Abstract
Haemophilus ducreyi previously has been shown to inhibit the phagocytosis of both secondary targets and itself by certain cells in vitro. Wild-type H. ducreyi strain 35000HP contains two genes, lspA1 and lspA2, whose encoded protein products are predicted to be 456 and 543 kDa, respectively. An isogenic mutant of H. ducreyi 35000HP with inactivated lspA1 and lspA2 genes has been shown to exhibit substantially decreased virulence in the temperature-dependent rabbit model for chancroid. This lspA1 lspA2 mutant was tested for its ability to inhibit phagocytosis of immunoglobulin G-opsonized particles by differentiated HL-60 and U-937 cells and by J774A.1 cells. The wild-type strain H. ducreyi 35000HP readily inhibited phagocytosis, whereas the lspA1 lspA2 mutant was unable to inhibit phagocytosis. Similarly, the wild-type strain was resistant to phagocytosis, whereas the lspA1 lspA2 mutant was readily engulfed by phagocytes. This inhibitory effect of wild-type H. ducreyi on phagocytic activity was primarily associated with live bacterial cells but could also be found, under certain conditions, in concentrated H. ducreyi culture supernatant fluids that lacked detectable outer membrane fragments. Both the wild-type strain and the lspA1 lspA2 mutant attached to phagocytes at similar levels. These results indicate that the LspA1 and LspA2 proteins of H. ducreyi are involved, directly or indirectly, in the antiphagocytic activity of this pathogen, and they provide a possible explanation for the greatly reduced virulence of the lspA1 lspA2 mutant.
Haemophilus ducreyi is a gram-negative, unencapsulated bacillus that is a strict human pathogen. This bacterium is the etiologic agent of chancroid, a sexually transmitted, genital ulcer disease that is quite prevalent in certain developing countries (reviewed in references 11, 49, and 55). Chancroid is very uncommon in the United States (45), although there are suggestions that it is underreported (36).
Information about the bacterial gene products essential to the pathogenesis of chancroid is limited at best (49). A number of H. ducreyi antigens have been suggested to be involved in the ability of this organism to produce disease, and these include both cell-associated and extracellular proteins. This organism synthesizes at least two toxins (39, 40), as well as a copper-zinc superoxide dismutase (43, 44), that could be involved in virulence expression. A number of outer membrane proteins (19, 20, 50, 51) as well as lipooligosaccharide (13, 15, 16, 22, 24, 52, 53) have been proposed as possible virulence factors. To date, however, only three outer membrane antigens have been shown to be essential to the ability of H. ducreyi to produce an infection in human volunteers. These include the peptidoglycan-associated lipoprotein (23), the hemoglobin-binding outer membrane protein HgbA (6), and the DsrA protein, which is involved in serum resistance (12, 20).
In addition to these specific gene products, several functional attributes of H. ducreyi have been proposed to be related to disease production. This organism has been shown to attach to extracellular matrix components in vitro, including type I and type III collagen, fibronectin, and laminin (9), and to colocalize with collagen and fibrin in infected sites in human volunteers (8). Several studies have indicated that H. ducreyi can attach to or invade human epithelial cell lines, fibroblasts (2, 32, 33, 54), or keratinocytes (14, 24, 29) in vitro, although a recent study in the human model for experimental chancroid indicated that, at least through the pustular stage of disease, H. ducreyi apparently remains extracellular (8). It has been suggested that H. ducreyi survives in vivo by resisting phagocytic killing (49), and two other laboratories recently confirmed that H. ducreyi can resist phagocytosis in vitro (1, 58).
Our laboratory recently reported that mutations in the H. ducreyi lspA1 and lspA2 genes result in much-reduced virulence of this organism in the temperature-dependent rabbit model for experimental chancroid (56). These two very large open reading frames encode proteins whose function was unknown but which can be detected as 260-kDa macromolecules in H. ducreyi culture supernatant fluid by Western blot analysis (57). In the present study, we show that mutations in these two genes eliminate the ability of H. ducreyi to inhibit the phagocytic activity of granulocyte- and macrophage-like cell lines in vitro. Moreover, we show that this LspA1- and LspA2-dependent ability to inhibit phagocytic activity is associated with intact bacterial cells and, under certain conditions, can be detected in concentrated H. ducreyi culture supernatant fluid.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. ducreyi strain 35000 (27) and its human-passaged variant 35000HP (7) have been previously described. The relevant mutants constructed in the present study and in previous studies are listed in Table 1. H. ducreyi strains were cultivated on chocolate agar plates (41) or in Columbia broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.1% (wt/vol) Trizma base (Sigma, St. Louis, Mo.), hemin (25 μg/ml), 1% (vol/vol) IsoVitaleX (Becton Dickinson, Cockeysville, Md.) and 2.5% (vol/vol) heat-inactivated fetal bovine serum (FBS; HyClone, Logan, Utah). Agar-solidified media were incubated at 33°C in a humidified atmosphere of 95% air-5% CO2, whereas liquid cultures were incubated at the same temperature in a water bath with agitation at 150 rpm. When necessary, chloramphenicol (1 μg/ml), kanamycin (30 μg/ml), or dihydrostreptomycin sulfate (100 μg/ml) was added to culture media.
TABLE 1.
H. ducreyi strains used in this study
| H. ducreyi strain | Description | Reference or source |
|---|---|---|
| 35000 | Wild-type strain | 27 |
| 35000HP | Human-passaged variant of strain 35000 | 7 |
| 35000HP.1 | lspA1 mutant; kanr | 56 |
| 35000HP.2 | lspA2 mutant; chlorr | 56 |
| 35000HP.12 | lspA1 lspA2 mutant; kanr chlorr | 56 |
| FX517 | dsrA mutant of 35000; chlorr | 20 |
| RO18 | Wild-type strain | 41 |
| Cha-I | Wild-type strain | 28 |
| 35000.88 | Isogenic lspB mutant; chlorr | Ward et al., submitted for publication |
| 35000.88(pCW225) | 35000.88 containing the wild-type lspB gene on a plasmid | Ward et al., submitted for publicatoin |
| 35000.38 | Isogenic cdtABC hhdAB deletion mutant; chlorr kanr | 56 |
Growth and differentiation of mammalian cells in tissue culture.
Three different eukaryotic cell lines were used in this study. The human promyelocytic leukemia cell line HL-60 (CCL-240; American Type Culture Collection, Manassas, Va.) was cultured in RPMI 1640 (BioWhittaker, Walkersville, Md.) supplemented with 4 mM GlutaMAX (Gibco-BRL, Rockville, Md.) and 20% (vol/vol) heat-inactivated FBS at 37°C in a humidified atmosphere of 95% air-5% CO2. These cells were differentiated into granulocytes by culturing in the same medium supplemented with 100 mM N,N-dimethylformamide (Sigma) for a period of 5 days as described previously (34, 42). HL-60 cells were harvested by centrifugation at 160 × g for 10 min and suspended in RPMI containing 20% (vol/vol) heat-inactivated FBS (RPMI-F) at a concentration of 108 cells/ml.
The human macrophage-like U-937 cell line (CRL-1593.2; American Type Culture Collection) was cultured in RPMI 1640 supplemented with 2 mM GlutaMAX, 10% (vol/vol) heat-inactivated FBS, 10 mM HEPES, and 1 mM sodium pyruvate. To induce differentiation and adherence, U-937 cells were seeded in 96-well tissue culture plates (Falcon; Becton Dickinson, Franklin Lakes, N.J.) or chamber slides (Nunc; Nalge Nunc International, Naperville, Ill.) at a density of 2 × 105 cells per well in culture medium containing 100 ng of phorbol myristate acetate/ml (Sigma). After 48 h, the nonadherent cells were removed by aspiration and the adherent U-937 cells were incubated overnight in medium lacking phorbol myristate acetate. Immediately prior to use, these cells were washed twice with RPMI-F.
