Abstract
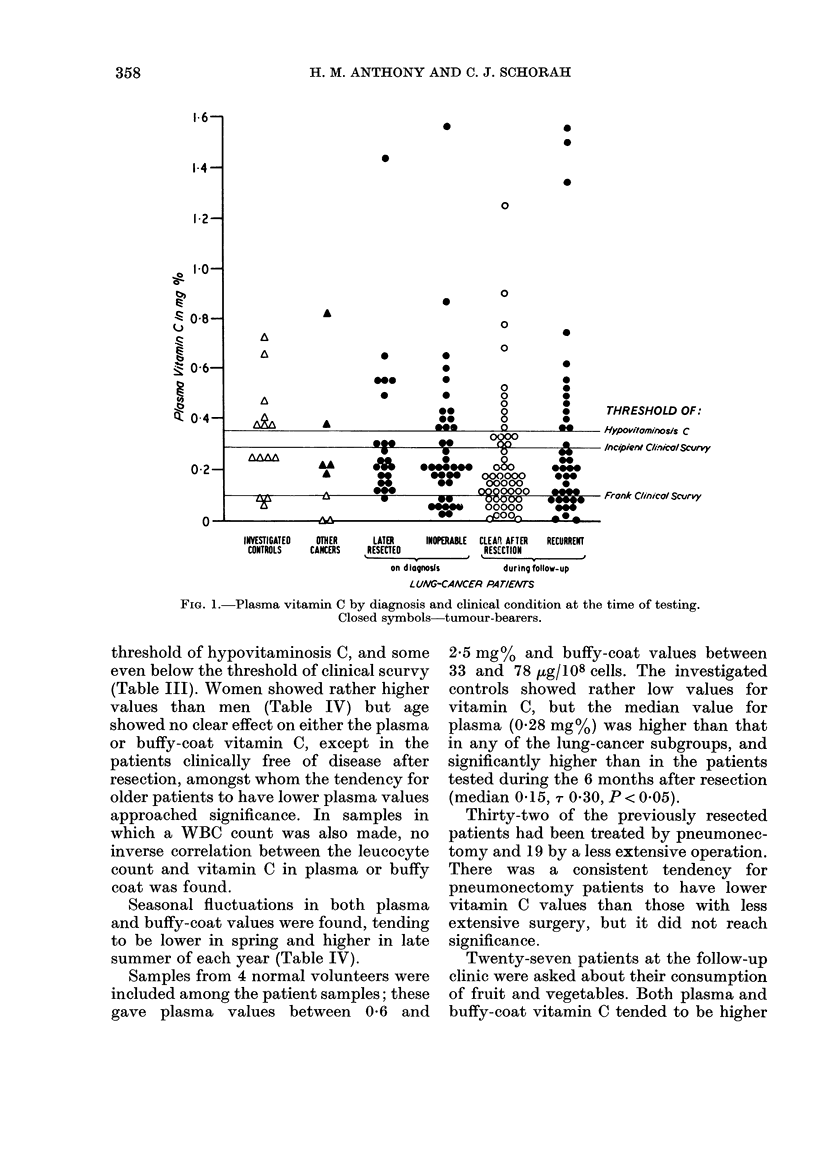
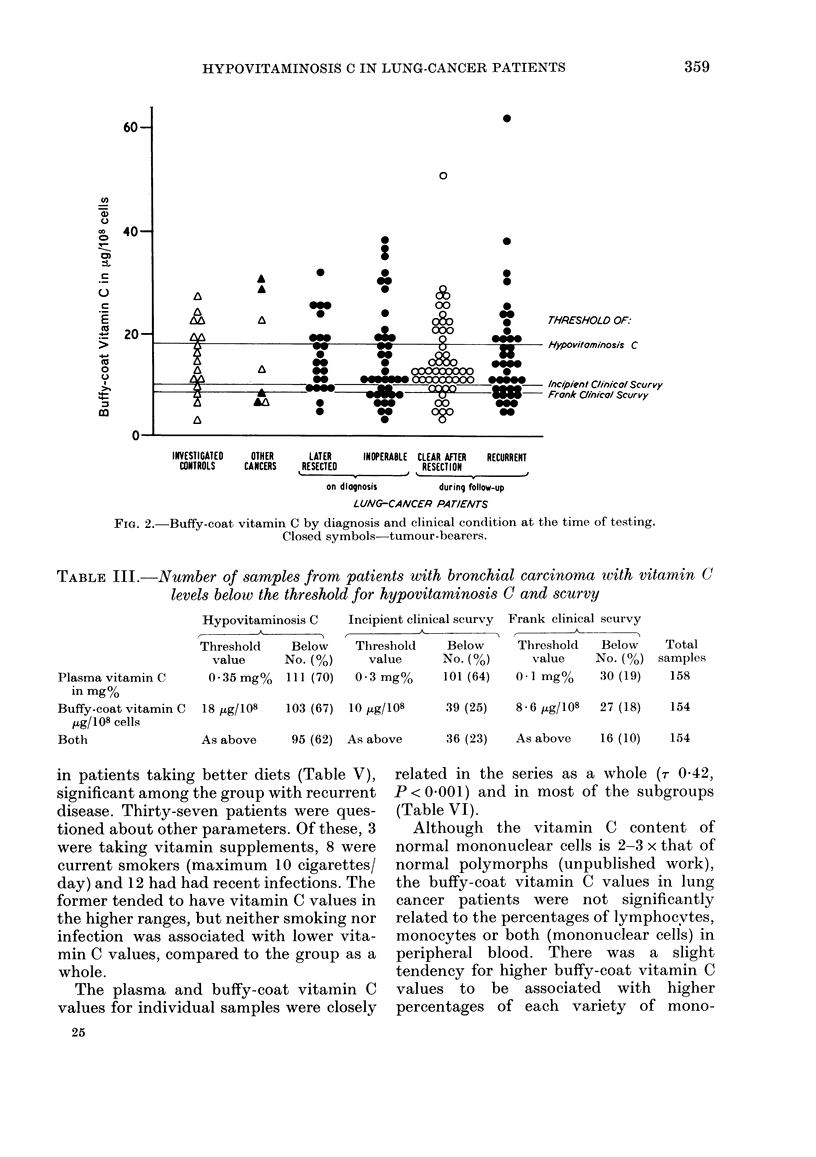
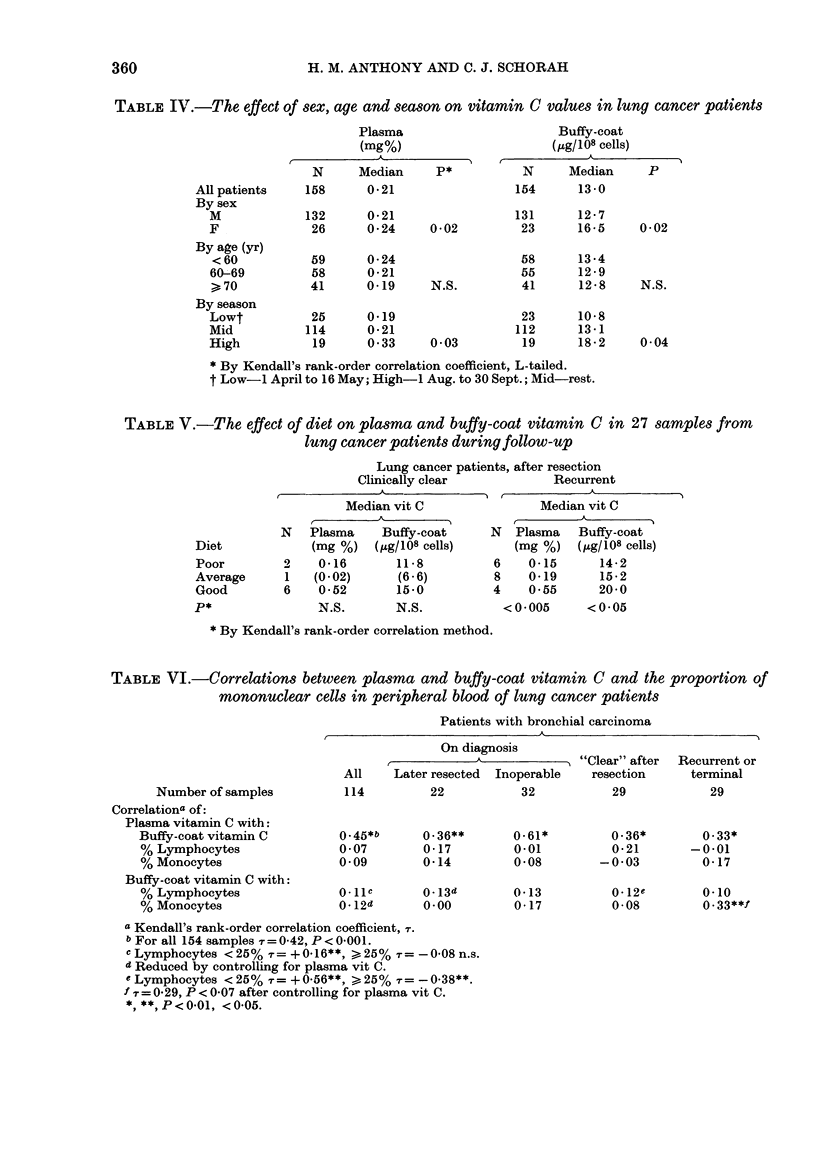
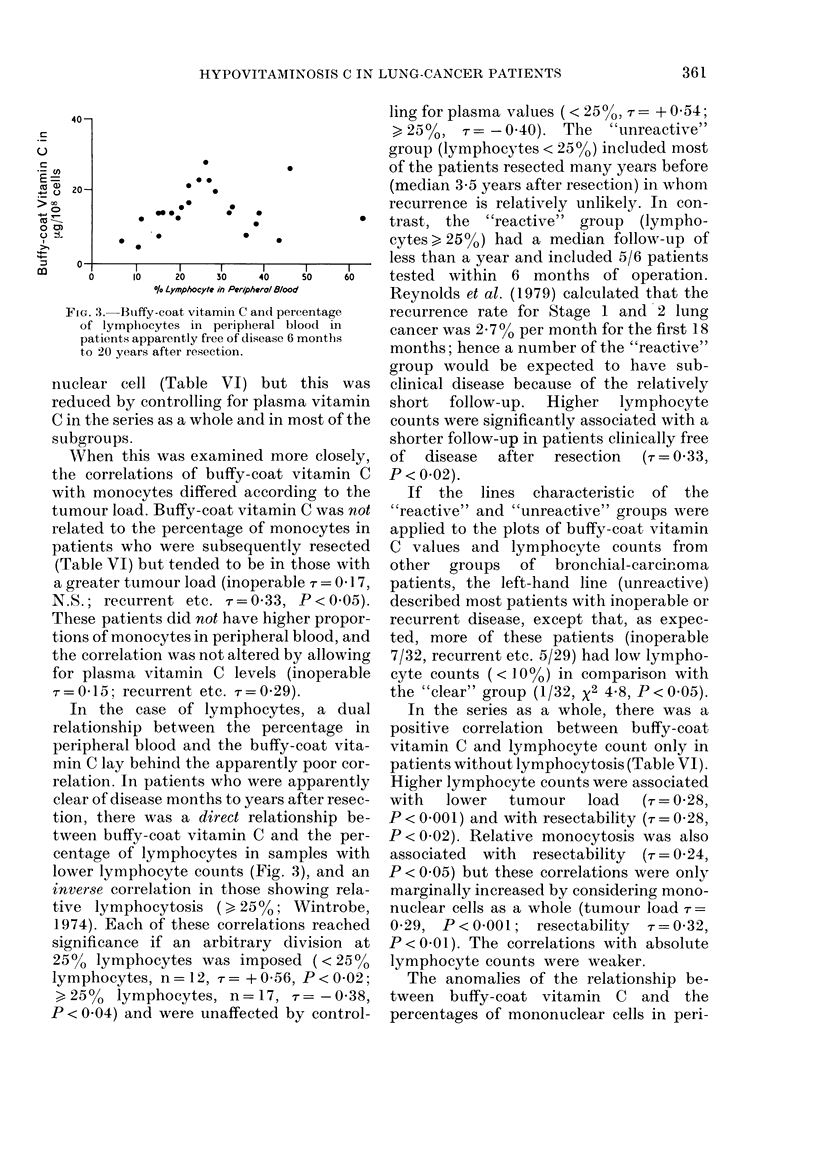
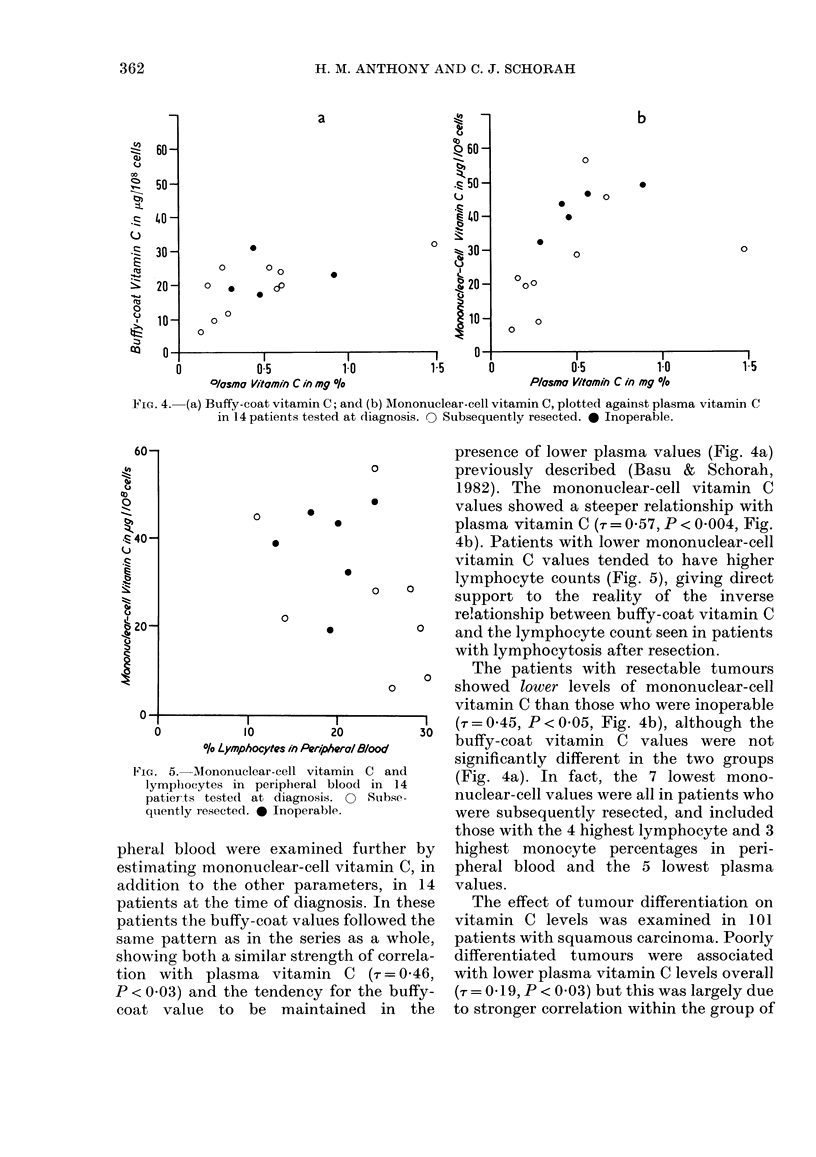
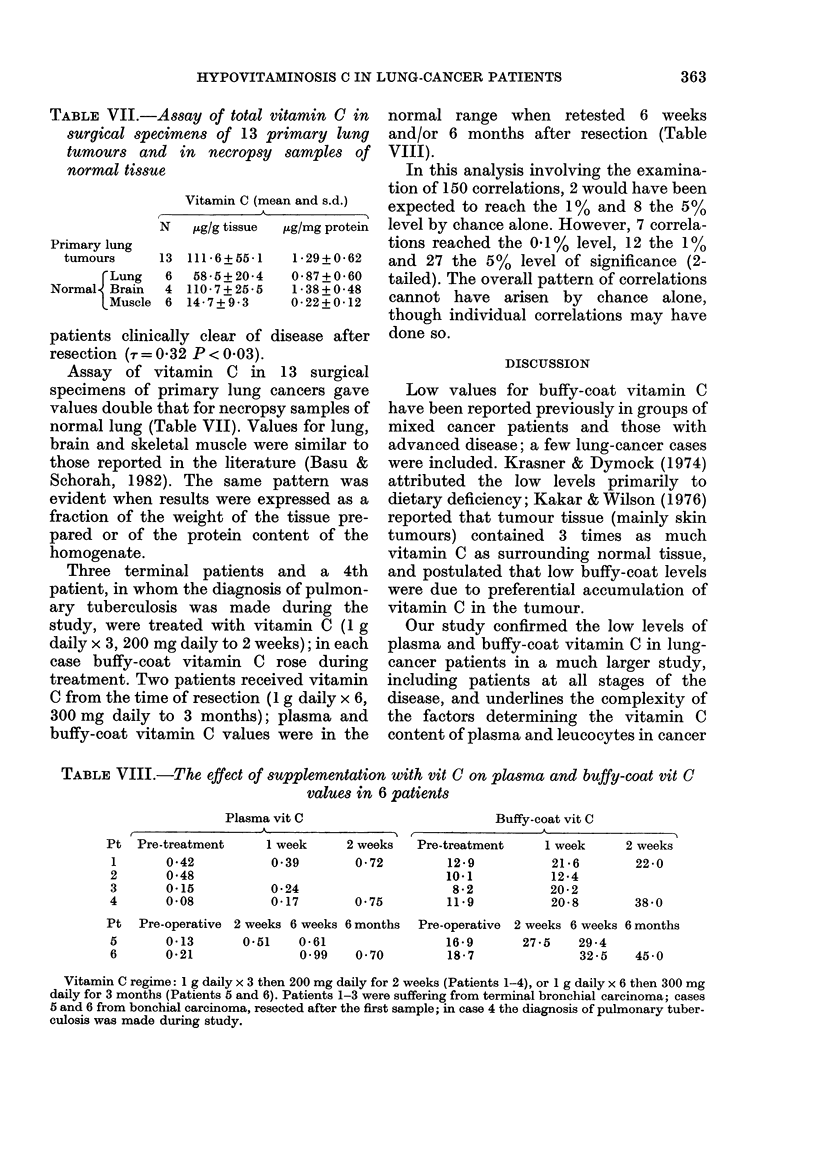
Plasma and buffy-coat vitamin C were estimated in 158 samples from 139 lung-cancer patients, at all stages of the disease. Most samples showed hypovitaminosis C in both estimations: 64% had plasma, and 25% buffy-coat values below the thresholds for incipient clinical scurvy (0.3 mg% and 10 micrograms/10(8) cells respectively). Levels were diet-dependent and could be increased by oral supplements. Levels were low both in tumour-bearing patients and in those clinically free of disease after resection. The latter had particularly low values during the first 6 months, indicating the utilization of vitamin C in surgical repair. The vitamin C content of 13 primary lung tumours was assayed: tumours had a higher vitamin C content (mean 111.6 +/- 55.1 micrograms/g tissue) than normal lung (58.5 +/- 20.4 micrograms/g). Mononuclear cells from normal individuals show a higher vitamin C content than polymorphs, but in lung-cancer patients the expected correlation of buffy-coat vitamin C with the proportion of lymphocytes in peripheral blood was