Abstract
In vitro levofloxacin exhibits both potent or intermediate activity against most of the pathogens frequently responsible for acute bacterial meningitis and synergistic activity with some beta-lactams. Since levofloxacin was shown to penetrate the cerebrospinal fluid (CSF) during meningeal inflammation both in animals and in humans, the disposition of levofloxacin in CSF was studied in 10 inpatients with external ventriculostomy because of communicating hydrocephalus related to subarachnoid occlusion due to cerebral accidents who were treated with 500 mg of levofloxacin intravenously twice a day because of extracerebral infections. Plasma and CSF concentration-time profiles and pharmacokinetics were assessed at steady state. Plasma and CSF levofloxacin concentrations were analyzed by high-pressure liquid chromatography. The peak concentration of levofloxacin at steady state (Cmax ss)was 10.45 mg/liter in plasma and 4.06 mg/liter in CSF, respectively, with the ratio of the Cmax ss in CSF to the Cmax ss in plasma being 0.47. The areas under the concentration-time curves during the 12-h dosing interval (AUC0-τs) were 47.69 mg · h/liter for plasma and 33.42 mg · h/liter for CSF, with the ratio of the AUC0-τ for CSF to the AUC0-τ for plasma being 0.71. The terminal-phase half-life of levofloxacin in CSF was longer than that in plasma (7.02 ± 1.57 and 5.51 ± 1.36 h, respectively; P = 0.034). The ratio of the levofloxacin concentration in CSF to the concentration in plasma progressively increased with time, from 0.30 immediately after dosing to 0.99 at the end of the dosing interval. In the ventricular CSF of patients with uninflamed meninges, levofloxacin was shown to provide optimal exposure, which approximately corresponded to the level of exposure of the unbound drug in plasma. The findings provide support for trials of levofloxacin with twice-daily dosing in combination with a reference beta-lactam for the treatment of bacterial meningitis in adults. This cotreatment could be useful both for overcoming Streptococcus pneumoniae resistance and for enabling optimal exposure of the CSF to at least one antibacterial agent for the overall treatment period.
Levofloxacin is a fluoroquinolone antibiotic characterized by a broad antimicrobial spectrum that covers, among other organisms, the pathogens most frequently responsible for acute bacterial meningitis (Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, and Escherichia coli) and also other sporadic agents of central nervous system (CNS) infections, such as Streptococcus agalactiae (13).
In in vitro studies based on the time-kill curve method, levofloxacin was recently shown to exhibit synergistic (or at least additive) activity with some beta-lactam antibiotics against both gram-positive and gram-negative microorganisms (11, 27, 33, 35). The degree of this synergy was sometimes of the same extent as that which occurs between beta-lactams and aminoglycosides (4).
Previous pharmacokinetic studies documented that levofloxacin could adequately penetrate the cerebrospinal fluid (CSF) in the presence of meningeal inflammation both in animals and in humans (8, 32). However, ratios of the concentration in CSF to the concentration in plasma (CSF to plasma concentration ratios) may vary substantially according both to different sampling times and to the status of the blood-CSF barrier. For example, Ohi et al. (23) found that 3 h after the administration of a single 200-mg dose of levofloxacin to healthy volunteers with uninflamed meninges, the CSF to plasma ratio ranged between 0.08 and 0.24. Although assessment of the level of antibiotic exposure in CSF on the basis of a single sampling time might provide incomplete information (19), an evaluation of exposure based on multiple sampling times covering the whole dosing interval should be more meaningful (19).
Moreover, although most fluoroquinolones are lipophilic agents whose penetration into CSF should be only minimally affected by the degree of meningeal inflammation, assessment of the penetration of levofloxacin into the CSF in the presence of an uninflamed blood-CSF barrier would be more informative. Finally, considering that antibiotic concentrations may be severalfold higher at the lumbar CSF level than at the ventricular CSF level (21), it might be more appropriate to assess the level of exposure in the latter compartment. The purpose of this study was to assess levofloxacin ventricular CSF concentrations during the whole dosing interval in patients with minimal alteration of the blood-brain barrier, namely, hydrocephalic patients with external ventriculostomies.