The mouse monocyte-macrophage cell line J774A.1 (TIB-67; American Type Culture Collection) was cultured in Dulbecco's modified Eagle's medium (BioWhittaker) supplemented with 4 mM GlutaMAX, 10% (vol/vol) heat-inactivated FBS, and 1 mM sodium pyruvate. Twenty hours before infection, the cells were allowed to adhere to chamber slides (2 × 105 cells/well) or glass coverslips (1 × 105 cells/well) placed in 24-well tissue culture plates. The next day, nonadherent cells were removed by washing twice with RPMI-F. Careful attention was paid to the passage level of all cell lines. The cells were used only at low passage level, and they were kept in culture for less than 1 month. The HL-60 and U-937 cell lines were used primarily for experiments involving quantitative measurement of phagocytic activity. The J774A.1 cell line has certain properties (i.e., tenacious adherence to glass or plastic) that facilitate obtaining detailed images (i.e., photomicrographs or fluorescent micrographs) of these cells.
Opsonization of fluorescent microspheres.
Green fluorescent or nonfluorescent streptavidin-coated microspheres (Bangs Laboratories, Inc., Fishers, Ind.) with a nominal diameter of 0.56 μm were used as the secondary target in some phagocytosis assays. After washing twice with a 10-fold volume of phosphate-buffered saline (PBS), microspheres (0.5 mg/ml) were opsonized with monospecific rabbit streptavidin antiserum (Rockland Immunochemicals, Gilbertsville, Pa.) for 30 min at room temperature with gentle mixing, following the manufacturer's instructions. These particles were then washed twice and suspended in PBS to a concentration of 0.5 mg/ml (≈5 × 109 particles/ml).
Phagocytosis assays using microspheres as a secondary target.
Bacteria grown for 15 h in liquid culture were used in these assays. These H. ducreyi cells were harvested by centrifugation for 10 min at 6,500 × g and suspended in RPMI-F to an optical density at 600 nm (OD600) of 0.5 (for all H. ducreyi wild-type and mutant strains except 35000HP.1 and 35000HP.12) or to an OD600 of 0.7 (for strains 35000HP.1 and 35000HP.12). The use of these two different suspensions was the result of the tendency of the latter two strains to agglutinate very rapidly. Escherichia coli HB101 was suspended to an OD600 of 0.23. For each strain, these particular suspensions were determined to be equivalent to ∼5 × 107 CFU/ml.
A 100-μl volume of bacterial suspension (∼5 × 106 CFU) was added to each well of a 96-well tissue culture plate containing 5 μl (5 × 105 cells) of differentiated HL-60 cells per well; the multiplicity of infection (MOI) was approximately 10. Similarly, 60 μl of bacterial suspension (∼3 × 106 CFU) was added to U-937 macrophages (2 × 105 cells), and 100 μl of bacterial suspension (∼5 × 106 CFU) was added to J774A.1 macrophages (2 × 105 cells) to obtain MOIs of approximately 15 and 25, respectively. These tissue culture plates were then incubated at 33°C for 1 h. A 10-μl volume of opsonized or nonopsonized fluorescent microspheres (∼5 × 107 particles) was then added to each well. In some experiments, 10 μl of normal rabbit serum (NRS) was included. In assays using HL-60 cells (which are nonadherent), the samples were incubated at 37°C for 1 h with gentle shaking. In assays using U-937 or J774A.1 cells, the tissue culture plates were centrifuged for 2 min at 150 × g followed by incubation of the samples for 1 h (U-937) or 40 min (J774A.1) at 37°C. The phagocytosis reaction was stopped by placing the tissue culture plates on crushed ice.
To remove unbound microspheres and bacteria, the samples involving HL-60 cells were transferred to microcentrifuge tubes and washed four times (130 × g; 7 min) with PBS containing 1% (wt/vol) bovine serum albumin. The washed cells were then suspended in 100 μl of PBS and centrifuged to the bottom of the wells of 96-well microtiter plates at 160 × g for 5 min. The samples involving adherent phagocytes (U-937 and J774A.1) were washed four times with PBS and viewed by fluorescence microscopy. The percentage of phagocytic cells containing fluorescent microspheres was determined by counting at least 100 cells in each sample.
In some experiments, heat-killed (65°C, 90 min) bacteria or live bacteria separated from the phagocytes by filter supports with a 0.2-μm-pore-size filter (Nunc) were incubated with the HL-60 or U-937 cells. In addition, unconcentrated and 40-fold-concentrated supernatant fluids from 15-h H. ducreyi cultures were tested for their ability to inhibit the phagocytosis of microspheres. Concentration of culture supernatant fluids was performed as previously described (57).
Phagocytosis assays using red blood cells as a secondary target.
These assays were performed using J774A.1 cells attached to glass coverslips in 24-well plates (105 cells/well) in tissue culture medium without FBS. The cells were incubated for 1 h at 33°C with H. ducreyi cells (MOI = 25). Sheep erythrocytes opsonized with rabbit immunoglobulin G (EIgG) were added to each well and incubated for 30 min at 37°C as described elsewhere (25). To measure ingestion, extracellular erythrocytes were lysed with 0.2% saline for 1 min. The cells were fixed with 2% glutaraldehyde for 10 min at room temperature and viewed by light microscopy. For each well, 100 cells were counted from at least five separate high-power fields that were selected at random. This experiment was performed twice.
Assay for direct phagocytosis of H. ducreyi.
Bacteria containing the green fluorescent protein-expressing plasmid pRB157K (R. J. Blick and E. J. Hansen, unpublished data) were grown for 15 h in liquid culture and then centrifuged at 6,500 × g for 10 min. Strains 35000HP(pRB157K) and 35000.88(pRB157K) were suspended to an OD600 of 0.2, and strain 35000HP.12(pRB157K) was suspended to an OD600 of 0.25 to obtain suspensions containing approximately 107 CFU/ml. A 900-μl portion of these bacteria was mixed with 100 μl of rabbit antiserum to H. ducreyi 35000, and this suspension was incubated for 30 min at room temperature. This suspension was then subjected to centrifugation, and the bacterial pellet was resuspended in 900 μl of RPMI-F. A 200-μl portion of these opsonized bacteria (2 × 106 CFU) was added to HL-60 or J774A.1 cells (2 × 105 cells; MOI = 10). Bacteria were incubated with HL-60 cells for 15 min at 33°C followed by phagocytosis at 37°C for 1 h with gentle agitation. With J774A.1 cells, the tissue culture plate was subjected to centrifugation at 150 × g for 2 min, and then phagocytosis was allowed to proceed at 37°C for 40 min.
Differential staining of intracellular and extracellular bacteria and microspheres.
To determine whether phagocyte-associated bacteria and microspheres were localized intracellularly or extracellularly, a differential staining method was used (26). Bacteria and microspheres were opsonized as described above using rabbit antiserum to H. ducreyi and streptavidin, respectively. To stain extracellular targets, the phagocytosis samples were covered for 1 h at room temperature with Alexa Fluor 568-conjugated goat anti-rabbit IgG (10 μg/ml; Molecular Probes). After four washes with PBS, the samples were fixed with 3.7% (vol/vol) formaldehyde and permeabilized by treatment with ice-cold methanol for 90 s. The samples were then covered for 1 h at room temperature with Alexa Fluor 488-conjugated goat anti-rabbit IgG (20 μg/ml; Molecular Probes) to stain both intracellular and extracellular targets. After another washing, the slides were mounted in Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, Calif.) and examined by fluorescence microscopy. Extracellular bacteria and microspheres stained with both Alexa Fluor 568 (red) and 488 (green), whereas ingested bacteria and microspheres stained with Alexa Fluor 488 only.
