Abstract
At infection sites, polymorphonuclear leukocyte (PMN) function is enhanced (“primed”) by granulocyte-macrophage colony-stimulating factor (GM-CSF) or lipopolysaccharide (LPS) and activated by formyl peptides. In this study, GM-CSF or LPS alone had no significant effects on PMN ciprofloxacin transport. Through a mechanism involving protein kinase C, activation by formyl-Met-Leu-Phe (fMLP) significantly decreased the Km of ciprofloxacin transport and enhanced ciprofloxacin accumulation. This effect was dramatically enhanced when PMNs were primed with GM-CSF or LPS prior to activation by fMLP.
Polymorphonuclear leukocytes (PMNs), the host's primary defense against bacterial infections, are directed to infection sites by formyl peptides and other chemoattractants. As they approach these sites, their microbicidal functions are “primed” by exposure of specific surface receptors to bacterial lipopolysaccharide (LPS), granulocyte-macrophage colony-stimulating factor (GM-CSF), and other inflammatory mediators (16). Priming is an important protective response that enhances degranulation and superoxide production when PMNs eventually encounter bacteria or high concentrations of chemoattractants. While PMNs provide a very effective defense against bacteria, some pathogens resist phagocytic killing. One example is Actinobacillus actinomycetemcomitans, which possesses several virulence factors and is associated with aggressive periodontitis (19). Antimicrobial agents can help the host cope with an overwhelming infection. Ciprofloxacin and other fluoroquinolones are actively taken up by phagocytes (1, 7, 17) and possess activity that augments PMN killing of some difficult pathogens. Recent work has shown that ciprofloxacin enhances PMN killing of A. actinomycetemcomitans when the bacterium-to-PMN ratio exceeds 10:1 (3). Since PMNs rapidly infiltrate an infection site in great numbers, their ability to accumulate fluoroquinolones could potentially enhance resolution of an infection by increasing local antimicrobial concentrations at these sites (11). PMNs possess at least two saturable systems for taking up fluoroquinolones. One is a relatively low-affinity system that operates continuously and is relatively pH insensitive, while the other is a high-affinity, pH-sensitive system that is expressed in phorbol ester-activated PMNs (17).
Although phorbol esters are a useful laboratory tool for evaluating the role of protein kinase C (PKC) in cell regulation, they play no physiological role in regulating PMN function in vivo. To gain insight into the manner in which PMN fluoroquinolone transport is regulated at infection sites, we investigated the effects of chemoattractants and priming agents. We predicted that the chemoattractant formyl-Met-Leu-Phe (fMLP) would stimulate ciprofloxacin transport into PMNs by a process involving PKC. Because priming agents potentiate many chemoattractant-activated microbicidal functions in PMNs, we hypothesized that they would also enhance fMLP-stimulated ciprofloxacin transport. To test these hypotheses, human PMNs were isolated from citrated whole blood obtained from healthy volunteers. After purification by Ficoll/Hypaque density gradient centrifugation and dextran sedimentation (2), residual erythrocytes were eliminated by hypotonic lysis. The remaining PMNs were washed three times with phosphate-buffered saline and resuspended at a density of 5 × 106 cells/ml in Hank's balanced salts solution.
Ciprofloxacin transport was assayed by measuring cell-associated ciprofloxacin fluorescence as previously described (17). PMN suspensions were warmed to 37°C prior to the assay. In some experiments, PMNs were pretreated with 4 μg of cytochalasin B per ml to enhance their response to fMLP. At the indicated times after PMN activation with fMLP, 0.5-ml aliquots of cell suspension were rapidly withdrawn, layered over 0.3 ml of canola oil-dibutyl phthalate (3:10), and centrifuged for 45 s at 15,000 × g in a microcentrifuge. After removal of the aqueous and oil layers, the cell pellet was recovered by cutting off the end of the microcentrifuge tube. The pellet was lysed and dispersed in 1.5 ml of 100 mM glycine-HCl (pH 3.0) by agitation at room temperature. The samples were clarified by centrifugation at 5,600 × g for 5 min, and the fluorescence of the supernatant was measured with a fluorescence spectrometer. Excitation and emission wavelengths of 278 and 445 nm, respectively, were used. The detection limit for ciprofloxacin was below 1 ng/ml, and recovery from cell pellets was essentially quantitative. To determine the Km and Vmax of fluoroquinolone transport, transport activity was assayed during the initial linear phase of transport and analyzed by the Lineweaver-Burk method.
