Abstract
The purpose of this study was to determine the effects of various lipid and mixed-micelle formulations on the oral absorption and renal toxicity of amphotericin B (AMB) in rats. The maximum concentration of AMB in plasma and the area under the concentration-time curve for 0 to 24 h for AMB were elevated in rats administered triglyceride (TG)-rich AMB formulations in comparison to those in rats given (i) AMB preformulated as a micelle containing sodium deoxycholate with sodium phosphate as a buffer (DOC-AMB), (ii) an AMB-lipid complex suspension, or (iii) AMB solubilized in methanol. Furthermore, our findings suggest that AMB incorporated into TG-based oral formulations has less renal toxicity than DOC-AMB.
Despite the development of a number of new antifungal agents (6), amphotericin B (AMB) formulated as a micellar suspension (Fungizone; Bristol-Myers Squibb, Princeton, N.J.) remains one of the most effective agents in the treatment of systemic fungal infections (13). However, its use is often limited by the development of kidney toxicity manifested by renal vasoconstriction with a significant decrease in the glomerular filtration rate and renal plasma flow and by the wasting of renal potassium and magnesium (6, 13, 22). A number of studies have reported that monomeric AMB that is solubilized in methanol is poorly absorbed from the gastrointestinal (GI) tract (3, 10, 19), and therefore it is not commonly administered orally but intravenously (i.v.), which can result in the aforementioned renal toxicity.
Improved GI absorption of poorly absorbable drugs can be achieved by increasing the dissolution rate of the drug in the presence of bile acids. Within the GI tract, bile salts behave as biological detergents that, when mixed with phospholipids, form thermodynamically stable mixed micelles. Numerous studies have reported enhanced absorption of poorly absorbable drugs when administered as mixed micellar solutions (7, 14, 23). In addition, when AMB was incorporated into mixed micelles containing bile acids and phospholipids, it resulted in increased intestinal permeability and subsequent GI absorption when a rat intestinal-perfusion methodology was used (3). However, the limitation of that study was that various AMB mixed-micelle formulations were perfused through a cannulated upper intestine of an anesthetized rat. This model does not account for the effect of anesthesia and was not done in a whole-animal model. Furthermore, the toxicological consequences of improving GI absorption, specifically, the dose-dependent kidney toxicity of AMB which limits the use of this compound, were not investigated in this study.
Thus, the purpose of our study was to determine the effects of various lipid and mixed-micelle formulations on the oral absorption and renal toxicity of AMB in rats. Based on preliminary studies, our working hypothesis was that the incorporation of AMB into mixed micelles composed of mono- and diglycerides and phospholipids would significantly enhance GI tract absorption, resulting in increased concentration in plasma without the associated AMB-induced kidney toxicity.
AMB was administered i.v. to rats at a dose of 1 mg/kg of body weight. AMB was preformulated as a micelle which contained sodium deoxycholate with sodium phosphate as a buffer (DOC-AMB; Fungizone) and was reconstituted in sterile water (5 mg/ml); it was purchased from the Department of Pharmaceutical Services, Vancouver General Hospital. In addition, this solution was administered to rats by oral gavage at doses of 1, 5, and 50 mg/kg. The method of preparing AMB-lipid complex suspension (ABLC) (Abelcet; Enzon Inc., Nutley, N.J.) has been described previously (20, 21). This lipid suspension consists of AMB complexed with two nontoxic phospholipids, l-α-dimyristoyl phosphatidylcholine and l-α-dimyristoyl phosphatidylglycerol, in a 1:1 drug-to-lipid molar ratio and was reconstituted in sterile water to a concentration of 5 mg of drug/ml (20, 21). AMB formulated as a lipid suspension has a hydrophile-lipophile balance value between 7 and 8 (K. M. Wasan et al., unpublished results). This formulation serves as a lipid-soluble treatment group that does not contain triglycerides (TGs). The dispersion of lipid droplets into a high-surface-area emulsion is an essential step in the efficient intestinal absorption of lipids. Peceol is a readily dispersible, solubilizing agent comprised primarily of a mixture of mono- and diglycerides of oleic acid which closely resembles the end products of intestinal lipid digestion (7). Previous studies have demonstrated a significant increase in the absorption of the hydrophobic drug cyclosporine from predigested olive oil, when compared to that of a nondigested control (15).