Adherence assay.
Adherence of H. ducreyi cells to U-937 macrophages was evaluated with a modified version of a previously described attachment assay (38). The differentiation of the U-937 cells was induced as described above at 2 × 105 cells/well. The macrophages were treated with cytochalasin D (5.0 μg/ml; A.G. Scientific, San Diego, Calif.) for 1 h at 37°C prior to use in the adherence assay (to prevent or minimize interference by phagocytosis).
H. ducreyi strains were grown overnight in broth, harvested by centrifugation at 6,500 × g for 10 min, and suspended in RPMI-F to an OD600 of 1.0. Portions (5 μl) were added in duplicate to the wells of 96-well plates containing confluent layers of U-937 cells and 95 μl of RPMI-F. Bacteria were then centrifuged onto the U-937 cells at 200 × g for 5 min, and the plates were incubated at 33°C for 2 h. The wells were washed four times with 200 μl of RPMI-F to remove nonadherent bacteria. The cells were then incubated with 100 μl of trypsin-EDTA (Gibco-BRL) for 5 to 15 min at room temperature. The contents of each well were suspended, serially diluted, spread onto chocolate agar plates, and incubated for 48 h at 33°C. Control wells which contained the same number of bacteria and RPMI-F but no U-937 cells were incubated under the same conditions. The results for each strain were expressed as the percentage of bacteria attached to the eukaryotic cells relative to the number of bacteria present in the control wells.
RESULTS
Inhibitory effect of H. ducreyi on phagocytosis of a secondary target.
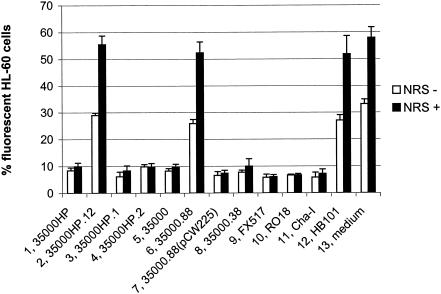
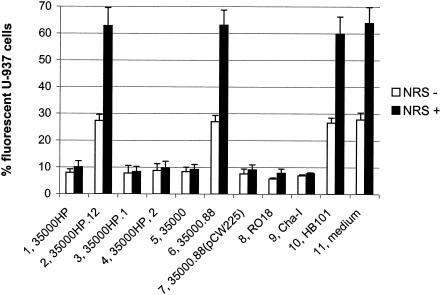
Wild-type H. ducreyi strains have been previously shown to inhibit phagocytosis of secondary targets and themselves by human macrophage-like cell lines and granulocytes from peripheral blood (1, 58). We tested the H. ducreyi wild-type strain 35000HP and its isogenic lspA1 lspA2 mutant 35000HP.12 (56) for their abilities to inhibit phagocytosis of opsonized fluorescent microspheres by differentiated HL-60 granulocytes (Fig. 1) and U-937 macrophages (Fig. 2). In this assay, the bacteria were incubated with the phagocytic cells for 1 h prior to the addition of the microspheres, as described in Materials and Methods. In contrast to the wild-type parent strain, the lspA1 lspA2 mutant was unable to inhibit phagocytosis of these microspheres. Fewer than 10% of the phagocytes incubated with the wild-type 35000HP strain (Fig. 1 and 2, column 1) engulfed the microspheres, whereas approximately 30% of the phagocytes exposed to the lspA1 lspA2 mutant (Fig. 1 and 2, column 2) or to an E. coli HB101 control (Fig. 1, column 12, and Fig. 2, column 10) contained microspheres. When NRS was added into the assay as a source of complement (NRS+ in Fig. 1 and 2), fewer than 15% of the phagocytes incubated with the wild-type strain 35000HP (Fig. 1 and 2, column 1) took up the microspheres. In contrast, 60% of these phagocytic cells engulfed microspheres after having been incubated with either the lspA1 lspA2 mutant (Fig. 1 and 2, column 2) or E. coli (Fig. 1, column 12, and Fig. 2, column 10).
FIG. 1.
H. ducreyi inhibits the phagocytic activity of HL-60 cells in vitro. Differentiated HL-60 granulocytes were incubated for 1 h at 33°C with H. ducreyi strains, E. coli HB101, or medium only (RPMI-F) followed by 1 h of incubation at 37°C with opsonized fluorescent microspheres in the presence and absence of 10% (vol/vol) NRS. The percentage of HL-60 cells containing intracellular fluorescent microspheres is shown. Each bar represents the mean with standard error from at least three independent experiments. Bars: 1, wild-type 35000HP; 2, lspA1 lspA2 double mutant 35000HP.12; 3, lspA1 mutant 35000HP.1; 4, lspA2 mutant 35000HP.2; 5, wild-type 35000; 6, lspB mutant 35000.88; 7, complemented lspB mutant 35000.88(pCW225); 8, cdtABC hhdAB deletion mutant 35000.38; 9, dsrA mutant FX517; 10, wild-type RO18; 11, wild-type Cha-I; 12, E. coli HB101; 13, medium control.
FIG. 2.
H. ducreyi inhibits the phagocytic activity of U-937 cells in vitro. Differentiated U-937 macrophages were incubated for 1 h at 33°C with H. ducreyi strains, E. coli HB101, or medium only (RPMI-F) followed by 1 h of incubation at 37°C with opsonized fluorescent microspheres in the presence and absence of 10% (vol/vol) NRS. The percentage of U-937 cells containing intracellular fluorescent microspheres is shown. Each bar represents the mean with standard error from at least three independent experiments. Bars: 1, 35000HP; 2, lspA1 lspA2 mutant 35000HP.12; 3, lspA1 mutant 35000HP.1; 4, lspA2 mutant 35000HP.2; 5, 35000; 6, lspB mutant 35000.88; 7, complemented lspB mutant 35000.88(pCW225); 8, RO18; 9, Cha-I; 10, E. coli HB101; 11, medium control.
We also tested a total of nine additional relevant H. ducreyi mutants and wild-type strains in this assay system. The lspA1 mutant 35000HP.1 (Fig. 1 and 2, column 3) expresses LspA2 but does not express LspA1, whereas the lspA2 mutant 35000HP.2 (Fig. 1 and 2, column 4) expresses only LspA1 (56). Both of these mutants exhibited wild-type levels of inhibition of phagocytic activity. The three other wild-type strains used in this assay system were 35000 (Fig. 1 and 2, column 5), RO18 (Fig. 1, column 10, and Fig. 2, column 8), and Cha-I (Fig. 1, column 11, and Fig. 2, column 9). Finally, three other mutants and a complemented mutant were also included. These were the lspB mutant 35000.88 (Fig. 1 and 2, column 6), the complemented lspB mutant 35000.88(pCW225) (Fig. 1 and 2, column 7), the cdtABC hhdAB deletion mutant 35000.38 (Fig. 1, column 8), and the dsrA mutant FX517 (20) (Fig. 1, column 9).
All nine of these strains inhibited phagocytosis, except for the lspB mutant 35000.88 that does not secrete LspA1 and LspA2 proteins across the outer membrane of H. ducreyi (C. K. Ward, J. R. Mock, and E. J. Hansen, submitted for publication). Inactivation of the lspB gene in the wild-type 35000 strain caused this mutant to lose its ability to inhibit phagocytic activity of HL-60 and U-937 cells. Complementation of the lspB mutation in strain 35000.88 with the H. ducreyi lspB gene provided in trans on plasmid pCW225 restored the ability of the strain to inhibit phagocytosis. These results indicated that the LspA1 and LspA2 proteins are involved, directly or indirectly, in the ability of wild-type H. ducreyi cells to inhibit phagocytic activity.