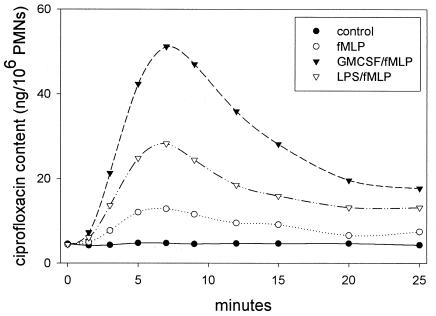
When resting PMN suspensions were incubated in medium containing 5 μg of ciprofloxacin per ml, they attained a steady-state intracellular ciprofloxacin content of approximately 4 ng/106 cells within 15 min. When treated with fMLP (100 nM; Sigma Chemical Co., St. Louis, Mo.), these cells underwent a transient, 2.5-fold increase in ciprofloxacin content that peaked within 7 min and remained above control levels for more than 25 min (Fig. 1). The increased intracellular content was facilitated by a significant decrease in the Km for ciprofloxacin transport induced by fMLP (P < 0.05) (Table 1). PMNs treated for 30 min with either of the priming agents GM-CSF (20 ng/ml; PeproTech, Rock Hill, N.J.) and Salmonella enterica serovar Minnesota Re 595 LPS (1 μg/ml; List Biological Laboratories, Campbell, Calif.) exhibited no significant changes in Km or Vmax of transport (Table 1) and no changes in intracellular ciprofloxacin content (data not shown). When primed cells were subsequently treated with fMLP, however, they underwent a dramatic increase in ciprofloxacin content that noticeably exceeded that induced by fMLP alone (Fig. 1). Under these conditions, the Km for ciprofloxacin transport was lower than in PMNs treated with fMLP alone. This effect was most significant in cells primed with GM-CSF (P < 0.05) (Table 1). In all cases, fMLP triggered a reduction in the Vmax of transport. fMLP interacts with formyl peptide chemoattractant receptors on the PMN surface to initiate chemotaxis and activate microbicidal functions, including production of reactive oxygen intermediates and release of lytic enzymes. At the concentrations used in these experiments, fMLP stimulates superoxide production and release of preformed lytic enzymes from granules. These substances are cytotoxic and could potentially compromise the velocity of ciprofloxacin transport by damaging membrane-associated transporters.
FIG. 1.
Kinetics of ciprofloxacin transport by resting and fMLP-activated PMNs. Cell suspensions were incubated in medium containing 5 μg of ciprofloxacin per ml for 15 min at 37°C to obtain steady-state intracellular levels of this agent. Where indicated, suspensions were pretreated with 20 ng of GM-CSF or 1 μg of Re 595 LPS per ml for 10 min prior to addition of ciprofloxacin. The assay was started by addition of medium (resting control) or fMLP (100 nM). Intracellular ciprofloxacin content was measured at the indicated time points. The results are representative of three experiments.
TABLE 1.
Effect of priming and activation on the kinetics of PMN ciprofloxacin transporta
| Priming agent | Activating agent | Km (μg/ml) (n) | Vmax (ng/min/106) (n) |
|---|---|---|---|
| None (control) | None | 322 ± 16.0 (10) | 58.3 ± 4.8 (10) |
| GM-CSF (20 ng/ml) | None | 385 ± 41.1 (5) | 69.1 ± 13.6 (5) |
| LPS (1 μg/ml) | None | 285 ± 30.2 (5) | 50.0 ± 5.9 (5) |
| None (control) | fMLP | 95.4 ± 14.7* (10) | 37.8 ± 2.3 (10) |
| GM-CSF (20 ng/ml) | fMLP | 15.6 ± 4.96* (5) | 37.6 ± 3.0 (5) |
| LPS (1 μg/ml) | fMLP | 69.1 ± 7.76 (5) | 31.8 ± 2.60 (5) |
Cells were pretreated for 30 min with the indicated priming agent or medium alone (control), and then half of the pretreated cells were activated with 100 nM fMLP. Results were derived from the initial linear phase of transport and are expressed as the means for n experiments ± standard errors of the means. Priming with GM-CSF or LPS had no significant effect on the Km or Vmax of ciprofloxacin transport in PMNs that were not activated by fMLP and no effect on Vmax in cells that were eventually activated by fMLP (P > 0.12, Kruskal-Wallis one-way analysis of variance on ranks). However, priming with GM-CSF or LPS had a significant effect on Km (P = 0.012, Kruskal-Wallis); *, significant difference (P < 0.05, Dunn's method). In every instance, activation with fMLP induced a significant reduction in Km and Vmax (P < 0.05, paired t test).