MATERIALS AND METHODS
Study entry criteria.
This study was performed with a cohort of 10 inpatients (four males and six females) who received external ventriculostomies during recovery at the Department of Anaesthesia and Intensive Care Unit (ICU), Regional Hospital Ca' Foncello, Treviso, Italy, because of communicating hydrocephalus related to subarachnoid occlusion due to cerebral accidents. The patients were treated with a standard high dose of 500 mg of levofloxacin (licensed in Italy) intravenously (i.v.) twice a day (b.i.d.) for suspected or microbiologically proven severe extracerebral infections. The patients' characteristics are depicted in Table 1.
TABLE 1.
Patient demographics on study day
| Parameter | Mean (range) |
|---|---|
| Age (yr) | 56 (26-74) |
| Sex (no. of males/no. of females) | 4/6 |
| Wt (kg) | 72.3 (60-102) |
| Level of bilirubinemia (mg/dl) | 0.6 (0.3-1.4) |
| Level of albuminemia (mg/dl) | 3.2 (2.5-3.8) |
| CLCRa (ml/min/kg) | 1.76 (1.11-3.36) |
CLCR was estimated by the formula of Cockcroft and Gault (6).
Study design.
The study protocol was approved by the local Ethics Committee of the Regional Hospital Ca' Foncello, and informed consent to assess levofloxacin pharmacokinetics in plasma and CSF was obtained from the nearest relative of each patient. Criteria for inclusion in the pharmacokinetic study were as follows: age, >18 years; estimated creatinine clearance (CLCR), determined by the formula of Cockcroft and Gault (6), > 50 ml/min; stable renal function (daily serum creatinine level fluctuation, <0.3 mg/dl); and levofloxacin treatment for extracerebral infections.
Levofloxacin disposition.
The disposition of levofloxacin was assessed under steady-state conditions, that is, after at least 3 days of unmodified treatment. Blood samples were collected through an arterial indwelling catheter before dosing and at 0, 0.5, 1, 2, 4, 6, 8, and 11 h after the morning 1-h i.v. infusion of 500 mg of levofloxacin. After centrifugation, the plasma samples were stored at −80°C until they were assayed.
CSF samples were collected simultaneously with each blood sample through an indwelling external drainage ventricular catheter (Ethicon SpA, Divisione Codman, Rome, Italy). This system was set as a sterile closed circuit consisting of nine consecutive taps, each of which was opened only one time (that is, each CSF sample was collected through a single tap) in order to avoid bacterial contamination as much as possible. Before collection, 1 to 2 ml of CSF was discharged to allow correct sampling. The CSF samples were then stored frozen (−80°C) until they were assayed.
Protein, glucose, red blood cell (RBC), and white blood cell (WBC) concentrations in CSF were determined to measure the degree of impairment of the blood-CSF barrier on the study day (see Table 2).
TABLE 2.
Patients' admission diagnosis, underlying infectious diseases, etiological agents of infection, and CSF chemical statusa
| Patient no. | Underlying CNS disease | Time from event (days) | Etiological agent(s) | Antibiotic cotreatment | HAD(s) | Protein concn in CSF (mg/liter) | WBC count in CSF (no. of cells/μl) | Glucose concn in CSF (mg/dl) |
|---|---|---|---|---|---|---|---|---|
| 1 | Extradural hematoma | 12 | P. aeruginosa, MS S. aureus | Dopamine, mannitol | 150 | 1 | 93 | |
| 2 | Subarachnoid hemorrhage | 11 | Enterobacter spp. MS S. aureus | Dopamine, mannitol | 270 | 1 | 63 | |
| 3 | Subarachnoid hemorrhage | 9 | H. influenzae | Vancomycin | Dopamine, mannitol | 220 | 3 | 110 |
| 4 | Intracerebral hemorrhage | 10 | Klebsiella oxytoca | Dopamine, mannitol | 201 | 3 | 77 | |
| 5 | Intracerebral hemorrhage | 11 | E. coli | Dopamine, mannitol | 141 | 86 | 93 | |
| 6 | Subarachnoid hemorrhage | 9 | Proteus mirabilis | Dopamine, mannitol | 383 | 0 | 103 | |
| 7 | Intracerebral hemorrhage | 10 | Furosemide, mannitol | 413 | 3 | 114 | ||
| 8 | Subdural hemorrhage | 9 | MS S. aureus | Oxacillin | Mannitol | 220 | 1 | 181 |
| 9 | Frontal meningioma | 8 | P. aeruginosa | Piperacillin- tazobactam | Furosemide | 63 | 109 | 75 |
| 10 | Thalamic hemorrhage | 10 | MS S. aureus | Teicoplanin | Mannitol | 188 | 218 | 79 |
| Total | 9.9 ± 1.2b | 224.9 ± 107.1b | 3 (0-218)c | 98.80 ± 33.19b |
All patients had ventilator-associated pneumonia. Abbreviations: HAD(s), hemodynamically active drug(s); MS, methicillin sensitive.
