Abstract
Monocytic cells exposed to Borrelia burgdorferi, through unknown receptors, overexpress the urokinase receptor (uPAR), a key mediator of the plasminogen activation system. We show that combined blockade of CD14 and TLR2 causes a significant inhibition of B. burgdorferi-induced uPAR in Mono Mac 6 (MM6) cells. Other pattern recognition receptors tested (CD11b/CD18, the mannose receptor, and the N-formyl-methionyl-leucyl-phenylalanine receptor) did not have demonstrated roles in B. burgdorferi-mediated uPAR induction. We dissected the result for CD14 andTLR2 by investigating the singular contributions of each. Independent functional blockade of CD14 or TLR2 failed to inhibit B. burgdorferi-mediated uPAR induction. 1,25-Dihydroxyvitamin D3 differentiation of MM6 cells increased CD14 expression 12-fold but did not augment B. burgdorferi-mediated uPAR expression. Peritoneal exudate macrophages (PEM) from CD14- or TLR2-deficient mice were not defective in B. burgdorferi-mediated synthesis of uPAR mRNA and protein. Increased uPAR mRNA or protein or both were apparent in PEM from transgenic and control mice, even at a ratio of one Borrelia spirochete per cell. We conclude that signaling for the uPAR response, as mediated by B. burgdorferi, proceeds with CD14 and TLR2 as partial contributors. That part under control of CD14 and TLR2 represents a new link between the host plasminogen activation and innate immunity systems.
The spirochete Borrelia burgdorferi, the etiologic agent of Lyme disease, is transmitted by Ixodes sp. ticks. The spirochete initially propagates locally in the skin, causing a characteristic lesion known as erythema migrans, and disseminates hematogenously to infect other organ systems (19). To promote its dissemination, B. burgdorferi can bind elements of the plasminogen activation system (PAS), including the zymogen plasminogen (PLG) and urokinase plasminogen activator (uPA) to its surface, leading to the formation of plasmin and providing the spirochete with host-derived proteases (9, 17, 18, 30, 32). The acquired proteolytic activity allows B. burgdorferi and related spirochetes to degrade components of the extracellular matrix (8, 18) and penetrate endothelial cell monolayers in vitro (9) and provides for efficient dissemination in mice (32, 43) and ticks (7). Additionally, live B. burgdorferi induces the expression and release of matrix metalloproteinase 1 (MMP-1) and MMP-9 in inflammatory and noninflammatory human (21) and rat primary neural cells (46) in vitro. The presence of monocytic cell-derived MMPs leads to enhanced penetration of tissue barriers by B. burgdorferi in vitro (21). Host plasmin and MMPs secreted by host cells may represent distinct factors to be exploited by B. burgdorferi and other bacterial pathogens. Alternatively, acquisition of plasmin may directly trigger further downstream proteolytic events, including cleavage and increased activation of pro-MMPs (21).
A key mediator of the PAS is the uPA receptor (CD87; uPAR) (40, 65, 68), a glycosylphosphatidylinositol (GPI)-anchored (48), highly glycosylated protein of 55 to 60 kDa (47), which focuses plasmin activity at the leading edge of migrating cells. Initially identified on monocytic cells (10, 42, 68), uPAR is also expressed by granulocytes, natural killer cells, and activated T cells (39, 44, 45). uPAR consists of three domains (D1 to D3), with D1 containing the binding sites for uPA and D3 being associated with the plasma membrane through the GPI anchor. uPAR is found also in a soluble form, which is overexpressed in tissue extracts and body fluids of patients with pathological conditions (4, 20, 62).
uPAR is induced by a variety of tumor promoters, including phorbol myristate acetate (37), proinflammatory mediators interleukin-1β (IL-1β) (25), transforming growth factor beta 1, tumor necrosis factor alpha (TNF-α) (36, 57), and nerve growth factor (13), as well as diverse bacterial products such as lipopolysaccharide (LPS), muramyl dipeptide (67), and lipoteichoic acid (6). In addition, we have recently reported that exposure to live B. burgdorferi, purified B. burgdorferi lipoprotein, and synthetic lipidated hexapeptides induces an increase in expression of both cellular uPAR and soluble uPAR (suPAR) in monocytic cells (6). uPAR also has significant roles in chemokinesis, chemotaxis, and adhesion of normal and tumor cells (11), and thus it is an important molecule in inflammation and infection and tumor metastasis. The receptor(s) used by B. burgdorferi for upregulation of uPAR could be among those involved in pathogen recognition which signal for the production of mediators of the innate immune response.
The B. burgdorferi genome encodes a large number of lipoproteins (16), which contribute to monocyte activation. B. burgdorferi, its lipoproteins, or synthetic lipoprotein analogs can activate monocytes through pattern recognition receptors (PRRs) (28, 35, 55, 56, 72). Because uPAR is induced by identical stimuli in monocytic cells (6), we hypothesized that PRRs can initiate the signaling mechanism for its synthesis. To test this, we evaluated the importance of several such receptors to the uPAR response in monocytes/macrophages during stimulation by live B. burgdorferi. We report that a statistically significant reduction in B. burgdorferi-induced uPAR synthesis occurred only when blocking antibodies (Abs) against CD14 and TLR2 are used in combination, confirming the presence of a CD14/TLR2-dependent pathway and providing a functional link between the PRRs of innate immunity and the PAS. None of the other receptors investigated were involved in signaling for uPAR. Vitamin D3 (1,25-dihydroxyvitamin D3) maturation of monocytic cells, after which CD14 (but not TLR2) expression is significantly increased, did not enhance B. burgdorferi-mediated synthesis of uPAR. Independent functional blockade of CD14 or TLR2 in MM6 cells was not sufficient to inhibit induction of uPAR by B. burgdorferi, and peritoneal exudate macrophages (PEM) from CD14- or TLR2-deficient mice were not defective in their potential for B. burgdorferi-mediated uPAR induction. This evidence suggests the utilization of additional pathways that are independent of these receptors.
MATERIALS AND METHODS
Mice.
Mice homozygous null for cd14 (CD14tm1Frm), in the C57BL/6J background, were purchased from Jackson Laboratories, Bar Harbor, Maine. Mice homozygous null for tlr2 (66) in the B6129PF2/J background were a gift from S. Akira, Osaka University, Osaka, Japan, through R. Medzhitov, Yale University, New Haven, Conn. The wild-type (WT) controls for cd14−/− and tlr2−/− mice were C57BL/6J and B6129PF2/J, respectively. PEM from cd14−/− and tlr2−/− mice were tested by Western blot analysis and were confirmed negative for expression of CD14 and TLR2, respectively.
Bacteria.
The infectious N40 strain of B. burgdorferi (1), derived from laboratory-infected mouse skin cultures (6), was used in all experiments. B. burgdorferi was cultured at 33°C in serum-free BSK-H medium (Sigma Chemical Co., St. Louis, Mo.).
MM6 cells.
The mature human monocyte-like cell line Mono Mac 6 (MM6) (73) was cultured in RPMI 1640 supplemented with 2 mM l-glutamine (Gibco-Invitrogen, Grand Island, N.Y.), 10% heat inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, Utah), penicillin (100 U/ml), streptomycin (100 μg/ml), 1% nonessential amino acids (Gibco), and 1× OPI medium supplement (Sigma).
Incubation of MM6 cells with B. burgdorferi in the presence of CD14 and TLR2 blocking Abs.