Peceol was chosen for the self-emulsifying drug delivery system (SEDDS) formulation because of the ability of this combination to solubilize AMB in high concentrations while providing an oral delivery system with rapid self-emulsifying properties (Wasan et al., unpublished). SEDDS formulations containing 10 mg of AMB/ml were prepared by dissolving AMB in 100% Peceol with stirring and gentle heating. AMB has a solubility in TG of 10 to 30 mg of AMB per mg of TG (Wasan et al., unpublished). A second TG-rich formulation that was tested incorporated AMB into 10% Intralipid. AMB (10 mg) was dissolved in 10 ml of 10% Intralipid and immediately administered to rats.
Adult male Sprague-Dawley rats (380 to 450 g) were used in this study. The rat is an appropriate animal model to investigate the GI absorption of AMB following oral administration due to similarities in rat and human intestinal characteristics (i.e., anatomical, metabolic, and biochemical characteristics) (5, 9, 11, 16). AMB levels in plasma and tissues were analyzed by high-pressure liquid chromatography (HPLC) as previously described (20-22). HPLC was performed on an LDC/Milton Roy pump with a Milton Roy variable-wavelength detector set at a wavelength of 405 nm. The mobile phase (0.005 M EDTA-methanol, 35:65 [vol/vol]) elutes on an LC-1 Supelco column (5-μm inside diameter; 4.6 by 150 mm) at a flow rate of 2 ml/min at ambient temperature. The retention time of AMB is 3.5 min with a run time of 4 min. The assay is sensitive to 50 ng/ml with a linear range of 50 to 1,000 ng/ml (the interday coefficient of variation is between 5 and 8%) (20-22). To assess renal function, plasma creatinine concentrations were measured by standard enzymatic reactions (Sigma Chemical, St. Louis, Mo.) prior to and 24 h following drug administration. Based on previous studies of rats (20, 21), the criterion for this study for measurable kidney toxicity was set as a 50% increase in plasma creatinine concentration from the baseline. The area under the plasma AMB concentration-time curve from 0 to 24 h (AUC0-24) was determined by using the trapezoidal rule.
All rats were cared for in accordance with the Canadian Council on Animal Care and the University of British Columbia guidelines. Adult male Sprague-Dawley rats were obtained from the University of British Columbia animal care unit (Vancouver, British Columbia, Canada). The rats were maintained under a 12-h-light-12-h-dark cycle (light exposure from 0700 to 1900 h) and supplied with a standard laboratory diet (PMI Feeds, Richmond, Va.) and water ad libitum. Following an overnight fast (12 to 16 h) and 48 h postsurgery, the rats were divided into six treatment groups and at 0700 h each rat received a single dose (total volume, 1 ml) by oral gavage of one of the following: AMB solubilized in methanol (1, 5, or 50 mg of AMB/kg), DOC-AMB (1, 5, or 50 mg of AMB/kg), ABLC (50 mg of AMB/kg), AMB incorporated into 10% Intralipid (50 mg of AMB/kg), or AMB incorporated into 100% Peceol (5 or 50 mg of AMB/kg). Six rats were used in each treatment group.
Blood samples (0.5 ml) were obtained before and 2, 4, 8, 10, and 24 h after the oral gavage. An equal volume of normal saline (1 ml) was administered i.v. to the animals following each blood draw to prevent fluid depletion during the course of the study. Plasma was immediately harvested by centrifugation and analyzed for drug content by HPLC. After the 24-h sample was collected following the last dose, each rat was humanely sacrificed and the liver, right kidney, spleen, heart, and lung were removed, dried, and weighed. Each organ was stored at −20°C until analysis. The animals were permitted free access to food at 4 h postdosing. A total lipid mass of 1.84 g/kg was administered with the lipid suspension. For the SEDDS formulations, a total lipid mass of 1.33 g/kg was administered. To aid in the interpretation of the effects of oral formulations on the plasma drug concentration versus time profiles, one group of three rats received a 1-mg/kg concentration of AMB in the DOC-AMB solution as a bolus i.v. dose through the left external jugular vein. Serial blood samples (300 μl) were collected in EDTA-coated tubes prior to and 2, 4, 8, 10, and 24 h postdosing. The 1-mg/kg dose has previously been shown to be therapeutically effective in rats (20, 21). In all studies, an equal volume of normal saline was administered to each rat after each blood sample was collected to prevent dehydration and minimize total blood volume depletion. All animals had free access to the electrolyte solution throughout the course of the study. All animals were allowed recovery from the surgery and given postsurgical analgesics (with a short half-life) 24 h prior to the start of the study if necessary. Allowing for analgesics only 24 h before the study ensured that the absorptive effects of AMB observed for the different formulations were not due to any analgesic-induced disturbances in oral bioavailability.