Data are expressed as means ± SDs.
Data are expressed as medians (ranges).
High-pressure liquid chromatography analysis.
Plasma and CSF levofloxacin concentrations were analyzed by a high-pressure liquid chromatography method validated in our laboratory, as described previously (28, 32). The analytical method chosen was not stereospecific, since levofloxacin has been shown to be stereochemically stable in body fluids without any metabolic inversion to d-ofloxacin (10, 34). The inter- and intraday coefficients of variation of the assay were less than 10%. The low limit of detection was 0.1 mg/liter.
Pharmacokinetic evaluations.
According to the Akaike information criterion (37), plasma concentration-versus-time data for individual patients were estimated by a two-compartment open model with first-order elimination by using the WinNonlin pharmacokinetic software package (Pharsight Corporation, Mountain View, Calif.). The pharmacokinetic parameters for levofloxacin in plasma explored in this study included the maximum concentration in plasma at steady state (Cmax ss), the volume of distribution at steady state (Vss), elimination half-life (t1/2β), total body clearance (CL), and the area under the plasma concentration-time curve (AUC) during the 12-h observational period (AUC0-τ).
The pharmacokinetic parameters of levofloxacin explored in CSF included Cmax ss in CSF (Cmax ss CSF), time to reach Cmax ss CSF (Tmax CSF), t1/2 in CSF (t1/2z CSF), and total exposure of CSF during the 12-h observational period (AUC0-τ CSF). The elimination rate constant for levofloxacin in CSF (λz CSF) was obtained by log-linear regression of the terminal portion of the CSF concentration-versus-time curve (on the basis of at least three datum points), while t1/2z CSF was calculated as ln 2/λz CSF. The AUC0-τ for levofloxacin in CSF was calculated by the linear trapezoidal method.
Since patients received standard levofloxacin dosages, to avoid bias due to interindividual differences in body weight, the dose-related pharmacokinetic parameters (Cmax ss, AUC0-τ) both in plasma and in CSF were also normalized with respect to the levofloxacin dose per kilogram of body weight and, consequently, to a dose of 1 mg of levofloxacin per kg every 12 h.
Theoretical pharmacodynamic breakpoints in CSF.
It is well known that the two most relevant pharmacodynamic parameters for the concentration-dependent bactericidal activity of levofloxacin are the ratio of the peak concentration in plasma to the MIC (Cmax/MIC) and the ratio of the daily AUC (AUC0-24) to the MIC (AUC0-24/MIC) (1). According to previous studies, a Cmax/MIC ratio of 12.2 and an AUC0-24/MIC ratio of 125 h were shown to be valid thresholds for optimal drug exposure with the intent of preventing the selection of resistant strains and/or obtaining a clinical and microbiological cure with levofloxacin (3, 12, 29). On this basis, the theoretical pharmacodynamic breakpoints of levofloxacin in CSF (PD BPCSF), defined as the theoretically highest MIC which might have enabled optimal drug exposure against a potential pathogen in CSF according to both of these thresholds (12.2 for the Cmax ss/MIC ratio and 125 h for the AUC0-24/MIC ratio) and the patients' observed Cmax ss CSF and AUC0-τ CSF, were calculated by the following formulas: PD BPCSF for Cmax = Cmax ss CSF/12.2, and PD BPCSF for AUC0-24 = (AUC0-τ CSF · 2)/125.