MM6 cells were washed twice in RPMI 1640 containing 10% FBS (RPMI-FBS) and resuspended at a density of 106 cells/ml in the same medium containing a saturating amount of Ab MY4 (Coulter Corp., Miami, Fla,) or N-17 (Santa Cruz Biotechnology, Santa Cruz, Calif.) (5 μg/ml each) or TL2.1 (eBioscience, San Diego, Calif.) (20 μg/ml). Control treatments included identical levels of mouse immunoglobulin G2b (IgG2b; Sigma; MY4), goat IgG (American Qualex, San Clemente, Calif.; N-17), mouse IgG2a (eBioscience; TL2.1), and medium alone. For experiments in which blocking Abs were combined, control Abs were also combined. The cells were then transferred to 24-well tissue culture plates (Costar, Cambridge, Mass.) and incubated at 37°C for 1 h. B. burgdorferi strain N40 spirochetes were washed twice in RPMI-FBS, resuspended in RPMI-FBS, and added directly to the cells at various ratios. A sham spirochete control preparation from BSK-H medium, subject to the same manipulations, was also included. The cocultures were then incubated for various lengths of time at 37°C and 5% CO2. Abs were present for the total incubation period. At the appropriate time point, the well contents were centrifuged individually and assayed as described below. Cell viability was routinely ≥90% as determined by trypan blue exclusion.
Detection of human cellular uPAR by FACS and human suPAR by quantitative capture ELISA.
MM6 cells were stained with a fluorescein isothiocyanate (FITC)-conjugated murine monoclonal Ab (MAb) against human uPAR (American Diagnostica, Greenwich, Conn.) and analyzed by fluorescence-activated cell sorting (FACS). suPAR in cell-free culture supernatants was measured with the IMUBIND Total uPAR Strip-well enzyme-linked immunosorbent assay (ELISA) kit (American Diagnostica). Both procedures have been described previously (6).
Other MM6 experiments.
In some instances, MM6 cells were treated for 96 h with 50 nM vitamin D3 (Biomol Research Laboratories Inc., Plymouth Meeting, Pa.). MM6 cells were washed with RPMI-FBS prior to the addition of B. burgdorferi and were tested for expression of CD14 and TLR2 by staining with MY4 and N-17. FITC-conjugated secondary Abs were goat anti-mouse and rabbit anti-goat IgG, respectively (Sigma). In other experiments, MM6 cells were pretreated with various concentrations of methyl α-d-mannopyranoside (0, 50, 100, 150, and 200 mM) or N-formyl-methionyl-leucyl-phenylalanine (fMLP; 0, 10−8, 10−6, 10−4, and 10−2 M) (both Sigma) or neutralizing MAb TSI/18 (25 μg/ml; gift from R. T. Coughlin, Cambridge Biotech, Worcester, Mass.) prior to addition of B. burgdorferi (50:1) to assess the contributions of the mannose receptor, the fMLP receptor, and CD11b/CD18, respectively. LPS from Salmonella enterica serovar Typhimurium was purchased from Sigma. Synthetic lipidated hexapeptides and purified native outer surface protein A (N-OspA) which were used to stimulate cells in some experiments, were obtained from Justin Radolf of the University of Connecticut, Storrs.
Isolation of murine PEM.
PEM from 8- to 12-week-old male cd14−/−, tlr2−/−, or respective WT mice were cultured as follows. Mice were injected intraperitoneally with 2 ml of 3% thioglycolate broth, Brewer modification (Difco, Detroit, Mich.). At 4 days postinjection, the mice were sacrificed by CO2 narcosis. PEM were harvested by peritoneal lavage with 10 ml of RPMI 1640 containing l-glutamine and penicillin-streptomycin. The PEM were washed twice with RPMI 1640-l-glutamine-penicillin-streptomycin and resuspended to a density of 2 × 106 cells/ml in the same medium supplemented with 1% autologous serum. PEM were added to the wells of a 24-well tissue culture plate (Costar) at a density of 106/well and incubated for 3 h at 37°C and 5% CO2. Nonadherent cells were removed by washing the monolayers twice with 1 ml of RPMI 1640/well prior to addition of stimuli.
Incubation of murine PEM with B. burgdorferi.
PEM were incubated with 1, 10, and 100 spirochetes/cell as well as with a sham spirochete preparation. Cocultures were incubated for 24 h at 37°C and 5% CO2. Some cocultures were done in the absence of serum. Murine PEM that adhered to 12-mm-diameter round coverglasses were stained indirectly for uPAR with a rabbit anti-mouse uPAR Ab (gift from Thomas H. Bugge, National Institutes of Health, Bethesda, Md.). The secondary Ab was goat anti-mouse IgG conjugated to FITC (Sigma).
Detection of murine uPAR transcript by RT-PCR.
Adherent PEM from PEM-B. burgdorferi cocultures were rinsed with RPMI 1640. Total RNA was isolated from the cells (RNeasy minikit; Qiagen, Valencia, Calif.), and RNA was quantified by spectrophotometry at 260 nm. Total cDNA synthesis was carried out with 0.4 μg of total RNA (RETROscript first-strand synthesis kit; Ambion, Austin, Tex.) and random decamer primers. Multiplex PCR for murine uPAR and β-actin was carried out with 5 μl of DNA from the reverse transcription (RT) reaction mixture and 30 pM primer in a Perkin-Elmer GeneAmp PCR System 9600 thermocycler (Applied Biosystyems, Foster City, Calif.). Primer sequences were as follows: mouse uPAR FWD, 5′-CAGGACCTCTGCAGGAC-3′; mouse uPAR REV, 5′-CCGGCCCCTCTCACAGCTC-3′ (270 bp); mouse β-actin-FWD, 5′-GACGAGGCCCAGAGCAAGAG-3′; mouse β-actin-REV, 5′-AGCCAGGTCCAGACGCAGGA-3′ (378 bp). PCR conditions consisted of 96°C for 1 min, followed by 25 cycles of 96°C for 30 s, 55°C for 30 s, and 72° for 1 min. The linearity of amplification of the β-actin product was the criterion for the selection of the total cycle number of 25. Following amplification, PCR products were separated on 2% agarose gels and stained with ethidium bromide.
Detection of murine suPAR by Western blotting.
Serum-free supernatants from PEM-B. burgdorferi cocultures were centrifuged at 4°C for 3 min at 10,000 × g and concentrated approximately eightfold (Amicon Microcon; Millipore Corp., Bedford, Mass.). Equivalent amounts of each sample were separated by nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis in gels of 12.5% polyacrylamide as has been described previously (6).
Detection of proinflammatory mediators by RT-PCR.
Following incubation of MM6 with B. burgdorferi, total RNA isolation and cDNA synthesis (from 2 μg of RNA) were carried out as described above. RT-PCR for IL-1β, IL-6, IL-8, (CXCL8), TNF-α, and gamma interferon was carried out with the Relative RT-PCR kit (Ambion) according to the manufacturer's instructions, with primers specific for each proinflammatory mediator type. The internal standard provided with the kit was 18S rRNA. RT-PCR products were electrophoresed and visualized as described above.
RESULTS
Exposure to B. burgdorferi results in an increase in cellular uPAR and suPAR in MM6 cells.