AMB concentrations in plasma and tissue, the maximum concentrations of AMB in plasma (Cmaxs), the times to Cmax (Tmaxs), and the AUC values were compared among the different treatment groups by the Mann-Whitney nonparametric test (1). Critical differences were assessed by the Newman-Keuls post hoc tests. A difference was considered significant if the probability of chance in explaining the results was reduced to <0.05. All data were expressed as means ± standard deviations.
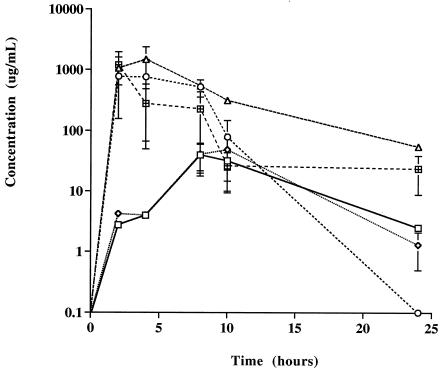
The mean weights of the rats prior to and following drug administration were not significantly different (data not shown). Similarly, kidney, liver, lung, spleen, and heart weights were not different between the control and drug treatment groups (data not shown). AMB concentrations in plasma and tissue were undetectable following oral administration of AMB solubilized in methanol at 1, 5, and 50 mg/kg or of DOC-AMB at 1 and 5 mg/kg (data not shown). No significant changes in plasma creatinine concentrations were observed prior to and 24 h following administration for these treatment groups (data not shown). Percent changes from baseline in plasma creatinine concentrations prior to and 24 h after the i.v. administration of DOC-AMB (1 mg/kg) were significantly greater than those after oral administration of DOC-AMB (1 or 50 mg/kg) (Table 1). A significant decrease from baseline in the plasma creatinine concentration was observed following administration of ABLC (50 mg/kg) (Table 1). However, no statistically significant differences from baseline were observed in the serum creatinine concentration prior to and 24 h after oral administration of Intralipid-AMB (50 mg/kg) or Peceol-AMB (5 or 50 mg/kg) (Table 1). Tmaxs ranged from 2 h (Intralipid-AMB) to 10 h (ABLC) following oral administration. The AMB Cmaxs following administration of Intralipid-AMB (50 mg/kg) and Peceol-AMB (5 or 50 mg/kg) were statistically significantly higher than that following administration of ABLC (50 mg/kg) (Fig. 1 and Table 1) or DOC-AMB (50 mg/kg) (Table 1). The plasma AMB Cmax following Peceol-AMB administration (50 mg/kg) was statistically significantly higher than that following Intralipid-AMB administration (50 mg/kg) (Fig. 1 and Table 1). AMB concentrations in kidney tissue were greater in rats following oral administration of DOC-AMB at 50 mg/kg than following administration of ABLC at 50 mg/kg, Intralipid-AMB at 50 mg/kg, or Peceol-AMB at 5 or 50 mg/kg (Table 2). AMB concentrations in kidney tissue were undetectable following oral administration of Intralipid-AMB at 50 mg/kg and Peceol-AMB at 5 or 50 mg/kg (Table 2). AMB concentrations in liver tissue were greater following oral administration of Intralipid-AMB at 50 mg/kg and Peceol-AMB at 5 or 50 mg/kg than those following administration of DOC-AMB at 50 mg/kg or ABLC at 50 mg/kg (Table 2). AMB concentrations in liver tissue were undetectable following oral administration of DOC-AMB at 50 mg/kg (Table 2). AMB concentrations in spleen tissue were undetectable following administration of all treatments except Peceol-AMB at 50 mg/kg (Table 2). AMB concentrations in heart tissue were undetectable following administration of all treatments except Peceol-AMB at 50 mg/kg and ABLC at 50 mg/kg (Table 2).