Statistical analysis.
According to the normal or the nonnormal distribution, as estimated by the Kolmogorov-Smirnov test, the findings were expressed as means ± standard deviations (SDs) or medians and ranges, respectively. Statistical analysis was performed by the t test and/or the Mann-Whitney rank sum test, as appropriate, by using SigmaStat software (Jandel Scientific, GmbH, Erkrath, Germany). A statistically significant difference was defined as a P value <0.05.
RESULTS
Patient characteristics and microbiology.
The patients' diagnoses on admission, the etiological agents for the extracerebral infections, and the chemical and cellular status of the CSF are shown in Table 2. Among the 10 patients included in the study, the diagnoses on admission to the ICU was cerebrovascular accident in nine patients and surgical ablation of frontal meningioma in one patient. All of the patients were treated with levofloxacin because of ventilator-associated pneumonia. Three of the 10 patients had normal ventricular CSF protein concentrations (50 to 150 mg/liter), whereas 7 patients had moderate elevations of CSF protein concentrations (>150 mg/liter); but the albumin concentration was <100 mg/liter in all patients. A moderate hyperglycorrhachia was observed in about half of the patients, whereas three patients (patients 5, 9, and 10) had CSF pleocytosis (presence of WBCs at >5 cells/μl), probably due to the underlying cerebrovascular disease and/or to a sterile inflammatory response, as confirmed by negative CSF cultures for all patients. RBCs were detectable in only small amounts in lysate form.
Pharmacokinetic analysis.
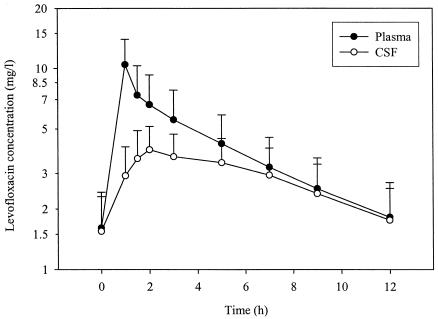
Mean ± SD plasma and CSF levofloxacin concentration-versus-time profiles are shown in Fig. 1. The average levofloxacin Cmax ss was 10.45 mg/liter in plasma and 4.06 mg/liter in CSF immediately and at 1.7 h after the end of the 1-h intravenous infusion, respectively, with an average ratio of the Cmax ss in CSF to the Cmax ss in plasma (CSF to plasma Cmax ss ratio) of 0.47.
FIG. 1.
Mean ± SD concentration-time profiles of levofloxacin in plasma and CSF of 10 patients with external ventriculostomies.
The pharmacokinetic parameters for levofloxacin in plasma and CSF are summarized in Tables 3 and 4, respectively. The average levofloxacin exposures (AUC0-τs) were 47.69 mg · h/liter for plasma and 33.42 mg · h/liter for CSF, with a mean ratio of the AUC0-τ for CSF to the AUC0-τ for plasma (CSF to plasma AUC0-τ ratio) of 0.71. The elimination of levofloxacin from CSF showed a log-linear decay, with a mean terminal half-life of 7.02 h.
TABLE 3.
Steady-state levofloxacin pharmacokinetic parameters in plasma during i.v. administration of 500 mg b.i.d. in 10 ICU patients with external ventriculostomies
| Parameter | Dose (mg/kg/12 h) | Cmax ss (mg/liter) | Vss (liters/kg) | t1/2β (h) | CL (ml/min/kg) | AUC0-τ (mg · h/liter) |
|---|---|---|---|---|---|---|
| Mean ± SD | 7.17 ± 1.30 | 10.45 ± 3.54 | 1.13 ± 0.30 | 5.51 ± 1.36 | 2.75 ± 1.18 | 47.69 ± 17.24 |
| CVa (%) | 18 | 34 | 27 | 25 | 43 | 36 |
CV, coefficient of variation.
TABLE 4.