obscured by an inverse correlation in patients with relative lymphocytosis (greater than or equal to 25% lymphocytes), confirmed by an inverse correlation of the proportion of lymphocytes in peripheral blood with mononuclear-cell vitamin C in 14 patients in whom this was measured. These correlations were unaffected by controlling for plasma values, and indicate the utilization of vitamin C in lymphocyte-related anti-tumour mechanisms. Vitamin C is necessary for phagocytosis and for the expression of cell-mediated immunity. In view of the increasing circumstantial evidence that immune mechanisms exert some measure of control on tumour extension and metastasis in man, the effect of supplementation with vitamin C in lung-cancer patients on survival should be tested in a clinical trial.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. Assessment of oral ascorbate in three children with chronic granulomatous disease and defective neutrophil motility over a 2-year period. Clin Exp Immunol. 1981 Jan;43(1):180–188. [PMC free article] [PubMed] [Google Scholar]
- Anderson R., Dittrich O. C. Effects of ascorbate on leucocytes: Part IV. Increased neutrophil function and clinical improvement after oral ascorbate in 2 patients with chronic granulomatous disease. S Afr Med J. 1979 Sep 1;56(12):476–480. [PubMed] [Google Scholar]
- Anthony H. M., Kirk J. A., Madsen K. E., Mason M. K., Templeman G. H. E and EAC rosetting lymphocytes in patients with carcinoma of bronchus. II. A sequential study of thirty patients: effect of BCG. Clin Exp Immunol. 1975 Apr;20(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Anthony H. M., Madsen K. E., Mason M. K., Templeman G. H. Lung cancer, immune status, histopathology and smoking. Is oat cell carcinoma lymphodependent? Br J Dis Chest. 1981 Jan;75(1):40–54. doi: 10.1016/s0007-0971(81)80006-x. [DOI] [PubMed] [Google Scholar]
- CRANDON J. H., LANDAU B., MIKAL S., BALMANNO J., JEFFERSON M., MAHONEY N. Ascorbic acid economy in surgical patients as indicated by blood ascorbic acid levels. N Engl J Med. 1958 Jan 16;258(3):105–113. doi: 10.1056/NEJM195801162580301. [DOI] [PubMed] [Google Scholar]
- Cameron E., Pauling L., Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979 Mar;39(3):663–681. [PubMed] [Google Scholar]
- Chretien J. H., Garagusi V. F. Correction of corticosteroid-induced defects of polymorphonuclear neutrophil function by ascorbic acid. J Reticuloendothel Soc. 1973 Sep;14(3):280–286. [PubMed] [Google Scholar]
- Chretien P. B., Crowder W. L., Gertner H. R., Sample W. F., Catalona W. J. Correlation of preoperative lymphocyte reactivity with the clinical course of cancer patients. Surg Gynecol Obstet. 1973 Mar;136(3):380–384. [PubMed] [Google Scholar]