TABLE 1.
Plasma creatinine and AMB concentrations after administration of a single i.v. dose of DOC-AMB and a single administration by oral gavage of DOC-AMB and various AMB lipid formulations to male Sprague-Dawley rats
| Treatment group and/or route of administration | Dose (mg/kg) | Plasma creatinine levela
|
Tmax (h) | Cmax (μg/ml) | AUC0-24 (μg · h/ml) | ||
|---|---|---|---|---|---|---|---|
| Concn prior to dose (mg/dl) | Concn 24 h after dose (mg/dl) | % Change from baseline | |||||
| DOC-AMB | |||||||
| i.v. | 1 | 0.38 ± 0.13 | 0.71 ± 0.10b | +87 | 4.3 ± 1.1 | ||
| Oral | 5 | 0.34 ± 0.07 | 0.29 ± 0.05 | −15 | NDc | ND | ND |
| Oral | 50 | 0.29 ± 0.07 | 0.42 ± 0.02b | +45 | 8 | 39.8 ± 22 | 519 ± 209d |
| AMB-lipid formulations | |||||||
| ABLC | 50 | 0.45 ± 0.01 | 0.24 ± 0.07b | −47 | 10 | 48.5 ± 26 | 542 ± 271 |
| Intralipid-AMB | 50 | 0.27 ± 0.02 | 0.38 ± 0.02b | +33 | 2 | 769 ± 213e | 5,984 ± 3,461e |
| Peceol AMB | 50 | 0.48 ± 0.10 | 0.65 ± 0.07 | +35 | 4 | 1,469 ± 891e | 11,407 ± 4,971e |
| 5 | 0.44 ± 0.06 | 0.39 ± 0.06 | −11 | 2 | 1,187 ± 409e | 4,415 ± 2,411e | |
Data presented as means ± standard deviations (n = 6).
Significantly difference from values prior to dose (P < 0.05).
ND, not determinable.
Significantly difference from DOC-AMB i.v. value (P < 0.05).
Significantly different from AUC0-24 value for ABLC (P < 0.05).
FIG. 1.
Plasma AMB concentration versus time curve on a log-linear graph following administrations by oral gavage of DOC-AMB (□), ABLC (◊), Intralipid-AMB at 50 mg of AMB/kg (○), and Peceol-AMB at 50 (▵) and 5 (⊞) mg of AMB/kg. Values are expressed as means ± standard deviations (six rats per treatment group).
TABLE 2.
Distribution of AMB in kidney, liver, spleen, heart, and lung 24 h after administration of a single dose of DOC-AMB and a single oral gavage of different AMB formulations to male Sprague-Dawley rats
| Tissue | AMB distribution in tissue (μg of AMB/g of tissue) after administration ofa:
|
|||||
|---|---|---|---|---|---|---|
| DOC-AMB
|
ABLC (50) | Intralipid-AMB (50) | Peceol-AMB (50) | Peceol-AMB (5) | ||
| i.v. (1)b | Oral (50) | |||||
| Kidney | 9.1 ± 1.1 | 9.0 ± 0.5 | NDc | ND | ND | ND |
| Liver | ND | ND | ND | 13.4 ± 3.5 | 10.9 ± 2.4 | 6.1 ± 0.2 |
| Spleen | ND | ND | ND | ND | 76.5 ± 0.7 | ND |
| Heart | ND | ND | 45.9 ± 34.5 | ND | 12.8 ± 3.0 | ND |
| Lung | 47 ± 8.7 | 731 ± 602d | 558 ± 48d | 43 ± 2 | ND | ND |
Data presented as means ± standard deviation (n = 6).
Values in parentheses are the AMB doses in milligram per kilogram.
ND, not detectable.
Significantly different from AMB concentration in lung tissue after DOC-AMB i.v. administration (AMB at 1 mg/kg) (P < 0.05).
The i.v. administration of AMB has been limited by its dose-dependent kidney toxicity that has not been predictable by monitoring plasma and/or serum drug concentrations (6, 13, 22). A number of studies have reported that AMB solubilized in methanol is poorly absorbed from the GI tract (3, 10, 17, 19), and therefore it is not commonly administered orally but i.v., which can result in the aforementioned renal toxicity. However, to date, few studies investigating the development and evaluation of an oral AMB formulation have been reported.