Levofloxacin pharmacokinetic and pharmacodynamic aspects in CSF of 10 patients with external ventriculostomy
| Patient no. | Cmax ss CSF (mg/liter) | Tmax CSF (h) | AUC0-τ CSF (mg · h/liter) | CSF to plasma AUC0-τ ratio | CSF to plasma Cmax ss ratio | t1/2z CSF (h) | PD BPCSF for AUC0-24 (mg/liter) | PD BPCSF for Cmax (mg/liter) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.66 | 2 | 21.05 | 0.69 | 0.41 | 4.91 | 0.34 | 0.22 |
| 2 | 2.55 | 2 | 20.31 | 0.79 | 0.43 | 4.99 | 0.32 | 0.21 |
| 3 | 6.14 | 1.5 | 42.69 | 0.83 | 0.79 | 7.70 | 0.68 | 0.50 |
| 4 | 3.21 | 3 | 28.68 | 0.66 | 0.43 | 6.52 | 0.46 | 0.26 |
| 5 | 3.63 | 2 | 21.14 | 0.73 | 0.49 | 6.02 | 0.34 | 0.30 |
| 6 | 5.52 | 2 | 41.36 | 0.68 | 0.52 | 8.31 | 0.66 | 0.45 |
| 7 | 3.31 | 5 | 31.02 | 0.77 | 0.51 | 8.15 | 0.50 | 0.27 |
| 8 | 4.68 | 5 | 45.28 | 0.64 | 0.39 | 8.66 | 0.72 | 0.38 |
| 9 | 5.31 | 3 | 48.73 | 0.65 | 0.32 | 9.17 | 0.78 | 0.44 |
| 10 | 3.56 | 1.5 | 33.90 | 0.67 | 0.39 | 5.74 | 0.54 | 0.29 |
| Mean ± SD | 4.06 ± 1.26 | 2.70 ± 1.32 | 33.42 ± 10.69 | 0.71 ± 0.07 | 0.47 ± 0.13 | 7.02 ± 1.57 | 0.53 ± 0.17 | 0.33 ± 0.10 |
| CVa (%) | 31 | 49 | 32 | 9 | 27 | 22 | 32 | 31 |
CV, coefficient of variation.
The average CSF-to-plasma-concentration ratios progressively increased with time, from 0.30 immediately after dosing to 0.99 at the end of the dosing interval.
Dose-normalized data showed that for each 1 mg of levofloxacin per kg/12 h, the mean ± SD dose-normalized Cmax sss were 1.47 ± 0.44 mg/liter in plasma and 0.59 ± 0.26 mg/liter in CSF, whereas the mean fractional AUC0-τs were 6.80 ± 2.44 mg · h/liter for plasma and 4.84 ± 1.90 mg · h/liter for CSF.
No correlation between AUC0-τ CSF or Cmax ss CSF and the protein concentration in CSF was found.
The mean theoretical PD BPCSF values for the optimal bactericidal efficacy of levofloxacin in CSF were 0.53 and 0.33 mg/liter for an AUC0-24/MIC ratio threshold of 125 h and a Cmax/MIC ratio threshold of 12.2, respectively (Table 4).
DISCUSSION
Our study investigated the ventricular CSF disposition of levofloxacin in patients with external ventriculostomies treated because of documented or suspected extracerebral infections.
The pharmacokinetics of levofloxacin in plasma confirmed our previous findings for patients with early-onset ventilator-associated pneumonia (28), suggesting that high b.i.d. dosages (500 mg b.i.d.) are needed to ensure optimal drug exposure in ICU patients, mainly because the renal clearance of levofloxacin, which is the most predictive parameter of its interindividual pharmacokinetic variability (28, 30), may be increased due either to the frequent hyperdynamic conditions or to cotreatment with hemodynamically active drugs.
When the dispositions of levofloxacin in CSF and plasma were compared, all the findings (the AUC corresponded to about 70% of the total exposure in plasma, the rapid achievement of a peak level in CSF corresponded to about one-half of that in plasma, and the terminal half-life was only slightly significantly longer than that in plasma) were consistent with the fact that levofloxacin freely crosses the blood-CSF barrier by passive diffusion (19, 21, 24), according to its physicochemical properties (namely, small size, moderate level of lipophilia, and negligible plasma protein binding [20 to 30%]).