- DENSON K. W., BOWERS E. F. The determination of ascorbic acid in white blood cells. A comparison of W.B.C. ascorbic acid and phenolic acid excretion in elderly patients. Clin Sci. 1961 Oct;21:157–162. [PubMed] [Google Scholar]
- Dallegri F., Lanzi G., Patrone F. Effects of ascorbic acid on neutrophil locomotion. Int Arch Allergy Appl Immunol. 1980;61(1):40–45. doi: 10.1159/000232413. [DOI] [PubMed] [Google Scholar]
- Di Paola M., Bertolotti A., Colizza S., Coli M. Histology of bronchial carcinoma and regional lymph nodes as putative immune response of the host to the tumor. J Thorac Cardiovasc Surg. 1977 Apr;73(4):531–537. [PubMed] [Google Scholar]
- Hume R., Weyers E. Changes in leucocyte ascorbic acid during the common cold. Scott Med J. 1973 Jan;18(1):3–7. doi: 10.1177/003693307301800102. [DOI] [PubMed] [Google Scholar]
- Hume R., Weyers E., Rowan T., Reid D. S., Hillis W. S. Leucocyte ascorbic acid levels after acute myocardial infarction. Br Heart J. 1972 Mar;34(3):238–243. doi: 10.1136/hrt.34.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachim H. L., Dorsett B. H., Paluch E. The immune response at the tumor site in lung carcinoma. Cancer. 