Effect of opsonization on inhibition of phagocytic activity by H. ducreyi.
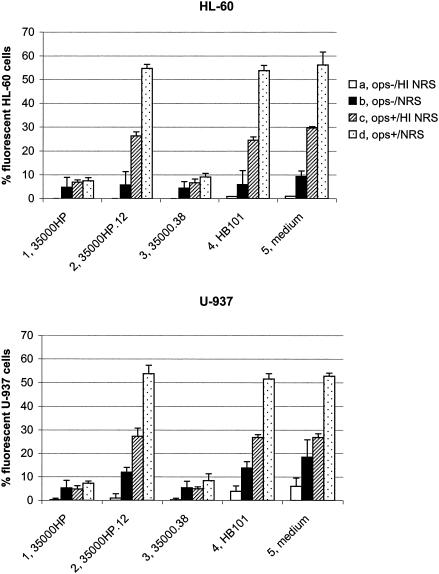
To investigate whether opsonization of the microspheres with specific antibodies or complement affected the inhibition of phagocytic activity by H. ducreyi, we performed experiments with HL-60 (Fig. 3, upper panel) and U-937 (Fig. 3, lower panel) phagocytes using nonopsonized (Fig. 3, columns a and b) or antibody-opsonized (Fig. 3, columns c and d) microspheres. These experiments were also performed in the presence of complement (i.e., NRS [Fig. 3, columns b and d]) and in the absence of complement (i.e., heat-inactivated NRS [Fig. 3, columns a and c]). No significant phagocytosis of microspheres by HL-60 or U-937 cells was observed in the absence of any type of opsonization (Fig. 3, column a). When nonopsonized microspheres were tested in the presence of NRS as a source of complement (Fig. 3, upper panel, column b), 5 to 10% of the HL-60 cells engulfed microspheres. There were no differences in uptake of these nonopsonized microspheres if the HL-60 cells were incubated with the H. ducreyi wild-type 35000HP strain (Fig. 3, upper panel, column 1b), the lspA1 lspA2 mutant 35000HP.12 (Fig. 3, upper panel, column 2b), the cdtABC hhdAB mutant 35000.38 (Fig. 3, upper panel, column 3b), or E. coli HB101 (Fig. 3, upper panel, column 4b). The cdtABC hhdAB deletion mutant is unable to express either the H. ducreyi cytolethal distending toxin (18) or the cell-associated hemolysin (3, 39) and was used here as a control. Under these same conditions involving nonopsonized microspheres in the presence of NRS, 5 to 10% of U-937 cells engulfed microspheres when incubated with the wild-type 35000HP strain (Fig. 3, lower panel, column 1b) or the cdtABC hhdAB mutant (Fig. 3, lower panel, column 3b), whereas up to 15% of the U-937 cells contained microspheres after exposure to the lspA1 lspA2 mutant (Fig. 3, lower panel, column 2b) or E. coli HB101 (Fig. 3, lower panel, column 4b).
FIG. 3.
Uptake of opsonized and nonopsonized microspheres by HL-60 and U-937 cells exposed to H. ducreyi. HL-60 granulocytes (upper panel) and U-937 macrophages (lower panel) were incubated for 1 h at 33°C with H. ducreyi strains, E. coli HB101, or medium only (RPMI-F) followed by 1 h of incubation at 37°C with the following: nonopsonized microspheres in the presence of heat-inactivated NRS (a); nonopsonized microspheres in the presence of NRS (b); opsonized microspheres in the presence of heat-inactivated NRS (c); or opsonized microspheres in the presence of NRS (d). The percentage of cells containing intracellular fluorescent microspheres is shown. Each bar represents the mean with standard error from three independent experiments. 1, wild-type 35000HP; 2, lspA1 lspA2 double mutant 35000HP.12; 3, cdtABC hhdAB deletion mutant 35000.38; 4, E. coli HB101; 5, medium control.
When microspheres opsonized with specific antibody were used in the absence or presence of active complement (i.e., NRS), the results were similar to those described in Fig. 1 and 2. In the presence of heat-inactivated NRS (Fig. 3, column c), fewer than 10% of HL-60 or U-937 phagocytes incubated with the wild-type 35000HP (Fig. 3, column 1c) or the cdtABC hhdAB mutant (Fig. 3, column 3c) took up these microspheres, whereas up to 30% of the phagocytic cells exposed to the lspA1 lspA2 mutant (Fig. 3. column 2c) or E. coli HB101 (Fig. 3, column 4c) engulfed these microspheres. Addition of active complement (Fig. 3, column d) had only a minor influence on the uptake of opsonized microspheres by HL-60 or U-937 cells incubated with the wild-type 35000HP (Fig. 3, column 1d) or the cdtABC hhdAB mutant (Fig. 3, column 3d). In contrast, up to 55% of the HL-60 and U-937 cells engulfed opsonized microspheres in the presence of the lspA1 lspA2 mutant (Fig. 3, column 2d) or E. coli HB101 (Fig. 3, column 4d). These data indicate that opsonization of the microspheres with specific antibodies was necessary to obtain significant uptake of these secondary targets by both HL-60 and U-937 cells. These data also suggest that the antiphagocytic activity of H. ducreyi affects both antibody- and complement-mediated pathways of phagocytosis (5, 17).
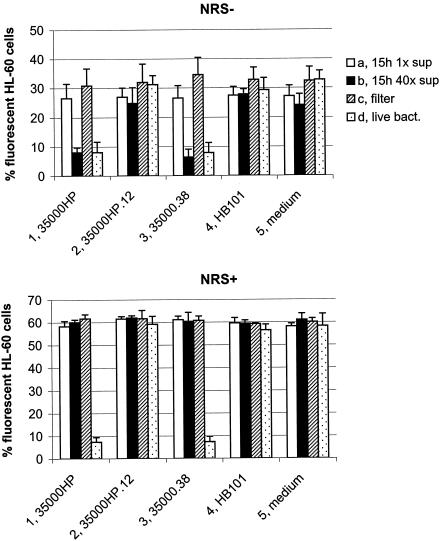
Characterization of the inhibitory activity.
To determine whether the antiphagocytic activity of H. ducreyi was cell associated or secreted, HL-60 granulocytes were exposed to culture supernatant fluids from overnight (15-h) cultures of H. ducreyi and to live H. ducreyi bacteria separated from the phagocytes by a filter support (0.2-μm pore size). The experiment was performed in the absence (Fig. 4, upper panel) and presence (Fig. 4, lower panel) of NRS as a source of complement. No inhibition of phagocytic activity was obtained with unconcentrated culture supernatant fluids of any strain tested (Fig. 4, column a). Forty-fold-concentrated culture supernatant fluids from the wild-type 35000HP (Fig. 4, upper panel, column 1b) and the cdtABC hhdAB mutant (Fig. 4, upper panel, column 3b) inhibited phagocytosis of opsonized microspheres in the absence of complement, whereas concentrated culture supernatant fluids of the lspA1 lspA2 mutant (Fig. 4, upper panel, column 2b) or E. coli HB101 (Fig. 4, upper panel, column 4b) had no effect on the phagocytic activity of HL-60 cells. These concentrated culture supernatant fluids were shown to be free of contamination with outer membrane fragments by Western blot analysis using a monoclonal antibody directed against the H. ducreyi major outer membrane protein (data not shown). Interestingly, if NRS was present as a source of complement, concentrated culture supernatant fluids from the wild-type 35000HP (Fig. 4, lower panel, column 1b) and the cdtABC hhdAB mutant (Fig. 4, lower panel, column 3b) were unable to inhibit phagocytic activity.
FIG. 4.