This substantial penetration of levofloxacin into CSF is in agreement with the findings of other investigators both in animals and in humans. In an experimental meningitis model, Destache and coworkers (8) found that the average level of CSF exposure to levofloxacin ranged between 53 and 76% of the corresponding level of plasma exposure in rabbits challenged with S. pneumoniae. Likewise, in a previous study (32), based on a single sampling time during a diagnostic lumbar puncture in patients with acute bacterial meningitis treated with a combination of a beta-lactam plus levofloxacin at 500 mg b.i.d. i.v., the CSF to plasma Cmax ss ratio 2 h after dosing averaged 35%, ranging between 23 and 42%. Finally, in six patients with external ventriculostomy, Nau and coworkers (20) demonstrated that after administration of a single 400-mg dose, the level of CSF penetration of ofloxacin, namely, the racemate of l- and d-ofloxacin, enabled an average CSF-to- serum-AUC ratio of 0.65 (range, 0.59 to 0.81) and a mean peak level in CSF of 2.04 mg/liter to be achieved 1.75 h after dosing.
The persistence in the ventricular CSF of effective concentrations that lasted for the entire dosing interval suggested that optimal exposure to levofloxacin may be achieved in all parts of the CSF system, considering that, in general, antibiotic concentrations were found to be severalfold lower at the ventricular CSF level than at the lumbar CSF level (21).
It is noteworthy that the free penetration of levofloxacin into CSF, irrespective of the barrier status, may have important consequences for the treatment of bacterial meningitis. In fact, although the penetration of the reference agents for the therapy of bacterial meningitis, namely, hydrophilic agents such as cefotaxime, ceftriaxone, and ampicillin, is expected to decrease day by day because of the progressive healing of the barrier promoted by the antimicrobial therapy and/or by corticosteroid-associated action (26), the unimpeded access of levofloxacin to the CNS might ensure a highly effective exposure lasting not only for the first days of therapy but also for the overall treatment period.
With the intent of preventing both clinical failure and the spread of resistance (9), according to the theoretical pharmacodynamic breakpoints for levofloxacin in CSF, a levofloxacin regimen of 500 mg b.i.d. i.v. may provide optimal exposure in CSF against microorganisms for which MICs are <0.5 mg/liter. This value is lower than the MIC at which 90% of strains are inhibited for most but not all of the etiological agents of spontaneous bacterial meningitis. For S. pneumoniae, the most frequent causative agent of meningitis, the levofloxacin MIC at which 90% of strains are inhibited is 1 mg/liter. Therefore, although AUC/MIC ratios as low as 30 to 50 h were recently postulated to be enough, both in vitro and in vivo, for the eradication of S. pneumoniae with fluoroquinolones (1, 16-18, 22), these data support the potential utility of levofloxacin in the treatment of spontaneous bacterial meningitis in combination with a reference beta-lactam. Other antipneumococcal fluoroquinolones that are more potent in vitro, namely, moxifloxacin and gatifloxacin, could also be useful for these purposes. The coadministration of an antipneumococcal fluoroquinolone for the treatment of meningitis might be especially helpful whenever S. pneumoniae strains intermediately susceptible or resistant to cefotaxime and/or ceftriaxone may be involved, as the low degree of susceptibility of this pathogen is a factor negatively associated with the outcome of pneumococcus-related meningitis (25, 36). This hypothesis is supported by the recent finding of synergism between ceftriaxone and levofloxacin in the treatment of experimental meningitis in rabbits challenged with penicillin-resistant S. pneumoniae strains (L. Flatz, M. Cottagnoud, J. M. Entenza, P. Moreillon, M. G. Tauber, and P. Cottagnoud, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1429, p. 56, 2002).
Although pharmacokinetic-pharmacodynamic relationships provide evidence for the potential utility of levofloxacin in the treatment of bacterial meningitis, the facts that fluoroquinolones are not licensed for pediatric use and are not devoid of side effects in the CNS should not be overlooked. However, the proconvulsant activities of fluoroquinolones were shown to be dependent on both chemical structure and dosages (7, 31), and among the various compounds, levofloxacin was shown to be one of the least epileptogenic, having excitatory activity even less than that of the ofloxacin racemate, probably due to its weaker binding affinity to the γ-aminobutyric acid receptor in the CNS (14). Accordingly, the low potential for neurotoxicity is confirmed by the very low incidence of convulsions during treatment in humans (2, 5, 15).