1976 Dec;38(6):2296–2309. doi: 10.1002/1097-0142(197612)38:6<2296::aid-cncr2820380617>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Irwin M. I., Hutchins B. K. A conspectus of research on vitamin C requirements of man. J Nutr. 1976 Jun;106(6):821–879. doi: 10.1093/jn/106.6.821. [DOI] [PubMed] [Google Scholar]
- Kalden J. R., Guthy E. A. Prolonged skin allograft survival in vitamin C-deficient guinea-pigs. Preliminary communication. Eur Surg Res. 1972;4(2):114–119. doi: 10.1159/000127607. [DOI] [PubMed] [Google Scholar]
- Kaufmann M., Wirth K., Scheurer J., Zimmermann A., Luscieti P., Stjernswärd J. Immunomorphological lymph node changes in patients with operable bronchogenic squamous cell carcinoma. Cancer. 1977 Jun;39(6):2371–2377. doi: 10.1002/1097-0142(197706)39:6<2371::aid-cncr2820390610>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kolb E., Müller E. Local responses in primary and secondary human lung cancers. II. Clinical correlations. Br J Cancer. 1979 Sep;40(3):410–416. doi: 10.1038/bjc.1979.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasner N., Dymock I. W. Ascorbic acid deficiency in malignant diseases: a clinical and biochemical study. Br J Cancer. 1974 Aug;30(2):142–145. doi: 10.1038/bjc.1974.