Inhibition of phagocytic activity by H. ducreyi culture supernatant fluids and by bacteria separated from the phagocytes by filter supports. HL-60 granulocytes were treated for 1 h with the following: culture supernatant fluid from overnight (15-h) cultures of H. ducreyi 35000HP (a); 40-fold-concentrated culture supernatant fluid (b); live H. ducreyi 35000HP separated from the phagocytes by filter supports (c); live H. ducreyi 35000HP (d). Phagocytosis of opsonized fluorescent microspheres was performed in the absence and presence of 10% (vol/vol) NRS. The percentage of HL-60 cells with intracellular fluorescent microspheres is shown. Each bar represents the mean with standard error of three independent experiments. Bar groups: 1, wild-type 35000HP; 2, lspA1 lspA2 mutant 35000HP.12; 3, cdtABC hhdAB mutant 35000.38; 4, E. coli HB101; 5, uninoculated culture medium control (i.e., supplemented Columbia broth for H. ducreyi culture supernatant fluids, or RPMI-F for live bacteria).
When live bacteria were incubated with these phagocytic cells, only the wild-type 35000HP strain (Fig. 4, column 1d) and the cdtABC hhdAB mutant (Fig. 4, column 3d) inhibited phagocytic activity. However, when these same bacteria were separated from the HL-60 cells by a membrane filter, neither strain exerted any detectable inhibition of phagocytic activity (Fig. 4, columns 1c and 3c). It can be inferred from these results that the factor(s) that inhibits phagocytic activity is associated primarily with H. ducreyi cells but can also be detected in soluble culture supernatant fluid under certain conditions if this latter material is concentrated sufficiently.
This same set of experiments involving concentrated culture supernatant fluids and filter supports was performed with U-937 macrophages. The results (data not shown) were similar to those obtained using HL-60 granulocytes, except for those involving the concentrated culture supernatant fluid. The concentrated culture medium control (i.e., sterile culture medium that had not been exposed to bacteria) completely inhibited the phagocytic activity of the U-937 cells in the absence and presence of complement, thus precluding interpretation of data from the experiments involving the concentrated culture supernatant fluid and these U-937 cells.
To determine whether viability was necessary for H. ducreyi bacterial cells to exert their inhibitory effect on phagocytosis, HL-60 cells were exposed to bacteria that had been killed by heating at 65°C for 90 min. Experiments performed in the absence and presence of NRS as the source of complement indicated that heat-killed H. ducreyi 35000HP cells were unable to inhibit the phagocytosis of opsonized microspheres by HL-60 cells (data not shown).
Resistance of H. ducreyi to phagocytosis by HL-60 and J774A.1 cells.
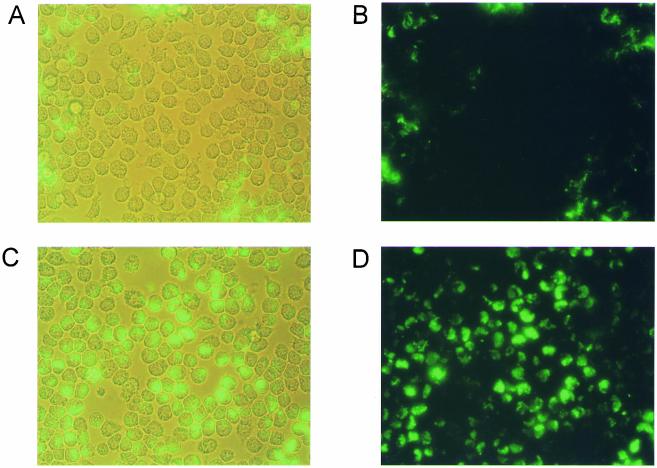
To confirm that the wild-type H. ducreyi 35000HP strain was also able to inhibit phagocytosis of itself (1, 58), a direct phagocytosis assay was performed with both HL-60 and J774A.1 cells. Wild-type H. ducreyi 35000HP, the lspA1 lspA2 mutant 35000HP.12, and the lspB mutant 35000.88, each carrying the gfp plasmid pRB157K, were opsonized with rabbit antiserum to H. ducreyi 35000 and then incubated with these two cell lines. Wild-type 35000HP inhibited its uptake by J774A.1 cells (Fig. 5A and B), whereas the lspA1 lspA2 mutant was engulfed efficiently by these phagocytes (Fig. 5C and D). To ensure that the difference observed between these two strains was not due to different autoagglutination patterns (56), the lspB mutant 35000.88, which does not secrete either LspA1 or LspA2 (Ward et al., submitted for publication) but which has an autoagglutination phenotype similar to that of wild-type 35000HP, was also tested in this assay. Similar to the lspA1 lspA2 mutant, this lspB mutant was unable to resist phagocytosis by J774A.1 cells (data not shown). Similar results were obtained with all three strains when HL-60 cells were used (data not shown). These data are in agreement with previous reports (1, 58) that wild-type H. ducreyi resists phagocytosis by macrophage-like cell lines and polymorphonuclear leukocytes (PMNs). In contrast, the lspA1 lspA2 mutant has lost this ability to resist phagocytosis.
FIG. 5.
Resistance of wild-type and mutant strains of H. ducreyi to phagocytosis by J774A.1 macrophages. These macrophages were incubated with antiserum-opsonized H. ducreyi 35000HP(pRB157K) (A [light microscopy] and B [fluorescence microscopy]) or the lspA1 lspA2 mutant 35000HP.12(pRB157K) (C [light microscopy] and D [fluorescence microscopy]) for 40 min at 33°C.
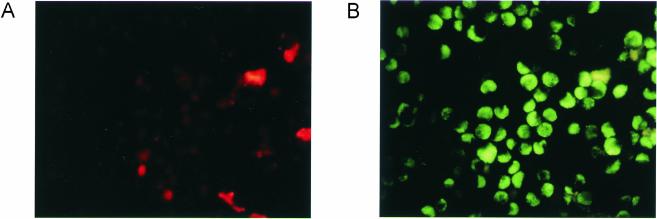
Use of differential staining to distinguish extracellular and intracellular bacteria.
Phagocytosis assays were performed using J774A.1 macrophages together with H. ducreyi wild-type 35000HP (data not shown) and lspA1 lspA2 mutant cells (Fig. 6) that had been opsonized with rabbit antiserum to H. ducreyi. Extracellular bacteria were stained with Alexa 568-conjugated goat anti-rabbit IgG (Fig. 6A). After fixing and permeabilization of the J774A.1 cells, intra- and extracellular bacteria were stained with Alexa 488-conjugated goat anti-rabbit IgG (Fig. 6B). Samples with wild-type 35000HP showed very little staining due to lack of phagocytosis (data not shown). The results obtained with the lspA1 lspA2 mutant showed that these bacteria were predominantly located intracellularly (Fig. 6B), although some extracellular bacteria were still adherent to the phagocytes despite washing (Fig. 6A). Experiments performed with opsonized microspheres as a secondary target gave similar results in that the microspheres were located intracellularly in phagocytic cells that had been incubated with the lspA1 lspA2 mutant (data not shown).
FIG. 6.
Differential staining to distinguish bacteria in intra- and extracellular locations. Murine J774A.1 macrophages were incubated with the antiserum-opsonized H. ducreyi lspA1 lspA2 mutant for 40 min at 37°C. (A) Extracellular bacteria were stained with Alexa 568 (red)-conjugated goat anti-rabbit IgG. (B) After fixing and permeabilization of the cells, intra- and extracellular bacteria were stained with Alexa 488 (green)-conjugated goat anti-rabbit IgG. The samples were examined by fluorescence microscopy.
Adherence of H. ducreyi to U-937 macrophages.
