Abstract
Solid-phase binding, competitive binding, and cytotoxicity neutralization assays indicate that the B pentamer and A subunit both contribute to human serum amyloid P (HuSAP) component binding to Stx2. A polyvalent globotriaosyl-ceramide receptor analog, Daisy, did not competitively inhibit HuSAP binding, implying that the two ligands bind to different Stx2 domains.
Enterohemorrhagic Escherichia coli (EHEC) strains cause hemorrhagic colitis (HC) and, occasionally, hemolytic-uremic syndrome (HUS) in humans. EHEC strains express two Shiga toxins, Stx1 and Stx2, which contribute to this pathogenesis. Epidemiological evidence indicates that EHEC strains expressing both Stx1 and Stx2 or Stx2 alone are more likely to cause HUS than those expressing only Stx1 (2, 6, 13). Although the reasons for the greater toxicity of Stx2 remain unclear, they probably relate to a combination of factors (3, 7, 14-17).
The cytotoxicity of Stx2, but not Stx1, is neutralized in cell culture assays by the human serum amyloid P (HuSAP) component (4). HuSAP is an acute-phase protein found in the immunoglobulin-depleted fraction of normal human serum (10). Kimura et al. (4) suggested that the association of Stx2 with HuSAP might also be relevant to EHEC pathogenicity. The experiments described herein represent our efforts to further understand the association of Stx2 with HuSAP.
For the purposes of this investigation, Stx1, Stx2, and their recombinant B pentamers were purified as described previously (9, 10). The Stx1 and Stx2 B pentamers and their respective A subunits were also prepared and reassembled into hybrid holotoxins as previously described (3). The Verocytotoxicity assay (1) was used to confirm the biological activities of these reassembled hybrid holotoxins.
The solid-phase binding assays were performed as described previously (4) with the following procedural modifications. The microtiter plate wells were coated with either Stx1, Stx2, or their high-performance liquid chromatography (HPLC)-separated or cloned subunits, and HuSAP binding was detected at 405 nm with the chromogenic substrate 2,2′azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS; Roche Diagnostics, Laval, Canada).
The competitive binding inhibition assays were performed as described above, with the exception that serial dilutions of the soluble Stx holotoxins, their HPLC-separated components, or the Daisy Gb3 receptor analog (11) were prepared in 3% bovine serum albumin-phosphate-buffered saline containing 0.1 μg of HuSAP per ml and incubated for 1 h at 37°C before adding the samples to the Stx2-coated microtiter plates.
The Ramos cell HuSAP Stx2 neutralization assay involved treating 5 × 105 Ramos cells as described previously (8), for 2 h with 0.1-ng/ml Stx holotoxins or hybrid toxins that had been preincubated for 15 min with various concentrations of HuSAP. The cells were then washed with RPMI medium, incubated for an additional 16 h, and finally labeled with fluorescein isothiocyanate (FITC)-conjugated Annexin V (BD Pharmingen, Mississauga, Ontario, Canada) and propidium iodide (Sigma Aldrich) as instructed by the manufacturer. Cellular apoptosis was quantified with a FACscan flow cytometer (Becton Dickinson, Mountain View, Calif.).
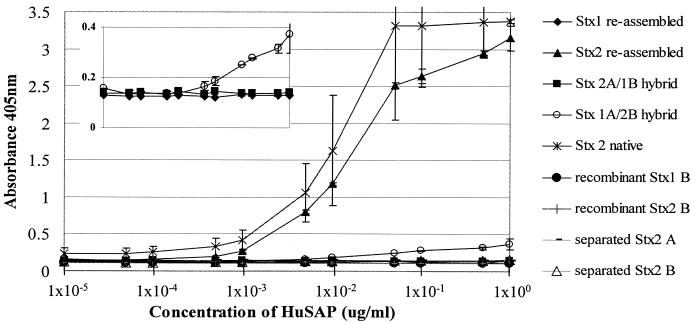
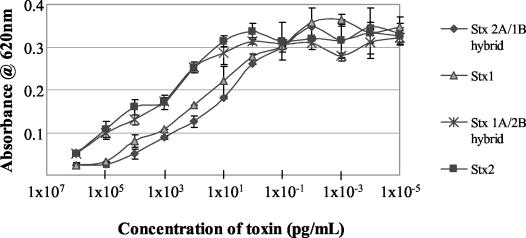
The data in Fig. 1 duplicate the results originally published by Kimura et al. (4) and demonstrate that native Stx2, but not Stx1 holotoxin, binds HuSAP in a solid-phase binding assay. In an extension of these experiments by Kimura et al. (4), we now show (also in Fig. 1) that HuSAP did not bind to any of the separated Stx1 or Stx2 subunits in this assay. However, HuSAP binding did occur when Stx2 holotoxin that had been reassembled from its separated A subunit and B pentamer was used in the assay. In contrast, we detected only minimal HuSAP binding to the hybrid holotoxin composed of the Stx1 A subunit and Stx2 B pentamer (Fig. 1, insert). HuSAP binding was not detected when the hybrid holotoxin composed of the Stx2 A subunit and Stx1 B pentamer was used in these experiments. The biological activity of all of these reassembled holotoxins was confirmed in the Verocytotoxicity assay. These data (Fig. 2) demonstrated that the separated Stx subunits had indeed reassembled into fully functional holotoxins.