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella J. P., Roberts N. J., Jr Human macrophage and lymphocyte responses to mitogen stimulation after exposure to influenza virus, ascorbic acid, and hyperthermia. J Immunol. 1979 Nov;123(5):1940–1944. [PubMed] [Google Scholar]
- Migliozzi J. A. Effect of ascorbic acid on tumour growth. Br J Cancer. 1977 Apr;35(4):448–453. doi: 10.1038/bjc.1977.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambisan B., Kurup P. A. Ascorbic acid and glycosaminoglycan and lipid metabolism in guinea pigs fed normal and atherogenic diets. Atherosclerosis. 1975 Nov-Dec;22(3):447–461. doi: 10.1016/0021-9150(75)90024-6. [DOI] [PubMed] [Google Scholar]
- Olusi S. O., Ojutiku O. O., Jessop W. J., Iboko M. I. Plasma and white blood cell ascorbic acid concentrations in patients with bronchial asthma. Clin Chim Acta. 1979 Mar 1;92(2):161–166. doi: 10.1016/0009-8981(79)90110-4. [DOI] [PubMed] [Google Scholar]
- Pelletier O. Vitamin C status of cigarette smokers and nonsmokers. Am J Clin Nutr. 1970 May;23(5):520–524. doi: 10.1093/ajcn/23.5.520. [DOI] [PubMed] [Google Scholar]
- Rebora A., Dallegri F., Patrone F. Neutrophil dysfunction and repeated infections: influence of levamisole and ascorbic acid. Br J Dermatol. 1980 Jan;102(1):49–56. doi: 10.1111/j.1365-2133.1980.tb05671.x. [DOI] [PubMed] [Google Scholar]
