Abstract
We identified a novel plasmid-borne gene (designated qacJ) encoding resistance to quaternary ammonium compounds (QACs) in three staphylococcal species associated with chronic infections in four horses. qacJ was located on a 2,650-bp plasmid (designated pNVH01), a new member of the pC194 family of rolling-circle replication plasmids. The 107-amino-acid protein, QacJ, showed similarities to known proteins of the small multidrug resistance family: Smr/QacC (72.5%), QacG (82.6%), and QacH (73.4%). The benzalkonium chloride MIC for a qacJ-containing recombinant was higher than those for otherwise isogenic recombinants expressing Smr, QacG, or QacH. Molecular epidemiological analyses by pulsed-field gel electrophoresis suggested both the clonal spread of a qacJ-harboring Staphylococcus aureus strain and the horizontal transfer of pNVH01 within and between different equine staphylococcal species. The presence of pNVH01 of identical nucleotide sequence in different staphylococcal species suggests that recent transfer has occurred. In three of the horses, a skin preparation containing cetyltrimethylammonium bromide had been used extensively for several years; this might explain the selection of staphylococci harboring the novel QAC resistance gene.
The coagulase-positive species Staphylococcus aureus and Staphylococcus intermedius are associated with various infectious diseases in horses, particularly skin and bone disorders (27, 30). Lesions of the limbs caused by trauma may be infected with staphylococci normally present on the skin, resulting in localized suppurating infections. Various pathogenic staphylococci are frequently isolated from skin lesions and are often mixed with other bacteria or dermatophytes (4).
Quaternary ammonium compounds (QACs) are widely used as disinfectants and antiseptics for control of bacterial growth in domestic households, health care settings, and the food industry. Benzalkonium chloride (BC) and cetyltrimethylammonium bromide (CTAB) are active components in various preparations commonly used in veterinary medicine (2; J. Bjorland, and M. Sunde, Abstr. Summer Conf. Antibiot. Biocide Resist. Bacteria, abstr. P45, 2001). Antiseptic preparations for horses are widely used to prevent or treat bacterial skin infections. Most of these preparations are commercially available without a prescription from a veterinarian, and information on the active disinfectant component is usually not supplied in the table of contents on the container label.
Staphylococcal resistance to QACs is known in human medicine and has been reported from the food industry (9, 15, 28, 29). Three different QAC genes from clinical human isolates of S. aureus and coagulase-negative staphylococci (CoNS) have been characterized: qacA, qacB, and smr (qacC) (13, 14, 19). Two additional genes, qacG and qacH, have been found in isolates of CoNS from the food industry (7, 8). Staphylococcal QAC resistance genes are, in general, plasmid borne; and the smr, qacG, and qacH genes are normally harbored by small plasmids less than 3 kb and encode efflux proteins belonging to the small multidrug resistance (SMR) family (7, 8, 22). The proteins expel hydrophobic drugs including QACs, the intercalating dye ethidium bromide (EBR), and some other cationic biocides by means of an H+/drug antiport mechanism (3).
Our knowledge about resistance to QACs in bacteria associated with disease in animals is limited. Recently, smr was found in bovine S. aureus isolates (2) and qacA was found in bovine and feline Staphylococcus haemolyticus isolates (1). Staphylococcus warneri, which contains both qacA/qacB and smr, and Staphylococcus epidermidis, which contains smr, were found to be associated with bovine clinical mastitis (Bjorland and Sunde, Abstr. Summer Conf. Antibiot. Biocide Resist. Bacteria, 2001). Here we report on the identification and characterization, as well as some epidemiological aspects, of a novel QAC resistance gene, designated qacJ, harbored by the novel staphylococcal plasmid pNVH01, present in both coagulase-positive staphylococci and CoNS associated with various infectious diseases in four horses.
MATERIALS AND METHODS
Animals and bacterial isolates.
During a 14-month period, 50 equine staphylococcal isolates recovered from clinical specimens subjected to bacteriological examination at the National Veterinary Institute or The Norwegian School of Veterinary Science, Oslo, Norway, were screened for QAC resistance as described below. Twelve isolates were QAC resistant and were further examined by PCR with plasmid DNA as the template. Seven of these isolates, in which neither qacA/qacB, smr, nor qacH was detected but in which a faint PCR product was obtained when qacG-specific primers were used, were selected for further genetic studies. The seven isolates were cultivated from single specimens from each of four horses (horses A, B, C, and D) on three equine operations in eastern Norway. Horses A and B (on the same operation) had dermatitis, horse C had a postoperative wound infection, and horse D had arthritis. According to information given by the care personnel in the different equine operations, a skin preparation containing CTAB had been used for several years on three of the horses (horses A, B, and D). For epidemiological purposes, two QAC-susceptible staphylococcal isolates from horse B, recovered from the same specimen as the QAC-resistant isolates, were also included in the study. The bacterial strains and plasmids used or identified in the study are shown in Table 1.