FIG. 1.
HuSAP binding to immobilized Stx proteins. The data represent the average (n = 3) for each HuSAP dilution, and the error bars represent the standard deviations. Missing error bars indicate that the deviations were too small to appear on the graph. The insert displays the data for the Stx1 holotoxin and the reassembled hybrid toxins plotted with an expanded y axis.
FIG. 2.
Cytotoxic activities of reassembled Stx1, Stx2, and hybrid holotoxins (Stx1A/Stx2B and Stx2A/Stx1B) in Vero cells (n = 2). Error bars represent standard errors.
We considered the possibility that HuSAP may have been unable to access its binding sites on the immobilized separated Stx subunits. To test this, we performed competitive binding inhibition experiments using Stx holotoxins, separated Stx subunits, and hybrid holotoxins in solution-based binding inhibition experiments. In these assays, the hybrid holotoxin composed of the Stx1 A subunit and Stx2 B pentamer and the HPLC-separated Stx2 B pentamer competitively inhibited HuSAP binding to immobilized Stx2 at 10- and 100-fold-higher concentrations, respectively, versus the Stx2 control or reassembled Stx2 holotoxin (Fig. 3). These results are consistent with the data in Fig. 4, illustrating that HuSAP neutralizes Stx2 and, to a lesser extent, the Stx 1A/2B hybrid toxin in the Ramos cell apoptosis assay.
FIG. 3.
HuSAP competitive binding to immobilized Stx2 in the presence of increasing concentration of soluble Stx proteins. The data represent the average (n = 3) for each dilution, and error bars represent standard deviations. Missing error bars indicate that the deviations were too small to appear on the graph.
FIG. 4.
HuSAP neutralization of apoptosis induced by Stx holotoxins and reassembled hybrid toxins in Ramos B cells. The data represent the average (n = 6 for no treatment and Stx2 with or without HuSAP, and n = 3 for the remaining inhibitors), and error bars represent standard deviations.
The simplest interpretation of these collective results is that, whereas the B pentamer appears to make the more significant contribution, HuSAP binding requires domains from both the Stx2 A subunit and the Stx2 B pentamer.
We rejected the notion that instability of the Stx2 B pentamer, absent the A subunit, contributed to the toxin's reduced ability to bind HuSAP, because we previously demonstrated (9), and have confirmed herein by HPLC size exclusion chromatography (data not shown), that the recombinant Stx2 B subunits remained stable as pentamers in solution. Absent any X-ray crystallographic evidence to the contrary, it is possible, however, that the tertiary conformation of the Stx2 B pentamer is slightly altered when it associates with the A subunit, thereby exposing cryptic HuSAP binding sites in the Stx2 B subunits.
The globotriaosyl-ceramide receptor-based polyvalent inhibitor called Daisy (11) was also used in the HuSAP competitive binding inhibition assays. We previously reported 50% binding inhibition constants for Daisy in the submicromolar range (11). Given its demonstrated high avidity for the Stx2 B pentamer, Daisy should have inhibited HuSAP binding to Stx2 if these two ligands indeed competed for the same binding sites in the toxin. However, even at the highest concentration that could be achieved (10 mM), Daisy failed to competitively inhibit HuSAP binding to Stx2 holotoxin (data not shown).
At present, it is uncertain if Stx2 binding to HuSAP is at all relevant to EHEC pathogenesis. It is possible that the neutralizing function of HuSAP might prevent the majority of EHEC patients from developing HUS (4). In this instance, the risk of HUS may be greater in EHEC-infected individuals expressing, for one reason or another, abnormally low concentrations of HuSAP in serum. However, in their more recent report, Kimura et al. (5) failed to obtain in vivo evidence for this intriguing hypothesis.
Nelson et al. (12) previously reported that the concentration of SAP in serum ranges from 0.19 to 0.26 μM in healthy human adults and is relatively unaffected by an individual's pathophysiological status. Assuming that a toxic concentration of Stx2 in humans is probably 2.6 pM (0.2 μg/kg of body weight) (11), the concentration of SAP in serum would be at least 104 to 105 times greater than any circulating Stx2 in an EHEC-infected subject. If so, any decrease in SAP concentration would have to be very dramatic if it was to influence whether an EHEC-infected subject developed HUS. Extrapolation of the same arguments to children, who are more at risk for developing the Stx-mediated systemic complications upon EHEC infection, may not be wise, however, because the concentration of SAP in serum is unknown in these subjects and may well be much lower than that in adults. Our continuing studies into the biochemical basis for and biological consequences of Stx2 binding to HuSAP are now focused on resolving these important issues.
Acknowledgments
This work was supported by operating grants MWS 56081 from the Canadian Institutes for Health Research (CIHR) and VP 17 from the Canadian Bacterial Diseases Network to G.D.A. P.M. was supported by Doctoral Scholarships from the CIHR and Alberta Heritage Foundation for Medical Research.