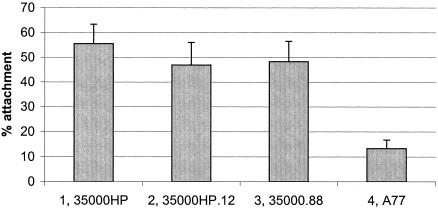
We previously showed that the H. ducreyi LspA1 and LspA2 proteins likely do not function as adhesins for human foreskin fibroblast cells and human HaCaT keratinocytes in vitro (56). To determine whether these proteins could be involved in the adherence of H. ducreyi to U-937 macrophages, we evaluated the ability of the wild-type 35000HP strain, the lspA1 lspA2 mutant, and the lspB mutant (which does not secrete the LspA proteins) to bind U-937 macrophages treated with cytochalasin D (to prevent interference by phagocytosis during the adherence assay). Both of these mutant strains (Fig. 7, columns 2 and 3) attached to the U-937 cells at levels similar to those obtained with the wild-type 35000HP strain (Fig. 7, column 1). H. ducreyi strain A77, which has been previously shown to be deficient in attachment to both human foreskin fibroblast cells and HaCaT cells (4, 56), was used as a negative control. Strain A77 (Fig. 7, column 4) attached significantly less to the U-937 cells than did the other strains. These results indicate that the LspA1 and LspA2 proteins likely are not involved in the adherence of H. ducreyi to U-937 macrophages in vitro.
FIG. 7.
Adherence of H. ducreyi strains to U-937 macrophages. U-937 macrophages treated with cytochalasin D were incubated for 2 h at 33°C with H. ducreyi strains grown overnight in broth cultures. The percentage of bacteria attached to the eukaryotic cells relative to controls with bacteria but no eukaryotic cells is shown. Each column represents the mean with standard error of three independent experiments. Columns: 1, wild-type 35000HP; 2, lspA1 lspA2 mutant 35000HP.12; 3, lspB mutant 35000.88; 4, A77.
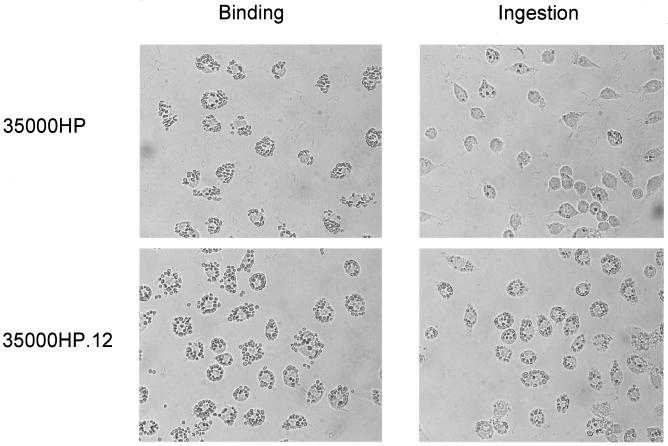
Phagocytosis of opsonized sheep erythrocytes by J774A.1 cells.
To determine whether H. ducreyi influenced the phagocytic efficiency of specific phagocytic receptors, we used IgG-opsonized sheep erythrocytes (EIgG) as a probe for receptors for the Fc portion of IgG. EIgG attached to J774A.1 cells at similar levels in the presence of either 35000HP or the lspA1 lspA2 mutant (Fig. 8). The average number (± standard error) of EIgG bound to J774A.1 cells was 12.1 (±0.4) in the presence of 35000HP, 13.0 (±0.45) in the presence of lspA1 lspA2 mutant, and 12.3 (±0.2) in the presence of E. coli HB101. These differences were not significant. However, phagocytes exposed to the lspA1 lspA2 mutant ingested substantially more EIgG than those exposed to the wild-type 35000HP strain (Fig. 8). The average number of ingested EIgG was 0.75 (±0.09) in the presence of 35000HP, 8.0 (±0.38) in the presence of the lspA1 lspA2 mutant, and 8.1 (±0.19) in the presence of E. coli HB101. The results obtained with the wild-type strain differed significantly from those obtained with both the lspA1 lspA2 mutant and E. coli (P < 0.01).
FIG. 8.
Binding and ingestion of opsonized sheep erythrocytes (EIgG) by J774A.1 macrophages. These macrophages were exposed for 1 h at 33°C to H. ducreyi 35000HP or to the lspA1 lspA2 mutant followed by 30 min of incubation at 37°C with EIgG. To measure ingestion (right-hand panels), extracellular erythrocytes were lysed with 0.2% saline. The samples were examined by light microscopy.
DISCUSSION
H. ducreyi has been shown to associate with human granulocytes and macrophages in chancroidal lesions that occur naturally (31, 37) and in the human challenge model of infection (8, 10, 49). At least during the early stages of experimental infection (i.e., through pustule development), H. ducreyi can be found associated with PMNs and macrophages but not with T cells, Langerhans' cells, or fibroblasts (8). Despite this close association with phagocytic cells, H. ducreyi apparently remains extracellular throughout the course of experimental infection (8, 10). Moreover, it has been reported that H. ducreyi can sometimes be isolated from naturally occurring chancroidal lesions for several weeks (37), a finding which suggests that this pathogen can survive in vivo despite the presence of various phagocytic cells in the lesion. It has been documented that H. ducreyi does not survive inside phagocytic cells (1, 58), a finding which makes it unlikely that the persistence of viable H. ducreyi in these lesions is the result of growth within phagocytic cells. Most recently, studies of the interaction between H. ducreyi and phagocytic cells performed in vitro have shown that this organism is not only resistant to phagocytosis but also able to inhibit the phagocytosis of secondary targets (1, 58).
In the present study, we tested the wild-type H. ducreyi strain 35000HP and its isogenic lspA1 lspA2 double mutant for their abilities to inhibit phagocytosis of both themselves and secondary targets by several different cell lines, including HL-60, U-937, and J774A.1 cells. Our laboratory previously showed that this lspA1 lspA2 mutant was almost avirulent in the temperature-dependent rabbit model for experimental chancroid (56). When tested for its ability to inhibit phagocytosis of opsonized fluorescent microspheres (Fig. 1 and 2), this lspA1 lspA2 mutant was unable to impede this process, whereas the wild-type parent strain did effectively prevent phagocytosis of these secondary targets. Expression of either LspA1 (i.e., by the lspA2 mutant 35000HP.2) or LspA2 (i.e., by the lspA1 mutant 35000HP.1) was sufficient to cause this inhibitory effect. That the wild-type 35000HP strain did not exert a greater inhibitory effect than either of these mutants is likely due to the fact that this wild-type strain readily expresses LspA1 but very little LspA2 (56). (It should be noted that expression of LspA2 in the lspA1 mutant 35000HP.1 is much increased relative to that of the wild-type parent strain [56].)
We also tested several other wild-type and mutant H. ducreyi strains for their inhibitory activity and found that all of these strains except the lspB mutant retained the ability to inhibit phagocytosis (Fig. 1 and 2). This lspB mutant, which was unable to inhibit the phagocytic activity of HL-60 and U-937 cells, is also not able to secrete the LspA1 and LspA2 proteins across the outer membrane of H. ducreyi (Ward et al., submitted for publication). However, complementation of the lspB mutation with the wild-type lspB gene on a plasmid restored both the ability of this mutant to secrete the LspA1 and LspA2 proteins (Ward et al., submitted for publication) and to inhibit phagocytosis (Fig. 1 and 2). Taken together, these results indicate that the LspA1 and LspA2 proteins are involved, directly or indirectly, in the ability of wild-type H. ducreyi to inhibit phagocytic activity, at least in vitro. Attempts to complement the lspA1 and lspA2 mutations directly have been unsuccessful to date because of the difficulties inherent in cloning these extremely large genes into a plasmid vector.