- Reynolds R. D., Pajak T. F., Bateman J. R., Greenberg B. R., Sun N. C., Frank J. G., Shirley J. H., Lucas R. N., O'Dell S. E. Considerations in designing and analyzing surgical adjuvant study in resected stage I and II carcinoma of the lung. Cancer. 1979 Oct;44(4):1201–1210. doi: 10.1002/1097-0142(197910)44:4<1201::aid-cncr2820440406>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Schorah C. J., Zemroch P. J., Sheppard S., Smithells R. W. Leucocyte ascorbic acid and pregnancy. Br J Nutr. 1978 Jan;39(1):139–149. doi: 10.1079/bjn19780020. [DOI] [PubMed] [Google Scholar]
- Shukla S. P. Plasma and urinary ascorbic acid levels in the postoperative period. Experientia. 1969;25(7):704–704. doi: 10.1007/BF01897573. [DOI] [PubMed] [Google Scholar]
- Thomas W. R., Holt P. G. Vitamin C and immunity: an assessment of the evidence. Clin Exp Immunol. 1978 May;32(2):370–379. [PMC free article] [PubMed] [Google Scholar]
- Tuderman L., Myllylä R., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Eur J Biochem. 1977 Nov 1;80(2):341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Zweiman B., Besdine R. W., Hildreth E. A. The effect of the scorbutic state on tuberculin hypersensitivity in the guinea pig. II. In vitro mitotic response of lymphocytes. J Immunol. 1966 Apr;96(4):672–675. [PubMed] [Google Scholar]