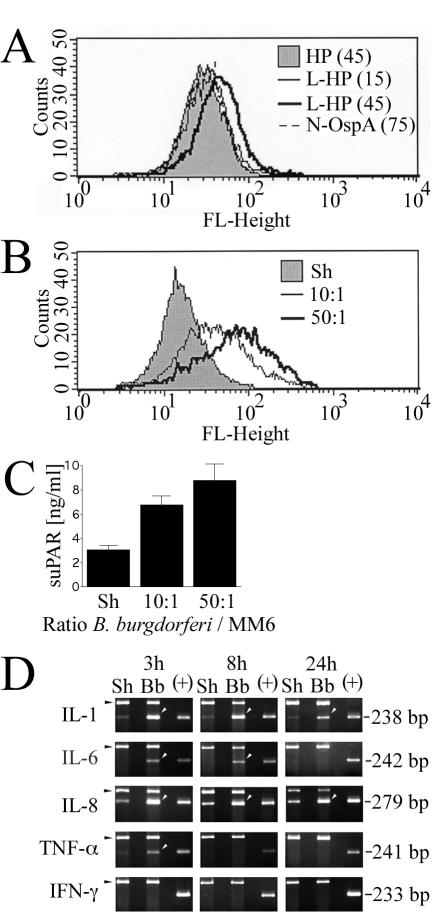
We have previously reported that exposure of human monocytic cells to B. burgdorferi in vitro results in an increase in both cellular uPAR and suPAR protein as well as uPAR mRNA (6). For this series of studies, we used the human cell line MM6, which exhibits phenotypic and functional characteristics of mature monocytes (73). The original objective was to determine the role of PRRs of the innate immune system in overexpression of uPAR by B. burgdorferi-stimulated monocytic cells. For these experiments we used both purified native outer surface protein A (N-OspA), a synthetic lipidated hexapeptide corresponding the N terminus of OspA, and live spirochetes. A nonstimulatory nonlipidated OspA control hexapeptide was also included. Cellular uPAR and suPAR levels were measured and compared to those for unstimulated control cells. The N-OspA and the synthetic peptide analogs were used at concentrations similar to those used previously to elicit the production of proinflammatory cytokines (56). Although measurable, the uPAR-stimulating capacity of these preparations was poor in comparison to that of whole B. burgdorferi (Fig. 1A and B); consequently, all experiments were carried out with live organisms. The utility of the whole-B. burgdorferi/MM6 cell model is shown in Fig. 1, in which undifferentiated MM6 cells were coincubated for 24 h with several concentrations of B. burgdorferi and the uPAR response was measured. This treatment resulted in a dose-dependent cellular uPAR increase (Fig. 1B). suPAR released into the conditioned medium was measured by a specific capture ELISA (Fig. 1C), and the level of suPAR followed a pattern similar to that for cellular uPAR.
FIG. 1.
uPAR response and proinflammatory mediator profile of MM6 cells exposed to B. burgdorferi components and/or live B. burgdorferi. uPAR in MM6 cells was determined by staining cells with a FITC-labeled murine MAb against human uPAR, followed by FACS. An isotype-matched irrelevant Ab conjugated to FITC was used to control for nonspecific Ab binding. (A) Expression of cellular uPAR in MM6 cells exposed to a nonlipidated OspA hexapeptide (HP) at 45 μg/ml, a lipidated hexapeptide (L-HP) at 15 and 45 μg/ml, and a purified native OspA lipoprotein (N-OspA) at 75 ng/ml. (B) Expression of cellular uPAR in MM6 cells exposed to B. burgdorferi at ratios of 10 and 50 spirochetes/cell or a sham B. burgdorferi preparation (Sh). (C) Measurement of release of suPAR into conditioned medium as measured by specific capture ELISA. Results shown are mean values ± standard deviations of duplicate samples from one of two experiments in which the results were consistent. (D) Cytokine/chemokine-specific mRNA responses of MM6 cells exposed to B. burgdorferi over time. MM6 cells (106/well) were coincubated with 50 B. burgdorferi spirochetes/cell for 24 h. Black arrowheads, 18S rRNA internal induction controls (495 bp); white arrowheads, cytokine transcript; +, kit positive cytokine PCR control. Data shown are from one of two independent experiments in which the results were similar.
The kinetics of induction of specific proinflammatory mediators after exposure to B. burgdorferi of MM6 cells was investigated by relative (semiquantitative) RT-PCR (Fig. 1D). Both IL-1β and IL-8 (CXCL8) mRNAs were consistently expressed at low levels in uninduced controls but were greatly increased by exposure to B. burgdorferi at every time point tested (3, 8, and 24 h). IL-6 and TNF-α mRNAs were differentially expressed, with IL-6 being induced at 3 and 8 h but turned off at 24 h. TNF-α was detectable at 3 h but was turned off at all later time points. Gamma interferon was not expressed at any time point by either control MM6 cells or MM6 cells exposed to B. burgdorferi. These experiments (Fig. 1) show that MM6 cells respond to B. burgdorferi by upregulating cellular uPAR and suPAR, as well as a variety of proinflammatory mediators.
Evaluation of several PRRs for roles in B. burgdorferi-mediated uPAR induction in MM6 cells: only functional inhibition of CD14 and TLR2 results in a decrease in uPAR expression.
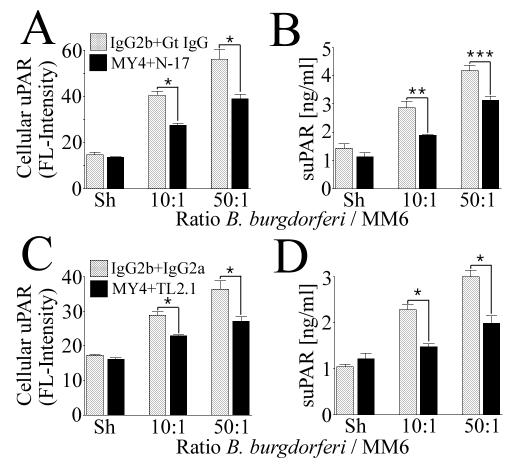
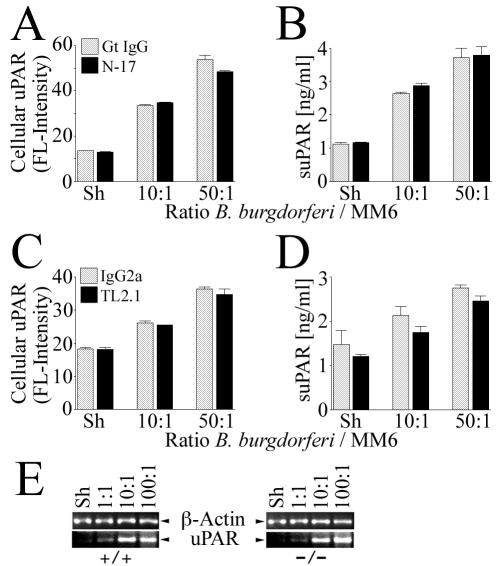
In a number of studies utilizing B. burgdorferi lipoproteins and/or whole-cell lysates, it has been shown that PRRs are important inflammatory cell receptors for spirochete-mediated cell signaling (28, 35, 55, 56). We hypothesized that PRRs could also be important for uPAR induction and tested their role with MM6 cells, using Ab neutralization and competitive-inhibition approaches. As a result of the functional blockade of CD11b/CD18 with a neutralizing Ab, of the mannose receptor with four concentrations of methyl α-d-mannopyranoside (ranging from 50 to 200 mM), and of the formyl peptide fMLP receptor with four concentrations of fMLP (ranging from 10−8 to 10−2 M), there was no discernible effect on either B. burgdorferi-mediated cellular uPAR or suPAR levels (data not shown). Consequently, these PRRs were not considered further. The only PRRs for which roles in B. burgdorferi-mediated uPAR synthesis could be identified were CD14 and TLR2. For these experiments, MM6 cells were treated with saturating concentrations of MY4, a murine MAb known to interfere with CD14 signaling (72), and N-17, a TLR2-neutralizing polyclonal Ab (15) or MY4 and TL2.1, a TLR2-neutralizing murine MAb (14, 35), prior to and during coincubation with B. burgdorferi. Functional blockade of CD14 and TLR2 resulted in a statistically significant and reproducible decrease in both cellular uPAR and suPAR (Fig. 2). The inhibition occurred at both concentrations of B. burgdorferi tested (10 and 50 B. burgdorferi spirochetes/cell). Inhibition approached reduction to baseline levels only with suPAR and MY4 and TL2.1 (Fig. 2D).