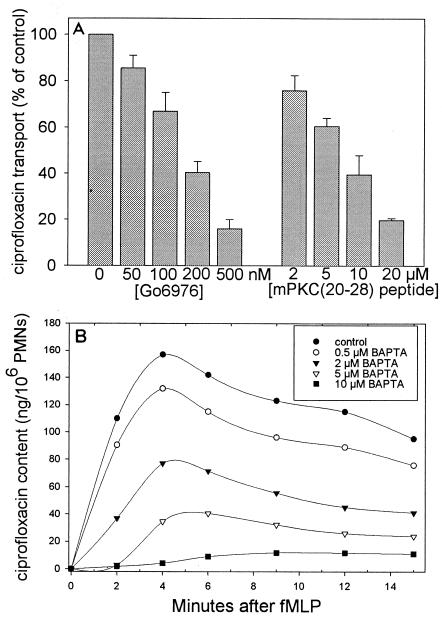
When occupied, formyl peptide receptors trigger cell activation by inducing formation of diacylglycerol (which activates PKC) and inositol 1,4,5-trisphosphate (which mobilizes intracellular Ca2+ stores) (reviewed in reference 10). PKC is an essential transducer of signals for PMN activation (10, 15). Previous work has identified several PKC isotypes in leukocytes, including α, βI, and βII (which are most prevalent and are activated by Ca2+, diacylglycerol, and phosphatidylserine), ζ (which is activated by phosphatidylserine and unsaturated fatty acids), and δ (which is activated by phosphatidylserine and diacylglycerol) (9, 14). Go6976 can be used to selectively inhibit the Ca2+-dependent α and β isotypes without affecting the Ca2+-independent ζ and δ isotypes (12). In this study, Go6976 (Biomol Laboratories, Plymouth Meeting, Pa.) inhibited fMLP-stimulated ciprofloxacin transport in a dose-dependent manner, producing 50% inhibition at approximately 150 nM (Fig. 2A). Similarly, myristoylated PKC(20-28) (Biomol Laboratories), a pseudosubstrate peptide sequence that inhibits PKC α and β isotypes but has no effect on other protein kinase systems (5, 18), produced dose-dependent inhibition of fMLP-stimulated transport (50% inhibitory concentration = 8 μM) (Fig. 2). Neither agent inhibited transport by resting control PMNs, and neither had any direct effect on ciprofloxacin fluorescence. In contrast, a ζ isotype-selective myristoylated PKC peptide inhibitor (Biomol) produced only half the inhibition observed with equimolar concentrations of PKC(20-28) (data not shown).
FIG. 2.
Effect of PKC inhibition and intracellular Ca2+ chelation on ciprofloxacin transport by fMLP-activated PMNs. (A) PMNs were pretreated for 10 min with 4 μg of cytochalasin B per ml and the indicated concentrations of Go6976 or myristoylated PKC(20-28) cell-permeative PKC inhibitor. The assay was initiated by the simultaneous addition of 10 μg of ciprofloxacin per ml and 100 nM fMLP and was terminated after 3 min. Results are expressed as means ± standard errors of the means for three experiments. (B) PMNs were pretreated for 10 min with 4 μg of cytochalasin B per ml and the indicated concentrations of BAPTA/AM. The assay was started by the simultaneous addition of 10 μg of ciprofloxacin per ml and 100 nM fMLP. The data are representative of three experiments.