Daisy was kindly provided by David R. Bundle, Department of Chemistry, University of Alberta, Edmonton, Canada.
Paola Marcato and Kathleen Vander Helm contributed equally to this report.
Editor: J. T. Barbieri
REFERENCES
- 1.Armstrong, G. D., E. Fodor, and R. Vanmaele. 1991. Investigation of Shiga-like toxin binding to chemically synthesized oligosaccharide sequences. J. Infect. Dis. 164:1160-1167. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto, H., K. Mizukoshi, M. Nishi, T. Kawakita, S. Hasui, Y. Kato, Y. Ueno, R. Takeya, N. Okuda, and T. Takeda. 1999. Epidemic of gastrointestinal tract infection including hemorrhagic colitis attributable to Shiga toxin 1-producing Escherichia coli O118:H2 at a junior high school in Japan. Pediatrics 103:E2. [Online.] [DOI] [PubMed]
- 3.Head, S. C., M. A. Karmali, and C. A. Lingwood. 1991. Preparation of VT1 and VT2 hybrid toxins from their purified dissociated subunits. Evidence for B subunit modulation of a subunit function. J. Biol. Chem. 266:3617-3621. [PubMed] [Google Scholar]
- 4.Kimura, T., S. Tani, Y. Matsumoto Yi, and T. Takeda. 2001. Serum amyloid P component is the Shiga toxin 2-neutralizing factor in human blood. J. Biol. Chem. 276:41576-41579. [DOI] [PubMed] [Google Scholar]
- 5.Kimura, T., S. Tani, M. Motoki, and Y. Matsumoto. 2003. Role of Shiga toxin 2 (Stx2)-binding protein, human serum amyloid P component (HuSAP), in Shiga toxin-producing Escherichia coli infections: assumption from in vitro and in vivo study using HuSAP and anti-Stx2 humanized monoclonal antibody TMA-15. Biochem. Biophys. Res. Commun. 305:1057-1060. [DOI] [PubMed] [Google Scholar]
- 6.Kleanthous, H., H. R. Smith, S. M. Scotland, R. J. Gross, B. Rowe, C. M. Taylor, and D. V. Milford. 1990. Haemolytic uraemic syndromes in the British Isles, 1985-8: association with verocytotoxin producing Escherichia coli. Part 2. Microbiological aspects. Arch. Dis. Child. 65:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louise, C. B., and T. G. Obrig. 1995. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J. Infect. Dis. 172:1397-1401. [DOI] [PubMed] [Google Scholar]
- 8.Marcato, P., G. Mulvey, and G. D. Armstrong. 2002. Cloned Shiga toxin 2 B subunit induces apoptosis in Ramos Burkitt's lymphoma B cells. Infect. Immun. 70:1279-1286. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Marcato, P., G. Mulvey, R. J. Read, K. Vander Helm, P. N. Nation, and G. D. Armstrong. 2001. Immunoprophylactic potential of cloned Shiga toxin 2 B subunit. J. Infect. Dis. 183:435-443. [DOI] [PubMed] [Google Scholar]
- 10.Mulvey, G., R. Vanmaele, M. Mrazek, M. Cahill, and G. D. Armstrong. 1998. Affinity purification of Shiga-toxin I and Shiga-like toxin II. J. Microbiol. Methods 32:247-252. [Google Scholar]
- 11.Mulvey, G. L., P. Marcato, P. I. Kitov, J. Sadowska, D. R. Bundle, and G. D. Armstrong. 2003. Assessment in mice of the therapeutic potential of tailored, multivalent Shiga toxin carbohydrate ligands. J. Infect. Dis. 187:640-649. [DOI] [PubMed] [Google Scholar]
- 12.Nelson, S. R., G. A. Tennent, D. Sethi, P. E. Gower, F. W. Ballardie, S. Amatayakul-Chantler, and M. B. Pepys. 1991. Serum amyloid P component in chronic renal failure and dialysis. Clin. Chim. Acta 200:191-199. [DOI] [PubMed] [Google Scholar]
- 13.Ostroff, S. M., P. I. Tarr, M. A. Neill, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 14.Rutjes, N. W., B. A. Binnington, C. R. Smith, M. D. Maloney, and C. A. Lingwood. 2002. Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse animal model. Kidney Int. 62:832-845. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki, A., H. Doi, F. Matsuzawa, S. Aikawa, K. Takiguchi, H. Kawano, M. Hayashida, and S. Ohno. 2000. Bcl-2 antiapoptotic protein mediates verotoxin II-induced cell death: possible association between Bcl-2 and tissue failure by E. coli O157:H7. Genes Dev. 14:1734-1740. [PMC free article] [PubMed] [Google Scholar]
- 16.Thorpe, C. M., W. E. Smith, B. P. Hurley, and D. W. Acheson. 2001. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect. Immun. 69:6140-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki, C., Y. Natori, X. T. Zeng, M. Ohmura, S. Yamasaki, and Y. Takeda. 1999. Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 442:231-234. [DOI] [PubMed] [Google Scholar]