FIG. 2.
Combined functional blockade of CD14 and TLR2 results in significant, but not total, inhibition of B. burgdorferi-dependent uPAR induction in MM6 cells. MM6 cells were cocultured for 24 h with B. burgdorferi at ratios of 10 and 50 spirochetes/cell or a sham B. burgdorferi preparation (Sh). (A and B) B. burgdorferi-mediated expression of cellular and suPAR in the presence of neutralizing Abs MY4 and N-17 as detected by staining of cells with a MAb against human uPAR and analysis by FACS or analysis of conditioned medium by uPAR-specific capture ELISA. (C and D) The same experiment was carried out with neutralizing Abs MY4 and TL2.1. Data shown are from one of two independent experiments in which the results were similar. Data were tested for statistical significance (asterisks) by a two-tailed Student t test (*, P < 0.05; ***, P < 0.01) or the alternate Welch t test (**, P < 0.05).
Vitamin D3 maturation of MM6 cells does not result in an increased uPAR response to B. burgdorferi.
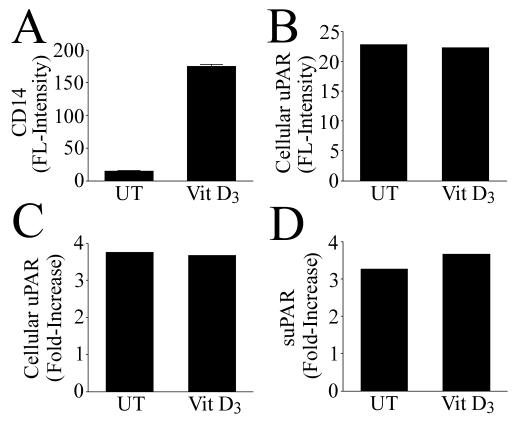
Blockade of CD14 and TLR2 in MM6 cells resulted in a partial inhibition of uPAR levels after exposure to B. burgdorferi, implicating either or both of these receptors, but also indicating a potential for diverse signaling pathways leading to uPAR synthesis. We next attempted to dissect the result shown in Fig. 2 by determining the singular contributions of CD14 and TLR2. We investigated the role of CD14 in B. burgdorferi-induced uPAR synthesis by subjecting MM6 cells to vitamin D3 maturation for 96 h prior to the addition of B. burgdorferi. Vitamin D3 maturation by itself resulted in an approximately 12-fold increase in cell surface CD14 expression as measured by FACS (Fig. 3A) but did not result in an increase in TLR2 expression (not shown). Cellular uPAR levels also were unaffected by vitamin D3 alone (Fig. 3B). Coincubation of vitamin D3-matured MM6 cells with B. burgdorferi did not result in an increase in cellular uPAR or suPAR in comparison to levels in undifferentiated MM6 cells (Fig. 3C and D). B. burgdorferi alone had no effect on CD14 levels (not shown).
FIG. 3.
Vitamin D3 maturation results in an increase in CD14 but does not affect B. burgdorferi-mediated uPAR induction in MM6 cells. (A) Cell surface CD14 expression by MM6 after pretreatment with vitamin D3, as measured by FACS. (B) Cellular uPAR expression by MM6 after pretreatment with vitamin D3 (FACS). (C) Increase of cellular uPAR in B. burgdorferi-induced over sham-induced MM6 cells in the presence or absence of vitamin D3 (FACS). (D) Increase of suPAR in B. burgdorferi-induced over sham-induced MM6 cells in the presence or absence of vitamin D3 (ELISA). UT, dimethyl sulfoxide alone. The ratio of B. burgdorferi spirochetes to MM6 cells was 50:1. Data are from one of two independent experiments in which the results were similar.
Functional blockade of CD14 alone does not inhibit B. burgdorferi-induced uPAR upregulation but does inhibit LPS-induced uPAR upregulation in MM6 cells.
Initial evidence indicated that increased CD14 expression in MM6 cells did not result in increased B. burgdorferi-induced uPAR induction by the cells (Fig. 3). It is possible that the presence of additional CD14 on the monocytic cell surface was not necessary for uPAR upregulation and that constitutive levels of CD14 would be sufficient to drive the response. We used MY4 to block CD14 functionally prior to and during coincubation with either 10 or 50 B. burgdorferi spirochetes/cell. The difference in B. burgdorferi-induced cellular uPAR (Fig. 4A) and suPAR (Fig. 4B) expression by MM6 cells treated with mouse IgG2b and that by cells treated with MY4 was not statistically significant (P > 0.05).
FIG. 4.
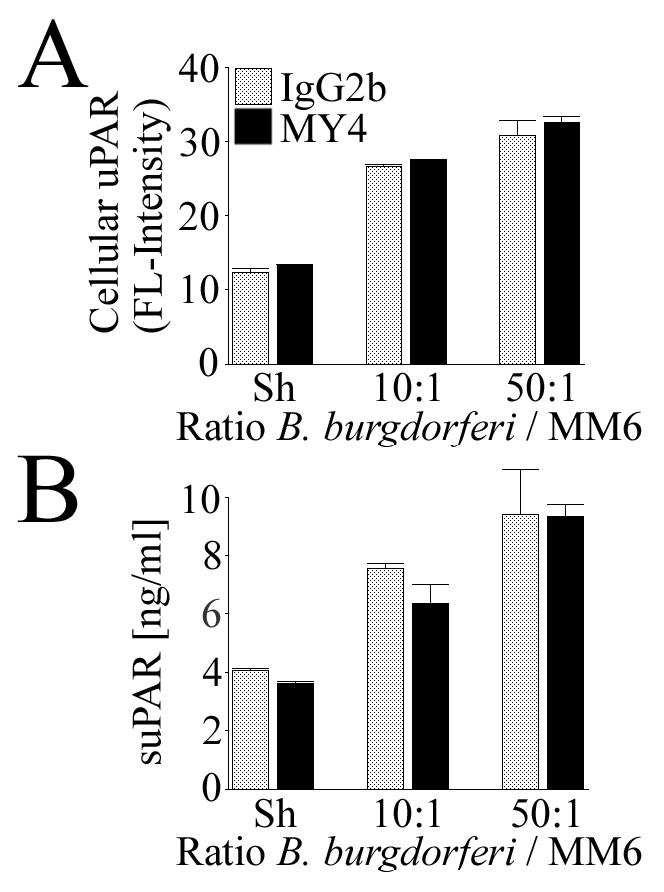
Functional blockade of CD14 does not significantly inhibit B. burgdorferi-dependent uPAR induction in MM6 cells. MM6 cells were cocultured for 24 h with B. burgdorferi at ratios of 10 and 50 spirochetes/cell or a sham B. burgdorferi preparation (Sh) in the presence or absence of MY4. (A) Expression of cellular uPAR as detected by staining of cells with a MAb against human uPAR and analysis by FACS. (B) Expression of suPAR in conditioned medium as measured by specific capture ELISA. Data are from one of two independent experiments in which the results were similar.
