgert K. D. Inhibition of mitogen and immune blastogenesis by two distinct populations of supressor cells present in the spleens of fibrosarcoma-bearing mice: adoptive transfer of suppression. Int J Cancer. 1978 Aug 15;22(2):142–151. doi: 10.1002/ijc.2910220207. [DOI] [PubMed] [Google Scholar]
- Glaser M., Kirchner H., Herberman R. B. Inhibition of in vitro lymphoproliferative responses to tumor-associated antigens by suppressor cells from rats bearing progressively growing Gross leukemia virus-induced tumors. Int J Cancer. 1975 Sep 15;16(3):384–393. doi: 10.1002/ijc.2910160305. [DOI] [PubMed] [Google Scholar]
- Haskill J. S., Häyry P., Radov L. A. Systemic and local immunity in allograft and cancer rejection. Contemp Top Immunobiol. 1978;8:107–170. doi: 10.1007/978-1-4684-0922-2_5. [DOI] [PubMed] [Google Scholar]
- Haskill S., Becker S., Fowler W., Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br J Cancer. 1982 May;45(5):728–736. doi: 10.1038/bjc.1982.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Koren H., Becker S., Fowler W., Walton L. Mononuclear-cell infiltration in ovarian cancer. II. Immune function of tumour and ascites-derived inflammatory cells. Br J Cancer. 1982 May;45(5):737–746. doi: 10.1038/bjc.1982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden H. T., Haskill J. S., Kirchner H., Herberman R. B. Two functionally distinct anti-tumor effector cells isolated from primary murine sarcoma virus-induced tumors. J Immunol. 1976 Aug;117(2):440–446. [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G. L., McCoy J. L., Herberman R. B. Role of suppressor cells in depression of in vitro lymphoproliferative responses of lung cancer and breast cancer patients. J Natl Cancer Inst. 1978 Oct;61(4):1001–1009. [PubMed] [Google Scholar]
- Key M., Haskill S. Macrophage-mediated, antibody-dependent destruction of tumor cells in DBA/2 mice: in vitro identification of an in situ mechanism. J Natl Cancer Inst. 1981 Jan;66(1):103–110. [PubMed] [Google Scholar]
- Kirchner H. Suppressor cells of immune reactivity in malignancy. Eur J Cancer. 1978 May;14(5):453–459. doi: 10.1016/0014-2964(78)90246-3. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sessa C., Bolis G., Mangioni C. Natural killer activity of lymphoid cells isolated from human ascitic ovarian tumors. Int J Cancer. 1980 May 15;25(5):573–582. doi: 10.1002/ijc.2910250505. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Polentarutti N., Peri G., Shavit Z. B., Vecchi A., Bolis G., Mangioni C. Cytotoxicity on tumor cells of peripheral blood monocytes and tumor-associated macrophages in patients with ascites ovarian tumors. J Natl Cancer Inst. 1980 Jun;64(6):1307–1315. doi: 10.1093/jnci/64.6.1307. [DOI] [PubMed] [Google Scholar]
- Parthenais E., Haskill S. Nonspecific T-cell reactivity in mice bearing autochthonous tumors or early-generation transplanted spontaneous mammary tumors. J Natl Cancer Inst. 1979 Jun;62(6):1569–1574. [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., Pace J. L. Evidence for mononuclear phagocytes in solid neoplasms and appraisal of their nonspecific cytotoxic capabilities. Contemp Top Immunobiol. 1980;10:143–166. doi: 10.1007/978-1-4684-3677-8_6. [DOI] [PubMed] [Google Scholar]
- Tötterman T. H., Parthenais E., Häyry P., Timonen T., Saksela E. Cytological and functional analysis of inflammatory infiltrates in human malignant tumors. III. Further functional investigations using cultured autochthonous tumor cell lines and freeze-thawed infiltrating inflammatory cells. Cell Immunol. 1980 Sep 15;55(1):219–226. doi: 10.1016/0008-8749(80)90153-7. [DOI] [PubMed] [Google Scholar]
- Vose B. M., Vánky F., Klein E. Human tumour--lymphocyte interaction in vitro. V. Comparison of the reactivity of tumour-infiltrating, blood and lymph-node lymphocytes with autologous tumour cells. Int J Cancer. 1977 Dec 15;20(6):895–902. doi: 10.1002/ijc.2910200612. [DOI] [PubMed] [Google Scholar]
- Werkmeister J. A., Pihl E., Nind A. P., Flannery G. R., Nairn R. C. Immunoreactivity by intrinsic lymphoid cells in colorectal carcinoma. Br J Cancer. 1979 Dec;40(6):839–847. doi: 10.1038/bjc.1979.274. [DOI] [PMC free article] [PubMed] [Google Scholar]