The intracellular Ca2+ chelator BAPTA/AM (Sigma Chemical Co.) was used to assess the role of intracellular Ca2+ in fMLP-stimulated ciprofloxacin transport. Full activation of Ca2+-dependent PKC isotypes by diacylglycerol requires intracellular free Ca2+ levels of 100 nM or greater (13). In the present study, BAPTA/AM produced 50% inhibition at a concentration of 2 μM and nearly complete inhibition at 10 μM (Fig. 2B). Our previous work demonstrated that BAPTA inhibits PKC isolated from PMN lysates at similar doses (6). Interestingly, BAPTA/AM (as well as Go6976) strongly inhibited GM-CSF-primed, fMLP-activated ciprofloxacin transport over the concentration ranges utilized for Fig. 2.
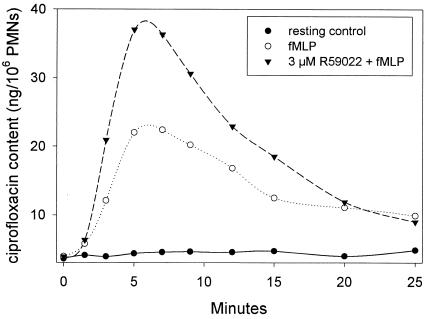
Diacylglycerol, the primary physiological activator of α and β PKC isotypes, is rapidly inactivated in intact PMNs by diacylglycerol kinase. Inhibition of diacylglycerol kinase potentiates many PKC-mediated events, including activation of PMN superoxide production by fMLP (8). We used the diacylglycerol kinase inhibitor R59022 (4) to further assess the role of PKC in up-regulating ciprofloxacin transport. When used at a concentration that minimizes nonspecific effects (3 μM), pretreatment with R59022 (Sigma Chemical Co.) enhanced the stimulation of PMN ciprofloxacin accumulation by fMLP (Fig. 3). Peak intracellular ciprofloxacin content increased nearly twofold, and the increase in content due to R59022 was sustained for nearly 20 min after cell activation.
FIG. 3.
Effect of the diacylglycerol kinase inhibitor R59022 on ciprofloxacin transport by fMLP-activated PMNs. The assay was conducted by using methods similar to those described for Fig. 1, except that 10 μg of ciprofloxacin per ml was added to the medium. Where indicated, PMNs were pretreated for 10 min with 3 μM R59022 prior to activation with 100 nM fMLP. The results are representative of four experiments. Control experiments ensured that R59022 did not directly alter ciprofloxacin fluorescence.
In conclusion, our findings suggest that formyl peptide chemoattractants enhance fluoroquinolone accumulation in PMNs by inducing an increase in the affinity of transport. PKC, especially the Ca2+- and diacylglycerol-dependent isotypes, appears to mediate this process. fMLP presumably activates the same high-affinity component of transport expressed in phorbol ester-stimulated PMNs (16), but its effects are transient, in contrast to the sustained response produced by phorbol myristate acetate. We have also shown that priming agents found at infection sites potentiate activation of high-affinity ciprofloxacin transport by fMLP. Collectively, these data suggest that PMNs could acquire an increased ability to take up and accumulate fluoroquinolones as they migrate from the bloodstream to infection sites. This could enhance their ability to deliver fluoroquinolones to infection sites and augment their capacity to cope with an overwhelming bacterial challenge. Since it may be possible to enhance the effectiveness of antimicrobial chemotherapy by exploiting PMN fluoroquinolone transport activity, further studies of the regulation of PMN fluoroquinolone transport are warranted.
Acknowledgments
This work was supported by U.S. Public Health Service grant R01 DE12601 from the National Institute for Dental and Craniofacial Research.
REFERENCES
- 1.Bounds, S. J., R. Nakkula, and J. D. Walters. Fluoroquinolone transport by human monocytes: characterization and comparison to other cells of myeloid lineage. Antimicrob. Agents Chemother. 44:2609-2614. [DOI] [PMC free article] [PubMed]
- 2.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human peripheral blood. Scand. J. Clin. Lab. Investig. 21:77-89. [PubMed] [Google Scholar]
- 3.Cacchillo, D. A., and J. D. Walters. 2002. Effect of ciprofloxacin on killing of Actinobacillus actinomycetemcomitans by polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 46:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeCaffoy de Courcelles, D. 1990. The use of diacylglycerol kinase inhibitors for elucidating the roles of protein kinase C, p. 491-496. In Y. Nishizuka (ed.), The biology and medicine of signal transduction. Raven Press, New York, N.Y.