Prior work in our laboratory has shown that LPS can induce the upregulation of uPAR in monocytic cells (6). To test the efficacy of MY4 in blocking CD14 signaling, MM6 cells were stimulated with LPS in the presence of MY4 and the levels of cellular uPAR and suPAR were measured. The presence of MY4 significantly inhibited the LPS-mediated induction of cellular uPAR (Fig. 5A) and suPAR (Fig. 5B) in these cells.
FIG. 5.
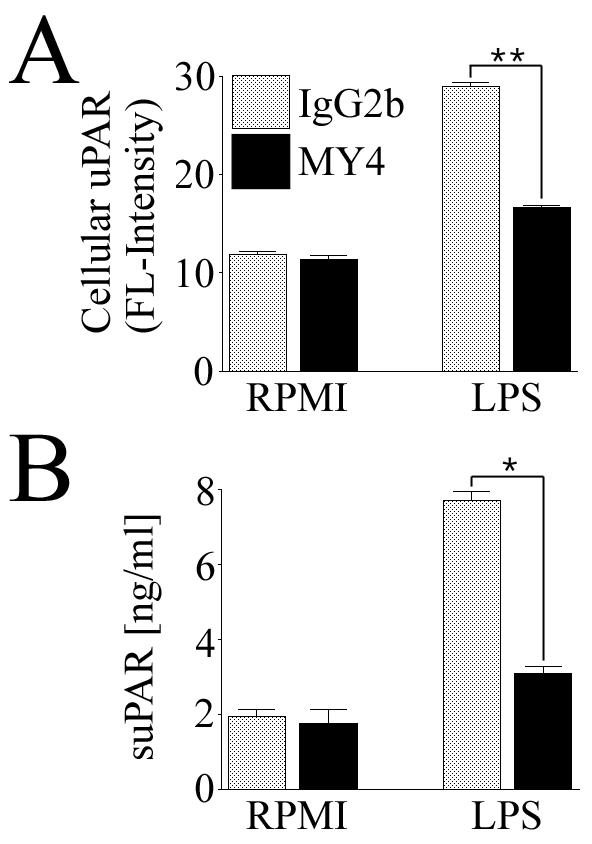
Functional blockade of CD14 significantly inhibits LPS-dependent uPAR induction in MM6 cells. MM6 cells were cultured for 24 h with LPS (500 ng/ml) in the presence or absence of MY4. Untreated cells received LPS carrier (RPMI 1640) alone. (A) Expression of cellular uPAR after staining of cells with a MAb against human uPAR and analysis by FACS. (B) Expression of suPAR in conditioned medium as measured by specific capture ELISA. Data are from one of two independent experiments in which the results were similar. Data were tested for statistical significance (asterisks) by a two-tailed Student t test (*, P < 0.001; **, P < 0.01).
Targeted disruption of cd14 in murine PEM does not decrease their potential for B. burgdorferi-mediated uPAR induction.
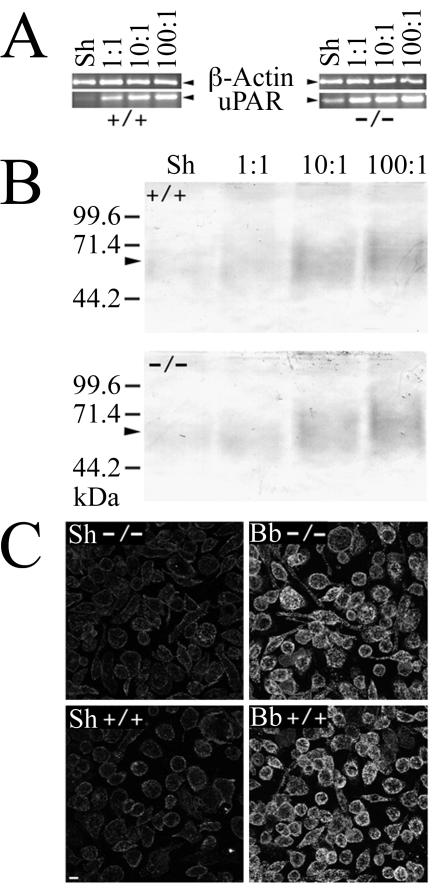
We sought additional information on the relevance of CD14 to B. burgdorferi-induced uPAR upregulation by measuring the uPAR response of PEM from mice genetically deficient in cd14. PEM from CD14-deficient and WT mice were incubated for 24 h with a range of B. burgdorferi concentrations. Following coincubation, RNA was isolated from the PEM, followed by RT-PCR to measure the uPAR transcript. There was no difference in the B. burgdorferi-induced uPAR response between CD14-deficient and WT control mice, as both responded in an equivalent and dose-dependent manner. A considerable increase in the uPAR transcript was detected in both experimental groups with as few as 1 spirochete per cell (Fig. 6A). A similar pattern was demonstrated for suPAR protein levels in conditioned medium (Fig. 6B). There was no difference in cellular uPAR expression between CD14-deficient and WT mouse-derived PEM as determined by indirect immunofluorescence (Fig. 6C).
FIG. 6.
PEM from CD14-deficient mice are not deficient in B. burgdorferi-mediated upregulation of cellular uPAR and suPAR. PEM isolated from CD14-deficient and WT mice were cocultured for 24 h with B. burgdorferi at ratios of 1, 10, and 100 spirochetes/cell or a sham B. burgdorferi preparation (Sh). (A) RT-PCR showing uPAR transcript in PEM from cd14+/+ and cd14−/− mice. (B) Western blots showing levels of suPAR in conditioned medium. (C) Confocal image showing expression levels of cellular uPAR by cd14+/+ and cd14−/− PEM. Bar, 10 μm.
Functional blockade of TLR2 by neutralizing Abs or targeted disruption of tlr2 does not inhibit B. burgdorferi-induced uPAR upregulation.
In a manner similar to that for CD14, we investigated the role served by TLR2 in B. burgdorferi-dependent uPAR induction in MM6 cells by use of TLR2-neutralizing Abs N-17 (15) and TL2.1 (14, 35). Blockade of TLR2 with a saturating concentration of N-17 (Fig. 7A and B) or TL2.1 (Fig. 7C and D) did not significantly inhibit the levels of induction of either cellular uPAR or suPAR in MM6 cells exposed to B. burgdorferi at levels of 10 or 50 spirochetes/cell (P > 0.05). Exposure of MM6 cells to B. burgdorferi alone had no effect on TLR2 expression (not shown). Targeted disruption of tlr2 in murine PEM did not alter their capacity for uPAR mRNA synthesis in response to B. burgdorferi (Fig. 7E).
FIG. 7.
Functional blockade or targeted disruption of TLR2 does not significantly inhibit B. burgdorferi-dependent uPAR induction. MM6 cells or murine PEM were cocultured for 24 h with various ratios of B. burgdorferi or a sham B. burgdorferi preparation (Sh). (A and B) B. burgdorferi-mediated expression of cellular uPAR and suPAR in the presence of neutralizing Ab N-17 as detected by staining of cells with a MAb against human uPAR and analysis by FACS or analysis of conditioned medium by uPAR-specific capture ELISA. (C and D) The same experiment was carried out with neutralizing Ab TL2.1. (E) RT-PCR showing B. burgdorferi-mediated uPAR transcripts in PEM from tlr2+/+ and tlr2−/− mice. Data are from one of two independent experiments in which the results were similar.