TABLE 1.
Bacterial strains and plasmids used or identified in the study
| Species and strain or plasmida | Remarks | Source or referenceb |
|---|---|---|
| S. intermedius | ||
| 119-r | Wild-type strain, pNVH01 | This study, A |
| 139-r | Wild-type strain, pNVH01 | This study, B |
| 139-s | Closely related to 139-r, but plasmid free | This study, B |
| S. aureus | ||
| 120-r | Wild-type strain, pNVH01 | This study, A |
| 120-c | Isogenic to 120-r but cured for plasmid | This study, A |
| 140-r | Wild-type strain, pNVH01 | This study, B |
| 140-s | Isogenic to 140-r but plasmid free | This study, B |
| 538-r | Wild-type strain, pNVH01 | This study, C |
| 538-c | Isogenic to 538-r but cured for plasmid | This study, C |
| 184-r | Wild-type strain, pNVH01 | This study, D |
| RN4220 | Restriction-deficient, plasmid-free host strain | 12 |
| RN4220(pSK265) | RN4220 containing cloning vector pSK265 | 11 |
| RN4220(pSK265) smr | RN4220 containing smr gene and promoter | 8 |
| RN4220(pSK265) qacG | RN4220 containing qacG gene and promoter | 8 |
| RN4220(pSK265) qacH | RN4220 containing qacH gene and promoter | 7 |
| RN4220(pSK265) qacJ | RN4220 containing qacJ gene and promoter | This study |
| S. simulans 138-r | Wild-type strain, pNVH01 | This study, B |
| E. coli DH5α | Host strain for pUC18 | Life Technologies |
| Plasmids | ||
| pNVH01 | 2.650-kb; contains qacJ | This study |
| pUC18 SmaI/BAP | 2.686-kb cloning vector | Amersham Biosciences |
| pSK265 | 3.0 kb cloning vector | 11 |
| pSK265/qacJ | 3.6 kb with qacJ gene and promoter | This study |
r, QAC resistant; s, QAC sensitive; c, cured for pNVH01.
A, B, C, and D refer to the horses from which the strains were isolated.
Screening procedure.
Mueller-Hinton (MH) agar plates containing BC or CTAB were used for screening as described previously (1), except that 50 μl of diluted culture (106 CFU/ml) was spread on each MH agar plate. Isolates showing confluent or semiconfluent growth on MH agar containing CTAB at ≥5.5 μg/ml and BC at ≥3.5 μg/ml were grown at 37°C on MH agar containing EBR (0.5 μg/ml), followed by inspection for fluorescence under UV light, as described previously (31). Cells accumulating EBR had a red fluorescence and were considered QAC sensitive; cells that did not accumulate EBR were white and were thus defined as QAC resistant.
DNA isolation and analysis.
Plasmid DNA was isolated with the QIAprep Spin Miniprep kit (QIAGEN, Hilden, Germany). Cells were lysed with lysostaphin (Sigma-Aldrich, St. Louis, Mo.), which was added to the resuspension buffer to a final concentration of 40 μg/ml, and were incubated at 37°C for 90 min. Plasmid DNA was restricted with HaeIII and SspI (Life Technologies, Paisley, United Kingdom). The sizes of the unrestricted plasmids and DNA fragments were estimated after agarose gel electrophoresis, with bacteriophage λ HindIII, φX174 HaeIII, and a supercoiled DNA ladder (Life Technologies) used as molecular weight markers.
Curing of QAC resistance plasmid.
Curing of plasmids from two S. aureus isolates (isolates 120-r and 538-r), one S. intermedius isolate (isolate 119-r), and the Staphylococcus simulans isolate was performed by plating out frozen stocks (−80°C) directly onto MH agar containing EBR (0.5 μg/ml). The plates were subsequently incubated at 37°C overnight. The colonies were inspected for fluorescence as described previously (31). Colonies that fluoresced red and that accumulated EBR and white colonies that did not accumulate EBR were analyzed for their plasmid contents and resistance to QAC.
Species determination.
Identification of staphylococci was based on standard laboratory criteria and the use of the Staph-Zym biochemical test kit (Rosco, Tåstrup, Denmark). Additionally, PCR amplification and sequencing of 583 bp of the 16S rRNA gene were carried out with the primers (Life Technologies) 16S rRNA-For (5′-CGGCGTGCCTAATACATGC-3′) and 16S rRNA-Rev (5′-CGTGGGCTTTCACATCAGAC-3′). The primers were designed after comparison of the 16S rRNA gene sequences from the topical species to ensure reliable species identification. Sequencing was carried out with an ABI PRISM 377 sequencer (ABI Biosystems, Perkin-Elmer Cetus Corp., Norwalk, Conn.), and the sequences were compared by use of the Sequencer (version 3.1) software package (Gene Codes Corporation, Ann Arbor, Mich.).