We investigated the effect of opsonization on inhibition of phagocytic activity by H. ducreyi and found that opsonization of the microspheres with specific antibodies was necessary to obtain significant uptake of these secondary targets by both HL-60 granulocytes and U-937 macrophages. When a source of complement (i.e., NRS) was also included in this assay system, the uptake of these secondary targets by HL-60 and U-937 cells exposed to the lspA1 lspA2 mutant or E. coli almost doubled (Fig. 3). In agreement with previous studies reporting inhibition by wild-type H. ducreyi of Fc receptor-mediated phagocytosis in vitro (1, 58), these results indicate that the inhibitory activity of H. ducreyi affected both antibody- and complement-mediated pathways of phagocytosis. This inhibitory activity was associated with live H. ducreyi cells and, if live bacterial cells were used in the assay, prevention of contact between the bacteria and the phagocytic cells resulted in no inhibition of phagocytic activity. Similar to the results of Totten and colleagues (58), we could not detect inhibitory activity with heat-killed H. ducreyi. However, we could detect this inhibitory activity in culture supernatant fluids from the wild-type 35000HP strain and from the cdtABC hhdA mutant 35000.38 if these were sufficiently concentrated (Fig. 4). This inhibitory activity in the concentrated culture supernatant fluids was eliminated by the presence of complement (Fig. 4), a finding which suggests that there is very little active inhibitory factor in these concentrated culture supernatant fluids.
In agreement with previous studies describing resistance of wild-type H. ducreyi strains to phagocytosis by macrophage-like cell lines and PMNs (1, 58), we found that the wild-type 35000HP strain was resistant to phagocytosis by J774A.1 macrophages and HL-60 granulocytes. In contrast, the lspA1 lspA2 mutant 35000HP.12 no longer had the ability to resist phagocytosis by these cells. Differential staining of J774A.1 cells from a direct phagocytosis assay confirmed that the lspA1 lspA2 mutants were primarily located intracellularly, with only a few extracellular bacteria attached to the phagocytes (Fig. 6). Experiments measuring the ability of these different strains to attach to U-937 macrophages showed no differences in the attachment levels obtained with the wild-type and lspA1 lspA2 mutant strains (Fig. 7), suggesting that the observed difference in phagocytosis of these two strains was not due to differential attachment. Additional experiments using opsonized erythrocytes as a secondary target showed similar levels of binding but substantially different levels of ingestion of these erythrocytes by J774A.1 macrophages exposed to the wild-type strain and the lspA1 lspA2 mutant (Fig. 8).
Exactly how the LspA1 and LspA2 proteins may exert their inhibitory effect on phagocytosis is not clear. There are multiple ways in which bacterial pathogens can avoid being engulfed by phagocytes (21), ranging from simple physical obstruction (e.g., by capsular polysaccharide) to sophisticated signaling stratagems involving effectors injected via type III secretion systems (e.g., YopE). Interestingly, both LspA1 and LspA2 contain a central 221-amino-acid region with 36% identity to the YopT protein of Yersinia enterocolitica (30, 57). YopT was first noted for its ability to exert a cytotoxic effect after it is injected into eukaryotic cells via a type III secretion system, altering the cell cytoskeleton and causing disruption of the actin filament structure (30). It was later shown that YopT causes release of the small GTP-binding protein RhoA from the cell membrane (48, 59). Most recently, it was shown that YopT is a cysteine protease (46) that cleaves a polybasic sequence N-terminal to the prenylated cysteine in RhoA, Cdc42, and Rac (47), and it was proposed that YopT is the prototypic member of a family of cysteine proteases involved in bacterial virulence (46).
Western blot analysis using polyclonal antiserum raised against a fusion protein containing the YopT-like region of LspA1 and LspA2 showed that this amino acid sequence is present in the 260-kDa soluble forms of LspA1 and LspA2 present in culture supernatant fluid (data not shown). If this YopT-like region of LspA1 and LspA2 is involved in the ability of H. ducreyi to inhibit phagocytic activity, it would not be unreasonable to expect that the phagocytic cell cytoskeleton would be altered in some manner. In preliminary experiments using opsonized erythrocytes (EIgG) as a secondary target, we observed extensive membrane ruffling and phagocytic cup formation beneath the EIgG in macrophages exposed to the lspA1 lspA2 mutant, whereas these rearrangements, typical for Fc receptor-mediated phagocytosis (35), were absent or much reduced in the phagocytes that had been exposed to the wild-type 35000HP strain (data not shown). These results suggest that LspA1 and LspA2 might be able to alter or block the changes that normally occur in the phagocyte cytoskeleton upon contact with an opsonized antigen. However, examination of the H. ducreyi genome indicates that there is no type III secretion system present (R. S. Munson, Jr., personal communication), and it is not clear how LspA1 and LspA2 (or the YopT-like region of LspA1 and LspA2) might gain access to the interior of the phagocyte. Alternatively, LspA1/LspA2 may exert its inhibitory effect on phagocytic activity by another means. Additional and extensive experimentation will be required to resolve these issues.
Acknowledgments
This study was supported by U.S. Public Health Service grants AI32011 (E.J.H.) and HL 54164 (S.G.).
We thank Christine Ward, Jo Latimer, Robert Blick, Jason Mock, Joseph Nika, Kaiping Deng, and Nicolai Van Oers for helpful discussions and Christopher Elkins for providing the dsrA mutant.
Editor: J. N. Weiser
REFERENCES
- 1.Ahmed, H. J., C. Johansson, L. A. Svensson, K. Ahlman, M. Verdrengh, and T. Lagergard. 2002. In vitro and in vivo interactions of Haemophilus ducreyi with host phagocytes. Infect. Immun. 70:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa, M. J., P. Degagne, and T. Hollyer. 1993. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect. Immun. 61:1735-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfa, M. J., P. Degagne, and P. A. Totten. 1996. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect. Immun. 64:2349-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfa, M. J., M. K. Stevens, P. Degagne, J. Klesney-Tait, J. D. Radolf, and E. J. Hansen. 1995. Use of tissue culture and animal models to identify virulence-associated traits of Haemophilus ducreyi. Infect. Immun. 63:1754-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen, L. A., and A. Aderem. 1996. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J. Exp. Med. 184:627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 8.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer, M. E., and S. M. Spinola. 1999. Binding of Haemophilus ducreyi to extracellular matrix proteins. Infect. Immun. 67:2649-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer, M. E., and S. M. Spinola. 2000. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 68:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bong, C. T., M. E. Bauer, and S. M. Spinola. 2002. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect. 4:1141-1148. [DOI] [PubMed] [Google Scholar]
- 12.Bong, C. T., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozue, J. A., M. V. Tullius, J. Wang, B. W. Gibson, and R. S. Munson, Jr. 1999. Haemophilus ducreyi produces a novel sialyltransferase. Identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J. Biol. Chem. 274:4106-4114. [DOI] [PubMed] [Google Scholar]
- 14.Brentjens, R. J., S. M. Spinola, and A. A. Campagnari. 1994. Haemophilus ducreyi adheres to human keratinocytes. Microb. Pathog. 16:243-247. [DOI] [PubMed] [Google Scholar]