DISCUSSION
In its capacity as the cellular receptor for uPA (40, 65, 68), uPAR has a stabilizing effect on its ligand, which allows the serine protease to convert bound PLG to plasmin. Enzymatically active plasmin, in turn, can degrade extracellular matrix proteins directly or indirectly through activation of pro-MMPs and can thereby promote the migration of inflammatory and tumor cells. The binding of uPA to uPAR is essential for tissue invasion by human monocytes (31), and uPAR expression has been associated with a negative prognosis in many human cancers (2). Furthermore, a consequence of targeted disruption of uPAR in mice is a significant decrease in uPA-mediated PLG activation (5), further confirming the importance of this molecule in plasmin formation. However, accumulating evidence has implicated uPAR in a variety of nonproteolytic roles in addition to its role in the acquisition of pericellular proteolytic activity. uPAR has a function in cellular adhesion by virtue of its affinity for vitronectin (69) and by complex formation with CD11b/CD18 (61), which modifies the expression of CD11B/CD18 to an adherence phenotype (60). uPAR is also involved in the recruitment of inflammatory and cancer cells through induction of chemotaxis in which both uPA-independent (24) and -dependent (50) mechanisms have been reported. Recently, a study has shown that suPAR binds to the G protein-coupled formyl peptide receptor FPRL1/LXA4R, which results in increased cell chemotaxis (51). The association of uPAR with the bacterial N-formyl peptide receptor provides evidence for a formal link between the PAS and inflammatory and/or infectious conditions at the signaling level. Finally, the association of uPAR with a variety of signaling molecules has led to the hypothesis that it can mediate signal transduction.
Although uPAR is anchored to the plasma membrane through its GPI linkage, the molecule can be cleaved by GPI-specific phospholipases (48, 71), leading to the release in soluble form of a full-length molecule containing D1, D2, and D3 (suPAR). In addition, a naturally occurring and therefore clinically relevant suPAR variant containing D2 and D3 can be found under physiologic and pathological conditions (4, 20, 58, 59, 62-64). uPAR can also be cleaved by uPA to release D1 (29, 63). In cancer, suPAR is thought to be of prognostic value (4).
The innate immune response includes macrophages and neutrophils that ingest and kill microbial pathogens, with the concomitant coordination of further host responses through production of a variety of proinflammatory mediators. Pathogens are identified through PRRs such as CD14 and Toll-like receptors (TLRs) on leukocytes, which recognize conserved pathogen-associated motifs such as formylated peptides, LPS, lipopeptides, peptidoglycan, and teichoic acids. Studies have shown that CD14 and TLR2 are important in bacterial lipoprotein-mediated induction of proinflammatory cytokines (22, 56, 72). The Toll gene, initially described as a dorsoventral patterning regulatory gene in Drosophila (41), controls the induction of antifungal peptides (33). Mutations in Toll result in Drosophila that cannot overcome fungal infection (33). The discovery of human TLR homologs (38, 53), 10 of which have been identified to date, and their expression primarily on macrophages and dendritic cells, suggested a function in vertebrate immunity. Recently, it has been shown that bacterial lipoproteins trigger host defense mechanisms through TLRs (3). More specifically, TLR2 has been identified as a mediator of inflammatory signaling by B. burgdorferi (28, 35), Treponema pallidum, Mycobacterium avium, Staphylococcus aureus (35), and Leptospira interrogans (70). In B. burgdorferi, TLR2-dependent signaling is facilitated by CD14 (28). Additionally, TLR2 is a signaling mediator for Listeria monocytogenes but not for group B streptococci (14), implying that it can distinguish between these two pathogens. Interestingly, it is LPS, not a lipoprotein, that is the primary signaling molecule in Leptospira interrogans (70). The fact that such a diverse collection of bacteria induces activation through TLR2 underscores its role as a PRR of central importance.
We have provided evidence for a CD14/TLR2-mediated signaling pathway controlling B. burgdorferi-dependent uPAR induction in monocytic cells, as well as evidence for a CD14/TLR2-independent mechanism. The CD14/TLR2-dependent pathway was not apparent in experiments that focused on these receptors individually. Vitamin D3 maturation of MM6 resulted in a 12-fold increase in cell surface CD14, but this increase did not translate to enhanced induction of uPAR in response to B. burgdorferi. Murine macrophages containing targeted disruptions in cd14 or tlr2 were not deficient in their capacity for increased uPAR synthesis in response to B. burgdorferi. Independent functional blockade of either CD14 or TLR2 by specific Abs failed to result in significant inhibition of the B. burgdorferi-mediated uPAR response. Only with combined Ab blockade was a statistically significant inhibition of uPAR realized. The reason for the combinatorial Ab synergism is unknown. The phenomenon has been reported previously, however, for Listeria monocytogenes-mediated TNF production in monocytic cells (14). One possible explanation is that steric interference caused by the presence of both Abs as opposed to each one individually prevents B. burgdorferi from binding to one or both receptors. The CD14/TLR2-independent result was unexpected, given the documented importance of these molecules for B. burgdorferi-mediated activation of macrophages (22, 28, 35, 55, 56). It must be noted, however, that studies on B. burgdorferi and CD14/TLR interactions published thus far have utilized purified lipoproteins, lipopeptide analogs, or whole-cell sonicates. It is conceivable that macrophages respond to live, motile spirochetes in a manner different from that in which they respond to purified spirochetal components. That is, exposure to a structurally complex pathogen results in a compound response by the macrophage. The concept of recognition redundancy of microbes is supported by previous studies in which it has been shown that CD14 and TLR2 are not critically involved in protection against Mycobacterium tuberculosis infections and that mice deficient in these PRRs are not defective in their resistance to a naturally acquired infection (49). In addition, TLR2 is not required for direct killing of Listeria monocytogenes by macrophages (12), moving the authors to hypothesize that one or more additional TLRs may be involved. Finally, we have previously shown that secondary messengers play a role in B. burgdorferi-mediated uPAR induction in monocytic cells (6), suggesting that its signaling may be more complex, and under the influence of inflammatory mediators induced by other receptors. Taken together, these results demonstrate that intact B. burgdorferi can stimulate monocytic cells through redundant cell surface signaling receptors. The elucidation of the CD14/TLR2-independent signaling pathway, utilizing an unknown monocytic cell signaling component(s) (which could be one or more additional TLRs), will require further investigation. This could be done by use of mice engineered to be deficient in multiple TLRs. It is also interesting to speculate as to which spirochetal components, in addition to lipoproteins, may be contributing factors. Peptidoglycan is probably not involved, due to its requirement for TLR2 (54), which was neutralized in the Ab experiments and absent in macrophages from transgenic mice. More-likely spirochete components are CG dinucleotides (CpGs) potentially contained in B. burgdorferi DNA, acting through macrophage TLR9 (27), and flagellin, as recognized by TLR5 (26). The CD14/TLR2-dependent signaling pathway that we have identified, however, demonstrates through its presence a clear signaling role for CD14 and TLR2 in B. burgdorferi-mediated uPAR induction. Significantly, the finding that uPAR synthesis can be induced through CD14 and TLR2 signaling establishes a new functional link between the PAS and the innate immune system. That both systems can be linked by common receptor utilization is of physiologic importance. Our data have demonstrated that there is cross talk between the multifunctional host PAS, which can also promote bacterial dissemination, and the innate immune response, a phylogenetically ancient system for microbial recognition and killing. In the context of infection, an appropriate question to ask is whether the coincident stimulation of these two systems works to the advantage of the host or B. burgdorferi. uPAR is required for adequate recruitment of neutrophils in pneumonia due to Streptococcus pneumoniae (52) and Pseudomonas aeruginosa (23). In these instances, the presence of uPAR is consistent with a role in host defense against bacteria. Conversely, it has been shown that overexpression of suPAR is a predictor of disease progression in human immunodeficiency virus infection (59), and pertussis toxin-mediated activation of uPAR, through CD14, can downregulate macrophage migratory capacity and thus promote Bordetella pertussis infection (34). Moreover, there is the possibility that uPA bound to suPAR can assist in the acquisition of bacterially bound plasmin that could in turn result in greater and more efficient dissemination (7-9, 18, 30, 32, 43). The existence of such contrasting evidence further emphasizes the requirement for further study to clarify the role(s) of uPAR in bacterial pathogenesis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR-40445 and AI-27044) and the Mathers Foundation (to J.L.B.).