In conclusion, both the favorable pharmacokinetics and the theoretical pharmacokinetic-pharmacodynamic analysis of levofloxacin in the ventricular CSF of patients with uninflamed meninges provide support for trials of levofloxacin with b.i.d. dosing in combination with a reference beta-lactam for the treatment of bacterial meningitis in adults. This cotreatment could be especially helpful both in overcoming S. pneumoniae resistance and in enabling optimal CSF exposure to at least one antibacterial agent for the overall treatment period.
Acknowledgments
This research was carried out spontaneously thanks to departmental funds (DPHSC, University of Udine).
The technical assistance of Eliana Di Terlizzi is gratefully acknowledged.
REFERENCES
- 1.Aminimanizani, A., P. Beringer, and R. Jelliffe. 2001. Comparative pharmacokinetics and pharmacodynamics of the newer fluoroquinolone antibacterials. Clin. Pharmacokinet. 40:169-187. [DOI] [PubMed] [Google Scholar]
- 2.Ball, P. 2000. Safety of the new fluoroquinolones compared with ciprofloxacin. J. Chemother. 12:8-11. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess, D., and S. Nathisuwan. 2002. Cefepime, piperacillin/tazobactam, gentamicin, ciprofloxacin, and levofloxacin alone and in combination against Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 44:35. [DOI] [PubMed] [Google Scholar]
- 5.Carbon, C. 2001. Comparison of side effects of levofloxacin versus other fluoroquinolones. Chemotherapy (Basel) 47(Suppl. 3):9-14. [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 7.De Sarro, A., and G. De Sarro. 2001. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects. Curr. Med. Chem. 8:371-384. [DOI] [PubMed] [Google Scholar]
- 8.Destache, C. J., C. B. Pakiz, C. Larsen, H. Owens, and A. K. Dash. 2001. Cerebrospinal fluid penetration and pharmacokinetics of levofloxacin in an experimental rabbit meningitis model. J. Antimicrob. Chemother. 47:611-615. [DOI] [PubMed] [Google Scholar]
- 9.Drusano, G. L. 2000. Fluoroquinolone pharmacodynamics: prospective determination of relationships between exposure and outcome. J. Chemother. 12:21-26. [DOI] [PubMed] [Google Scholar]
- 10.Fish, D. N., and A. T. Chow. 1997. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32:101-119. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, C. M., D. M. Johnson, and R. N. Jones. 1996. In vitro efficacy of levofloxacin alone or in combination tested against multi-resistant Pseudomonas aeruginosa strains. J. Chemother. 8:411-415. [DOI] [PubMed] [Google Scholar]
- 12.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst, M., H. M. Lamb, L. J. Scott, and D. P. Figgitt. 2002. Levofloxacin: an updated review of its use in the treatment of bacterial infections. Drugs 62:2127-2167. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi, T., K. Akahane, and N. Akaike. 1995. Attenuated inhibition by levofloxacin, l-isomer of ofloxacin, on GABA response in the dissociated rat hippocampal neurons. Neurosci. Lett. 193:81-84. [DOI] [PubMed] [Google Scholar]
- 15.Kahn, J. B. 2001. Latest industry information on the safety profile of levofloxacin in the US. Chemotherapy (Basel) 47:32-37. [DOI] [PubMed] [Google Scholar]
- 16.Lacy, M. K., W. Lu, X. Xu, P. R. Tessier, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob. Agents Chemother. 43:672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 43:79-86. [DOI] [PubMed] [Google Scholar]
- 18.Lister, P. D., and C. C. Sanders. 2001. Pharmacodynamics of moxifloxacin, levofloxacin and sparfloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 47:811-818. [DOI] [PubMed] [Google Scholar]
- 19.Lutsar, I., G. H. McCracken, Jr., and I. R. Friedland. 1998. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin. Infect. Dis. 27:1117-1127. [DOI] [PubMed] [Google Scholar]