- 15.Campagnari, A. A., R. Karalus, M. A. Apicella, W. Melaugh, A. J. Lesse, and B. W. Gibson. 1994. Use of pyocin to select a Haemophilus ducreyi variant defective in lipooligosaccharide biosynthesis. Infect. Immun. 62:2379-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campagnari, A. A., L. M. Wild, G. E. Griffiths, R. J. Karalus, M. A. Wirth, and S. M. Spinola. 1991. Role of lipopolysaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect. Immun. 59:2601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 18.Cope, L. D., S. R. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkins, C., C.-J. Chen, and C. E. Thomas. 1995. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect. Immun. 63:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkins, C., K. J. Morrow, Jr., and B. Olsen. 2000. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect. Immun. 68:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst, J. D. 2000. Bacterial inhibition of phagocytosis. Cell Microbiol. 2:379-386. [DOI] [PubMed] [Google Scholar]
- 22.Filiatrault, M. J., R. S. Munson, Jr., and A. A. Campagnari. 2001. Genetic analysis of a pyocin-resistant lipooligosaccharide (LOS) mutant of Haemophilus ducreyi: restoration of full-length LOS restores pyocin sensitivity. J. Bacteriol. 183:5756-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson, B. W., A. A. Campagnari, W. Melaugh, N. J. Phillips, M. A. Apicella, S. Grass, J. Wang, K. L. Palmer, and R. S. Munson, Jr. 1997. Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J. Bacteriol. 179:5062-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg, S., K. Burridge, and S. C. Silverstein. 1990. Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J. Exp. Med. 172:1853-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond, G. W., C. J. Lian, J. C. Wilt, and A. R. Ronald. 1978. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob. Agents Chemother. 13:608-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen, E. J., S. R. Lumbley, J. A. Richardson, B. K. Purcell, M. K. Stevens, L. D. Cope, J. Datte, and J. D. Radolf. 1994. Induction of protective immunity to Haemophilus ducreyi in the temperature-dependent rabbit model of experimental chancroid. J. Immunol. 152:184-192. [PubMed] [Google Scholar]
- 29.Hobbs, M. M., T. R. Paul, P. B. Wyrick, and T. H. Kawula. 1998. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction pattern in an in vitro human skin model. Infect. Immun. 66:2914-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 23:915-929. [DOI] [PubMed] [Google Scholar]
- 31.King, R., J. Gough, A. R. Ronald, J. Nasio, J. O. Ndinyaachola, F. Plummer, and J. A. Wilkins. 1996. An immunohistochemical analysis of naturally occurring chancroid. J. Infect. Dis. 174:427-430. [DOI] [PubMed] [Google Scholar]
- 32.Lagergard, T., M. Purven, and A. Frisk. 1993. Evidence of Haemophilus ducreyi adherence to and cytotoxin destruction of human epithelial cells. Microb. Pathog. 14:417-431. [DOI] [PubMed] [Google Scholar]
- 33.Lammel, C. J., N. P. Dekker, J. Palefsky, and G. F. Brooks. 1993. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J. Infect. Dis. 167:642-650. [DOI] [PubMed] [Google Scholar]
- 34.Martinez, J. E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G. M. Carlone. 1999. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab Immunol. 6:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May, R. C., and L. M. Machesky. 2001. Phagocytosis and the actin cytoskeleton. J. Cell Sci. 114:1061-1077. [DOI] [PubMed] [Google Scholar]
- 36.Mertz, K. J., D. L. Trees, W. C. Levine, J. S. Lewis, B. Litchfield, K. S. Pettus, S. A. Morse, M. E. St. Louis, J. B. Weiss, J. Schwebke, J. Dickes, R. Kee, J. Reynolds, D. Hutcheson, D. Green, I. Dyer, G. A. Richwald, J. Novotny, M. Goldberg, J. A. O'Donnell, and R. Knaup. 1998. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. J. Infect. Dis. 178:1795-1798. [DOI] [PubMed] [Google Scholar]
- 37.Morse, S. A. 1989. Chancroid and Haemophilus ducreyi. Clin. Microbiol. Rev. 2:137-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nika, J. R., J. L. Latimer, C. K. Ward, R. J. Blick, N. J. Wagner, L. D. Cope, G. G. Mahairas, R. S. Munson, Jr., and E. J. Hansen. 2002. Haemophilus ducreyi requires the flp gene cluster for microcolony formation in vitro. Infect. Immun. 70:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer, K. L., W. E. Goldman, and R. S. Munson, Jr. 1996. An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol. Microbiol. 21:13-19. [DOI] [PubMed] [Google Scholar]
- 40.Palmer, K. L., and R. S. Munson, Jr. 1995. Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol. Microbiol. 18:821-830. [DOI] [PubMed] [Google Scholar]
- 41.Purcell, B. K., J. A. Richardson, J. D. Radolf, and E. J. Hansen. 1991. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J. Infect. Dis. 164:359-367. [DOI] [PubMed] [Google Scholar]
- 42.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.San Mateo, L. R., M. M. Hobbs, and T. H. Kawula. 1998. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol. Microbiol. 27:391-404. [DOI] [PubMed] [Google Scholar]
- 44.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Neutropenia restores virulence to an attenuated Cu,Zn superoxide dismutase-deficient Haemophilus ducreyi strain in the swine model of chancroid. Infect. Immun. 67:5345-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulte, J. M., F. A. Martich, and G. P. Schmid. 1992. Chancroid in the United States, 1981-1990: evidence for underreporting of cases. Morb. Mortal. Wkly. Rep. 41:57-61. [PubMed] [Google Scholar]
- 46.Shao, F., P. M. Merritt, Z. Bao, R. W. Innes, and J. E. Dixon. 2002. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109:575-588. [DOI] [PubMed] [Google Scholar]
- 47.Shao, F., P. O. Vacratsis, Z. Bao, K. E. Bowers, C. A. Fierke, and J. E. Dixon. 2003. Biochemical characterization of the Yersinia YopT protease: cleavage site and recognition elements in Rho GTPases. Proc. Natl. Acad. Sci. USA 100:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorg, I., U. M. Goehring, K. Aktories, and G. Schmidt. 2001. Recombinant Yersinia YopT leads to uncoupling of RhoA-effector interaction. Infect. Immun. 69:7535-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spinola, S. M., M. E. Bauer, and R. S. Munson, Jr. 2002. Immunopathogenesis of Haemophilus ducreyi infection (chancroid). Infect. Immun. 70:1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spinola, S. M., T. J. Hiltke, K. R. Fortney, and K. L. Shanks. 1996. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL. Infect. Immun. 64:1950-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevens, M. K., S. Porcella, J. Klesney-Tait, S. R. Lumbley, S. E. Thomas, M. V. Norgard, J. D. Radolf, and E. J. Hansen. 1996. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect. Immun. 64:1724-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, S., N. K. Scheffler, B. W. Gibson, J. Wang, and R. S. Munson, Jr. 2002. Identification and characterization of the N-acetylglucosamine glycosyltransferase gene of Haemophilus ducreyi. Infect. Immun. 70:5887-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun, S., B. Schilling, L. Tarantino, M. V. Tullius, B. W. Gibson, and R. S. Munson, Jr. 2000. Cloning and characterization of the lipooligosaccharide galactosyltransferase II gene of Haemophilus ducreyi. J. Bacteriol. 182:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Totten, P. A., J. C. Lara, D. V. Norn, and W. E. Stamm. 1994. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect. Immun. 62:5632-5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward, C. K., J. L. Latimer, J. R. Nika, M. Vakevainen, J. R. Mock, K. Deng, R. J. Blick, and E. H. Hansen. 2003. Mutations in the lspA1 and lspA2 genes of Haemophilus ducreyi affect the virulence of this pathogen in an animal model system. Infect. Immun. 71:2478-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward, C. K., S. R. Lumbley, J. L. Latimer, L. D. Cope, and E. J. Hansen. 1998. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J. Bacteriol. 180:6013-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood, G. E., S. M. Dutro, and P. A. Totten. 2001. Haemophilus ducreyi inhibits phagocytosis by U-937 cells, a human macrophage-like cell line. Infect. Immun. 69:4726-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]