We thank S. Akira, Osaka University, and R. Medzhitov, Yale University, for their generous gift of tlr−/− mice. We thank Joseph Gebbia, State of New York Department of Health, Stony Brook University, Stony Brook, for helpful discussions and assistance with mouse macrophage experiments.
Editor: J. T. Barbieri
REFERENCES
- 1.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 2.Blasi, F. 1999. Proteolysis, cell adhesion, chemotaxis, and invasiveness are regulated by the u-PA-u-PAR-PAI-1 system. Thromb. Haemostasis 82:298-304. [PubMed] [Google Scholar]
- 3.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 4.Brunner, N., H. J. Nielsen, M. Hamers, I. J. Christensen, O. Thorlacius-Ussing, and R. W. Stephens. 1999. The urokinase plasminogen activator receptor in blood from healthy individuals and patients with cancer. APMIS 107:160-167. [DOI] [PubMed] [Google Scholar]
- 5.Bugge, T. H., T. T. Suh, M. J. Flick, C. C. Daugherty, J. Romer, H. Solberg, V. Ellis, K. Dano, and J. L. Degen. 1995. The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. J. Biol. Chem. 270:16886-16894. [DOI] [PubMed] [Google Scholar]
- 6.Coleman, J. L., J. A. Gebbia, and J. L. Benach. 2001. Borrelia burgdorferi and other bacterial products induce expression and release of the urokinase receptor (CD87). J. Immunol. 166:473-480. [DOI] [PubMed] [Google Scholar]
- 7.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 8.Coleman, J. L., E. J. Roemer, and J. L. Benach. 1999. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect. Immun. 67:3929-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubellis, M. V., M. L. Nolli, G. Cassani, and F. Blasi. 1986. Binding of single-chain prourokinase to the urokinase receptor of human U937 cells. J. Biol. Chem. 261:15819-15822. [PubMed] [Google Scholar]
- 11.Dear, A. E., and R. L. Medcalf. 1998. The urokinase-type-plasminogen-activator receptor (CD87) is a pleiotropic molecule. Eur. J. Biochem. 252:185-193. [DOI] [PubMed] [Google Scholar]
- 12.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 13.Farias-Eisner, R., L. Vician, A. Silver, S. Reddy, S. A. Rabbani, and H. R. Herschman. 2000. The urokinase plasminogen activator receptor (UPAR) is preferentially induced by nerve growth factor in PC12 pheochromocytoma cells and is required for NGF-driven differentiation. J. Neurosci. 20:230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 15.Frantz, S., R. A. Kelly, and T. Bourcier. 2001. Role of TLR-2 in the activation of nuclear factor κB by oxidative stress in cardiac myocytes. J. Biol. Chem. 276:5197-5203. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs, H., M. M. Simon, R. Wallich, M. Bechtel, and M. D. Kramer. 1996. Borrelia burgdorferi induces secretion of pro-urokinase-type plasminogen activator by human monocytes. Infect. Immun. 64:4307-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs, H., R. Wallich, M. M. Simon, and M. D. Kramer. 1994. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc. Natl. Acad. Sci. USA 91:12594-12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Monco, J. C., and J. L. Benach. 1995. Lyme neuroborreliosis. Ann. Neurol. 37:691-702. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Monco, J. C., J. L. Coleman, and J. L. Benach. 2002. Soluble urokinase receptor (uPAR, CD87) is present in serum and cerebrospinal fluid in patients with neurologic diseases. J. Neuroimmunol. 129:216-223. [DOI] [PubMed] [Google Scholar]
- 21.Gebbia, J. A., J. L. Coleman, and J. L. Benach. 2001. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun. 69:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 1999. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immun. 67:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyetko, M. R., S. Sud, T. Kendall, J. A. Fuller, M. W. Newstead, and T. J. Standiford. 2000. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J. Immunol. 165:1513-1519. [DOI] [PubMed] [Google Scholar]
- 24.Gyetko, M. R., R. F. Todd III, C. C. Wilkinson, and R. G. Sitrin. 1994. The urokinase receptor is required for human monocyte chemotaxis in vitro. J. Clin. Investig. 93:1380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa, T., L. Sorensen, M. Dohi, N. V. Rao, J. R. Hoidal, and B. C. Marshall. 1997. Induction of urokinase-type plasminogen activator receptor by IL-1 beta. Am. J. Respir. Cell Mol. Biol. 16:683-692. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 27.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 28.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 29.Hoyer-Hansen, G., M. Ploug, N. Behrendt, E. Ronne, and K. Dano. 1997. Cell-surface acceleration of urokinase-catalyzed receptor cleavage. Eur. J. Biochem. 243:21-26. [DOI] [PubMed] [Google Scholar]
- 30.Hu, L. T., G. Perides, R. Noring, and M. S. Klempner. 1995. Binding of human plasminogen to Borrelia burgdorferi. Infect. Immun. 63:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirchheimer, J. C., and H. G. Remold. 1989. Endogenous receptor-bound urokinase mediates tissue invasion of human monocytes. J. Immunol. 143:2634-2639. [PubMed] [Google Scholar]
- 32.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, R. Hu, S. A. Limentani, and R. A. Rogers. 1995. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 171:1258-1265. [DOI] [PubMed] [Google Scholar]
- 33.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 34.Li, H., and W. S. Wong. 2000. Mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of CD14 and urokinase receptor. Immunology 100:502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 36.Lund, L. R., J. Romer, E. Ronne, V. Ellis, F. Blasi, and K. Dano. 1991. Urokinase-receptor biosynthesis, mRNA level and gene transcription are increased by transforming growth factor beta 1 in human A549 lung carcinoma cells. EMBO J. 10:3399-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund, L. R., E. Ronne, A. L. Roldan, N. Behrendt, J. Romer, F. Blasi, and K. Dano. 1991. Urokinase receptor mRNA level and gene transcription are strongly and rapidly increased by phorbol myristate acetate in human monocyte-like U937 cells. J. Biol. Chem. 266:5177-5181. [PubMed] [Google Scholar]
- 38.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 39.Miles, L. A., and E. F. Plow. 1987. Receptor mediated binding of the fibrinolytic components, plasminogen and urokinase, to peripheral blood cells. Thromb. Haemostasis 58:936-942. [PubMed] [Google Scholar]
- 40.Min, H. Y., R. Semnani, I. F. Mizukami, K. Watt, R. F. Todd III, and D. Y. Liu. 1992. cDNA for Mo3, a monocyte activation antigen, encodes the human receptor for urokinase plasminogen activator. J. Immunol. 148:3636-3642. [PubMed] [Google Scholar]