- 20.Nau, R., M. Kinzig, T. Dreyhaupt, H. Kolenda, F. Sorgel, and H. W. Prange. 1994. Kinetics of ofloxacin and its metabolites in cerebrospinal fluid after a single intravenous infusion of 400 milligrams of ofloxacin. Antimicrob. Agents Chemother. 38:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nau, R., F. Sorgel, and H. W. Prange. 1998. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin. Pharmacokinet. 35:223-246. [DOI] [PubMed] [Google Scholar]
- 22.Nightingale, C. H., E. M. Grant, and R. Quintiliani. 2000. Pharmacodynamics and pharmacokinetics of levofloxacin. Chemotherapy (Basel) 46:6-14. [DOI] [PubMed] [Google Scholar]
- 23.Ohi, Y., T. Goto, K. Kawahara, et al. 1992. Penetration of fluoro-quinolones into human spinal fluid. Chemotherapy (Tokyo) 40:469-473. [Google Scholar]
- 24.Ooie, T., H. Suzuki, T. Terasaki, and Y. Sugiyama. 1996. Kinetics of quinolone antibiotics in rats: efflux from cerebrospinal fluid to the circulation. Pharm. Res. 13:1065-1068. [DOI] [PubMed] [Google Scholar]
- 25.Pallares, R. 1999. Treatment of pneumococcal pneumonia. Semin. Respir. Infect. 14:276-284. [PubMed] [Google Scholar]
- 26.Paris, M. M., S. M. Hickey, M. I. Uscher, S. Shelton, K. D. Olsen, and G. H. McCracken, Jr. 1994. Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob. Agents Chemother. 38:1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel, J. A., C. T. Pachucki, and J. R. Lentino. 1993. Synergy of levofloxacin (l-ofloxacin) and oxacillin against quinolone-resistant Staphylococcus aureus, measured by the time-kill method. Antimicrob. Agents Chemother. 37:339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pea, F., E. Di Qual, A. Cusenza, L. Brollo, M. Baldassarre, and M. Furlanut. 2003. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin. Pharmacokinet. 42:589-598. [DOI] [PubMed] [Google Scholar]
- 29.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 30.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, F. A. Wong, and M. Corrado. 1998. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob. Agents Chemother. 42:1098-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmuck, G., A. Schurmann, and G. Schluter. 1998. Determination of the excitatory potencies of fluoroquinolones in the central nervous system by an in vitro model. Antimicrob. Agents Chemother. 42:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotton, P. G., F. Pea, M. Giobbia, M. Baraldo, A. Vaglia, and M. Furlanut. 2001. Cerebrospinal fluid penetration of levofloxacin in patients with spontaneous acute bacterial meningitis. Clin. Infect. Dis. 33:E109-E111. [DOI] [PubMed] [Google Scholar]
- 33.Smith, C. E., B. E. Foleno, J. F. Barrett, and M. B. Frosco. 1997. Assessment of the synergistic interactions of levofloxacin and ampicillin against Enterococcus faecium by the checkerboard agar dilution and time-kill methods. Diagn. Microbiol. Infect. Dis. 27:85-92. [DOI] [PubMed] [Google Scholar]
- 34.Verho, M., V. Malerczyk, D. Damm, and K. H. Lehr. 1996. Pharmacokinetics of levofloxacin in comparison to the racemic mixture of ofloxacin in man. Drug Metab. Drug Interact. 13:57-67. [DOI] [PubMed] [Google Scholar]
- 35.Visalli, M. A., M. R. Jacobs, and P. C. Appelbaum. 1998. Determination of activities of levofloxacin, alone and combined with gentamicin, ceftazidime, cefpirome, and meropenem, against 124 strains of Pseudomonas aeruginosa by checkerboard and time-kill methodology. Antimicrob. Agents Chemother. 42:953-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, A. J., and S. Nadel. 2001. Bacterial meningitis: current controversies in approaches to treatment. CNS Drugs 15:909-919. [DOI] [PubMed] [Google Scholar]
- 37.Yamaoka, K., T. Nakagawa, and T. Uno. 1978. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165-175. [DOI] [PubMed] [Google Scholar]