The plasma AMB concentrations and tissue AMB distributions were considerably different following administration of Intralipid-AMB and Peceol-AMB than after administration of their nonlipid counterpart (DOC-AMB). Cmaxs (Table 1) and AUC0-24s (Fig. 1 and Table 1) were elevated in rats administered TG-rich AMB formulations (i.e., Intralipid-AMB and Peceol-AMB) compared to those in rats given DOC-AMB, ABLC, or AMB solubilized in methanol (data not shown). These results may be explained by the fact that lymphatic transport of many water-insoluble drugs occurs concurrently with TG absorption from the GI tract (7, 8, 14) and that Intralipid or Peceol could provide an efficiently absorbed source of lipids for promoting lymphatic drug transport, thus increasing systemic oral absorption of AMB. Previous studies have demonstrated a significant increase in absorption of the hydrophobic drug cyclosporine from predigested olive oil, when compared to that of a nondigested control (15). Further studies to test this hypothesis are warranted.
We further hypothesized that the incorporation of AMB into lipid-based formulations would have a major impact on the safety of this drug by altering the distribution of AMB in tissue. Our preliminary findings suggest that AMB incorporated into TG-based oral formulations (i.e., Intralipid and Peceol) has less renal toxicity than i.v. or orally administered DOC-AMB (Table 1) because it decreases the concentration of AMB recovered in the kidney and increases the concentration of AMB recovered in the liver (Table 2). Recently, others have reported that the incorporation of AMB into TG-rich parenteral formulations reduces AMB-induced toxicity without altering its antifungal activity (2, 4, 12, 18, 19). However, until now, few studies using oral TG-rich formulations to reduce AMB-induced toxicity have been reported. Taken together with our plasma AMB concentration findings (Table 1 and Fig. 1), these results suggest that the development of a TG-based oral formulation of AMB may address some of the limitations of the i.v. form of AMB.
In conclusion, we have demonstrated significant differences in the concentrations of AMB in plasma, the distributions of AMB in tissue, and the drug-induced renal toxicities of AMB following the administration of TG-rich oral formulations of AMB and i.v. and orally administered DOC-AMB, and we have demonstrated that altering the distribution of AMB in tissue can modify the induced renal toxicity.
Acknowledgments
This study was supported by an operating grant from the Canadian Institutes of Health Research (grant MOP-49432 to K.M.W.).
REFERENCES
- 1.Blom, G. 1958. Statistical estimates and transformed beta variables, p. 1-58. John Wiley and Sons, Inc., New York, N.Y.
- 2.Chavanet, P., V. Joly, D. Rigaud, J. Bolard, C. Carbon, and P. Yeni. 1994. Influence of diet on experimental toxicity of amphotericin B deoxycholate. Antimicrob. Agents Chemother. 38:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dangi, J. S., S. P. Vyas, and V. K. Dixit. 1998. Effects of various lipid-bile salt mixed micelles on the intestinal absorption of amphotericin-B in rat. Drug Dev. Ind. Pharm. 24:631-635. [DOI] [PubMed] [Google Scholar]
- 4.Egito, E. S. T., I. B. Araujo, P. G. Bolivar, L. Damasceno, and J. C. Price. 2002. Amphotericin B/emulsion admixture interactions: an approach concerning the reduction of amphotericin B toxicity. J. Pharm. Sci. 91:2354-2366. [DOI] [PubMed] [Google Scholar]
- 5.Fagerholm, U., M. Johansson, and H. Lennernas. 1996. Comparison between permeability coefficients in rat and human jejunum. Pharm. Res. 13:1336-1342. [DOI] [PubMed] [Google Scholar]
- 6.Gates, C., and R. J. Pinney. 1993. Amphotericin B and its delivery by liposomal and lipid formulations. J. Clin. Pharm. Ther. 18:147-153. [DOI] [PubMed] [Google Scholar]