- 41.Morisato, D., and K. V. Anderson. 1994. The spatzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell 76:677-688. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen, L. S., G. M. Kellerman, N. Behrendt, R. Picone, K. Dano, and F. Blasi. 1988. A 55,000-60,000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. J. Biol. Chem. 263:2358-2363. [PubMed] [Google Scholar]
- 43.Nordstrand, A., A. Shamaei-Tousi, A. Ny, and S. Bergstrom. 2001. Delayed invasion of the kidney and brain by Borrelia crocidurae in plasminogen-deficient mice. Infect. Immun. 69:5832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nykjaer, A., B. Moller, R. F. Todd III, T. Christensen, P. A. Andreasen, J. Gliemann, and C. M. Petersen. 1994. Urokinase receptor. An activation antigen in human T lymphocytes. J. Immunol. 152:505-516. [PubMed] [Google Scholar]
- 45.Nykjaer, A., C. M. Petersen, B. Moller, P. A. Andreasen, and J. Gliemann. 1992. Identification and characterization of urokinase receptors in natural killer cells and T-cell-derived lymphokine activated killer cells. FEBS Lett. 300:13-17. [DOI] [PubMed] [Google Scholar]
- 46.Perides, G., L. M. Tanner-Brown, M. A. Eskildsen, and M. S. Klempner. 1999. Borrelia burgdorferi induces matrix metalloproteinases by neural cultures. J. Neurosci. Res. 58:779-790. [DOI] [PubMed] [Google Scholar]
- 47.Ploug, M., H. Rahbek-Nielsen, P. F. Nielsen, P. Roepstorff, and K. Dano. 1998. Glycosylation profile of a recombinant urokinase-type plasminogen activator receptor expressed in Chinese hamster ovary cells. J. Biol. Chem. 273:13933-13943. [DOI] [PubMed] [Google Scholar]
- 48.Ploug, M., E. Ronne, N. Behrendt, A. L. Jensen, F. Blasi, and K. Dano. 1991. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J. Biol. Chem. 266:1926-1933. [PubMed] [Google Scholar]
- 49.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 50.Resnati, M., M. Guttinger, S. Valcamonica, N. Sidenius, F. Blasi, and F. Fazioli. 1996. Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO J. 15:1572-1582. [PMC free article] [PubMed] [Google Scholar]
- 51.Resnati, M., I. Pallavicini, J. M. Wang, J. Oppenheim, C. N. Serhan, M. Romano, and F. Blasi. 2002. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc. Natl. Acad. Sci. USA 99:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rijneveld, A. W., M. Levi, S. Florquin, P. Speelman, P. Carmeliet, and T. van Der Poll. 2002. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J. Immunol. 168:3507-3511. [DOI] [PubMed] [Google Scholar]
- 53.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 55.Sellati, T. J., D. A. Bouis, M. J. Caimano, J. A. Feulner, C. Ayers, E. Lien, and J. D. Radolf. 1999. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 163:2049-2056. [PubMed] [Google Scholar]
- 56.Sellati, T. J., D. A. Bouis, R. L. Kitchens, R. P. Darveau, J. Pugin, R. J. Ulevitch, S. C. Gangloff, S. M. Goyert, M. V. Norgard, and J. D. Radolf. 1998. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 160:5455-5464. [PubMed] [Google Scholar]
- 57.Shetty, S., A. Kumar, A. R. Johnson, S. Pueblitz, D. Holiday, G. Raghu, and S. Idell. 1996. Differential expression of the urokinase receptor in fibroblasts from normal and fibrotic human lungs. Am. J. Respir. Cell Mol. Biol. 15:78-87. [DOI] [PubMed] [Google Scholar]
- 58.Sidenius, N., C. F. Sier, and F. Blasi. 2000. Shedding and cleavage of the urokinase receptor (uPAR): identification and characterisation of uPAR fragments in vitro and in vivo. FEBS Lett. 475:52-56. [DOI] [PubMed] [Google Scholar]
- 59.Sidenius, N., C. F. Sier, H. Ullum, B. K. Pedersen, A. C. Lepri, F. Blasi, and J. Eugen-Olsen. 2000. Serum level of soluble urokinase-type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood 96:4091-4095. [PubMed] [Google Scholar]
- 60.Sitrin, R. G., P. M. Pan, H. A. Harper, R. F. Todd III, D. M. Harsh, and R. A. Blackwood. 2000. Clustering of urokinase receptors (uPAR; CD87) induces proinflammatory signaling in human polymorphonuclear neutrophils. J. Immunol. 165:3341-3349. [DOI] [PubMed] [Google Scholar]
- 61.Sitrin, R. G., R. F. Todd III, E. Albrecht, and M. R. Gyetko. 1996. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J. Clin. Investig. 97:1942-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slot, O., N. Brunner, H. Locht, P. Oxholm, and R. W. Stephens. 1999. Soluble urokinase plasminogen activator receptor in plasma of patients with inflammatory rheumatic disorders: increased concentrations in rheumatoid arthritis. Ann. Rheum. Dis. 58:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solberg, H., J. Romer, N. Brunner, A. Holm, N. Sidenius, K. Dano, and G. Hoyer-Hansen. 1994. A cleaved form of the receptor for urokinase-type plasminogen activator in invasive transplanted human and murine tumors. Int. J. Cancer 58:877-881. [DOI] [PubMed] [Google Scholar]
- 64.Speth, C., I. Pichler, G. Stockl, M. Mair, and M. P. Dierich. 1998. Urokinase plasminogen activator receptor (uPAR; CD87) expression on monocytic cells and T cells is modulated by HIV-1 infection. Immunobiology 199:152-162. [DOI] [PubMed] [Google Scholar]
- 65.Stoppelli, M. P., A. Corti, A. Soffientini, G. Cassani, F. Blasi, and R. K. Assoian. 1985. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc. Natl. Acad. Sci. USA 82:4939-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 67.Todd, R. F., III, P. A. Alvarez, D. A. Brott, and D. Y. Liu. 1985. Bacterial lipopolysaccharide, phorbol myristate acetate, and muramyl dipeptide stimulate the expression of a human monocyte surface antigen, Mo3e. J. Immunol. 135:3869-3877. [PubMed] [Google Scholar]
- 68.Vassalli, J. D., D. Baccino, and D. Belin. 1985. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J. Cell Biol. 100:86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei, Y., D. A. Waltz, N. Rao, R. J. Drummond, S. Rosenberg, and H. A. Chapman. 1994. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J. Biol. Chem. 269:32380-32388. [PubMed] [Google Scholar]
- 70.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 71.Wilhelm, O. G., S. Wilhelm, G. M. Escott, V. Lutz, V. Magdolen, M. Schmitt, D. B. Rifkin, E. L. Wilson, H. Graeff, and G. Brunner. 1999. Cellular glycosylphosphatidylinositol-specific phospholipase D regulates urokinase receptor shedding and cell surface expression. J. Cell. Physiol. 180:225-235. [DOI] [PubMed] [Google Scholar]
- 72.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 73.Ziegler-Heitbrock, H. W., E. Thiel, A. Futterer, V. Herzog, A. Wirtz, and G. Riethmuller. 1988. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int. J. Cancer 41:456-461. [DOI] [PubMed] [Google Scholar]