- 5.Eichholtz, T., D. B. A. de Bont, J. de Widt, R. M. F. Liskamp, and H. L. Ploegh. 1993. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J. Biol. Chem. 268:1982-1986. [PubMed] [Google Scholar]
- 6.Fernandez, M. C., P. T. Marucha, I. G. Rojas, and J. D. Walters. 2000. The role of protein kinase C and calcium in induction of human polymorphonuclear leukocyte IL-1β gene expression by GM-CSF. Cytokine 12:445-449. [DOI] [PubMed] [Google Scholar]
- 7.Garraffo, R., D. Jambou, R. M. Chichmanian, S. Ravoire, and P. Lapalus. 1991. In vitro and in vivo ciprofloxacin pharmacokinetics in human neutrophils. Antimicrob. Agents Chemother. 35:2215-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Cambronero, J., T. F. Molski, E. L. Becker, and R. I. Sha'afi. 1987. The diacylglycerol kinase inhibitor R59022 potentiates superoxide production but not secretion induced by fMet-Leu-Phe: effects of leupeptin and the protein kinase C inhibitor H-7. Biochem. Biophys. Res. Commun. 148:38-46. [DOI] [PubMed] [Google Scholar]
- 9.Kent, J. D., S. Sargent, D. J. Burns, and L. C. McPhail. 1996. Identification and regulation of protein kinase C-delta in human neutrophils. J. Immunol. 157:4641-4647. [PubMed] [Google Scholar]
- 10.Lew, D. P. 1989. Receptor signaling and intracellular calcium in neutrophil activation. Eur. J. Clin. Investig. 19:338-346. [DOI] [PubMed] [Google Scholar]
- 11.Mandell, G. L., and E. Coleman. 2001. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 45:1794-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martiny-Baron, G., M. G. Kazanietz, H. Mischak, P. M. Blumberg, G. Kochs, H. Hug, D. Marme, and C. Schachtele. 1993. Selective inhibition of protein kinase C isotypes by the indocarbazole Go6976. J. Biol. Chem. 268:9194-9197. [PubMed] [Google Scholar]
- 13.O'Flaherty, J. T., D. P. Jacobson, J. F. Redman, and A. G. Rossi. 1990. Translocation of protein kinase C in human polymorphonuclear neutrophils. Regulation by cytosolic Ca2+-independent and Ca2+-dependent mechanisms. J. Biol. Chem. 265:9146-9152. [PubMed] [Google Scholar]
- 14.Seibenhener, M. L., and M. W. Wooten. 1993. Heterogeneity of protein kinase C isoform expression in chemically induced HL-60 cells. Exp. Cell Res. 207:183-188. [DOI] [PubMed] [Google Scholar]
- 15.Smolen, J. E. 1992. Neutrophil signal transduction: Calcium, kinases, and fusion. J. Clin. Lab. Med. 120:527-532. [PubMed] [Google Scholar]
- 16.Steinbeck, M. J., and J. A. Roth. 1989. Neutrophil activation by recombinant cytokines. Rev. Infect. Dis. 11:549-568. [DOI] [PubMed] [Google Scholar]
- 17.Walters, J. D., R. J. Nakkula, and F. Zhang. 1999. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob. Agents Chemother. 43:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward, N. E., and C. A. O'Brian. 1993. Inhibition of protein kinase C by N-myristoylated peptide substrate analogs. Biochemistry 32:11903-11909. [DOI] [PubMed] [Google Scholar]
- 19.Zambon, J. J., V. I. Haraszthy, G. Hariharan, E. T. Lally, and D. R. Demuth. 1996. The microbiology of early-onset periodontitis: association of highly toxic Actinobacillus actinomycetemcomitans strains with localized juvenile periodontitis. J. Periodontol. 67:282-290. [DOI] [PubMed] [Google Scholar]