- 7.Hauss, D. J., S. E. Fogal, J. V. Ficorilli, C. A. Price, T. Roy, A. A. Jayaraj, and J. J. Kerns. 1998. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J. Pharm. Sci. 87:164-169. [DOI] [PubMed] [Google Scholar]
- 8.Holm, R., C. J. Porter, A. Mullertz, H. G. Kristensen, and W. N. Charman. 2002. Structured triglyceride vehicles for oral delivery of halofantrine: examination of intestinal lymphatic transport and bioavailability in conscious rats. Pharm. Res. 19:1354-1361. [DOI] [PubMed] [Google Scholar]
- 9.Kararli, T. T. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16:351-380. [DOI] [PubMed] [Google Scholar]
- 10.Lance, M. R., C. Washington, and S. S. Davis. 1995. Structure and toxicity of amphotericin B/triglyceride emulsion formulations. J. Antimicrob. Chemother. 36:119-128. [DOI] [PubMed] [Google Scholar]
- 11.Levet-Trafit, B., M. S. Gruyer, M. Marjanovic, and R. C. Chou. 1996. Estimation of oral drug absorption in man based on intestine permeability in rats. Life Sci. 58:PL359-PL363. [DOI] [PubMed] [Google Scholar]
- 12.Manfredi, R., and F. Chiodo. 1998. Case-control study of amphotericin B in a triglyceride fat emulsion versus conventional amphotericin B in patients with AIDS. Pharmacotherapy 18:1087-1092. [PubMed] [Google Scholar]
- 13.Meyer, R. D. 1992. Current role of therapy with amphotericin B. Clin. Infect. Dis. 14:S154-S160. [DOI] [PubMed] [Google Scholar]
- 14.Porter, C. J., and W. N. Charman. 2001. Intestinal lymphatic drug transport: an update. Adv. Drug Delivery Rev. 50:61-80. [DOI] [PubMed] [Google Scholar]
- 15.Reymond, J. P., H. Sucker, and J. Vonderscher. 1988. In vivo model for ciclosporin intestinal absorption in lipid vehicles. Pharm. Res. 5:677-679. [DOI] [PubMed] [Google Scholar]
- 16.Soria, I., and C. L. Zimmerman. 1996. The validation of the intestinal permeability approach to predict oral fraction of dose absorbed in humans and rats. Biopharm. Drug Dispos. 17:817-818. [DOI] [PubMed] [Google Scholar]
- 17.Souza, L. C., R. C. Maranhao, S. Schreier, and A. Campa. 1993. In-vitro and in-vivo studies of the decrease of amphotericin B toxicity upon association with a triglyceride-rich emulsion. J. Antimicrob. Chemother. 32:123-132. [DOI] [PubMed] [Google Scholar]
- 18.Souza, L. C., and A. Campa. 1999. Pharmacological parameters of intravenously administered amphotericin B in rats: comparison of the conventional formulation with amphotericin B associated with a triglyceride-rich emulsion. J. Antimicrob. Chemother. 44:77-84. [DOI] [PubMed] [Google Scholar]
- 19.Souza, L. C., P. H. Saldiva, and A. Campa. 2000. Lipid emulsion reduces subacute toxicity of amphotericin B: a histopathological study. Exp. Toxicol. Pathol. 52:169-175. [DOI] [PubMed] [Google Scholar]
- 20.Wasan, K. M., K. Vadiei, G. Lopez-Berestein, and D. R. Luke. 1990. Pharmacokinetics, tissue distribution, and toxicity of free and liposomal amphotericin B in diabetic rats. J. Infect. Dis. 161:562-566. [DOI] [PubMed] [Google Scholar]
- 21.Wasan, K. M., V. B. Grossie, Jr., and G. Lopez-Berestein. 1994. Concentrations in serum and distribution in tissue of free and liposomal amphotericin B in rats during continuous Intralipid infusion. Antimicrob. Agents Chemother. 38:2224-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasan, K. M., A. L. Kennedy, S. M. Cassidy, M. Ramaswamy, L. Holtorf, J. W.-L. Chou, and P. H. Pritchard. 1998. Pharmacokinetics, distribution in serum lipoproteins and tissues, and renal toxicities of amphotericin B and amphotericin B lipid complex in a hypercholesterolemic rabbit model: single-dose studies. Antimicrob. Agents Chemother. 42:3146-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasan, K. M. 2002. The role of lymphatic transport in enhancing oral protein and peptide drug delivery. Drug Dev. Ind. Pharm. 28:1047-1058. [DOI] [PubMed] [Google Scholar]