Cloning and sequencing of pNVH01.
Plasmids from all QAC-resistant isolates (except isolate 184-r) that harbored the novel QAC resistance determinant were cloned and sequenced. The 2.65-kb plasmids in isolates 138-r and 538-r were separated from the other plasmids by gel electrophoresis, followed by purification with the QIAgen Gel Extraction kit (QIAGEN). The plasmid was linearized with HaeIII (Life Technologies), followed by several unsuccessful attempts to clone the complete 2.65-kb plasmid into pUC18 digested with SmaI-bacterial alkaline phosphatase (Amersham Biosciences, Uppsala, Sweden). Restriction analysis with SspI (Life Technologies) demonstrated three fragments of approximately 1,450, 1,130, and 70 bp, respectively. Blunt-end-digested plasmid fragments were ligated into pUC18 digested with SmaI-bacterial alkaline phosphatase (Amersham Biosciences) and transformed into competent Escherichia coli DH5α as described previously (6). Transformants were selected by cultivation on Luria-Bertani agar containing ampicillin (50 μg/ml). Insert-containing vectors were isolated with the QIAprep Spin Miniprep kit (QIAGEN) and subjected to cycle sequencing with the BigDye Terminator kit (ABI Biosystems, Foster City, Calif.) and pUC18 universal primers (3.2 pmol/μl). All sequencing reaction mixtures were purified with the DyeEx Spin kit (QIAGEN) to remove unincorporated dye terminators prior to sequencing on an ABI 310 Genetic Analyzer (ABI Biosystems) with polymer POP-6. Complete double-stranded plasmid sequences were obtained by a primer walking strategy in combination with sequencing of the PCR products to obtain overlapping sequences between the various inserts. The raw data were exported from the ABI 310 analyzer into the Lasergene software package (DNASTAR, Madison, Wis.). All sequence assembly, comparisons, and alignments were done with this software, which additionally performed online searches for related sequences via the National Center for Biotechnology Information BLAST server.
Recombinant qacJ construct for promoter gene comparisons.
The S. aureus RN4220(pSK265) qacJ clone was constructed by standard molecular techniques. PCR was used to amplify a 677-bp fragment containing the qacJ gene and promoter region by using primers with BamHI and EcoRI restriction sites (in boldface): qacJ-BamHI (5′-ATCGGATCCATAAAAAGCCCCCAGTTTTG-3′) and qacJ-EcoRI (5′-TCAGTCGACGAGCTCGAATTCTTAATGACTTGATCCAAA-3′). The PCR product was digested (EcoRI and BamHI), purified with the QIAgen Gel Extraction kit (QIAGEN), and ligated into vector pSK265 before being electroporated into S. aureus RN4220 as described previously (23). In order to confirm that the qacJ and the promoter insert sequences were identical to the original plasmid sequence, both strands of the insert were sequenced.
Antimicrobial susceptibility.
The MICs of BC and CTAB were determined in a microtiter assay at 0.5-μg/ml concentration intervals from 0 to 18 μg/ml in MH broth as described previously (31) and were repeated at least twice. The MIC analyses were performed with wild-type staphylococci, plasmid-cured derivatives, and S. aureus RN4220 transformants. The MICs of the following antibiotics for the wild-type strains were determined by E-test (AB Biodisk, Solna, Sweden): benzylpenicillin, streptomycin, tetracycline, trimethoprim, chloramphenicol, enrofloxacin, gentamicin, cephalothin, clindamycin, erythromycin, fusidic acid, and oxacillin. Production of β-lactamase was determined by a nitrocefin test (AB Biodisk).
PFGE.
Molecular fingerprinting of staphylococci was performed by pulsed-field gel electrophoresis (PFGE) with a CHEF-DRIII apparatus (Bio-Rad Laboratories, Hercules, Calif.). SmaI-digested DNA was separated on 1% SeaKem Gold agarose (BioWhittaker Molecular Applications, Vallensbaek Strand, Denmark) for 18 h at 14°C and 6 V/cm with pulse times of 1 to 40 s at an angle of 120°. Low Range PFG Marker (New England BioLabs Ltd., Hitchin, United Kingdom) was used as a fragment size marker. The gel was stained with EBR and photographed under UV transillumination. PFGE patterns were compared by visual examination and were interpreted according to recommended guidelines (32).
Nucleotide sequence accession number.
The sequence reported in this paper was submitted to GenBank and assigned accession no. AJ512814.
RESULTS
Identification of a novel, plasmid-borne QAC resistance gene.
The screening of 50 staphylococcal isolates for QAC resistance revealed 12 resistant isolates and 38 sensitive isolates. Seven of the QAC-resistant isolates each contained an approximately 2.65-kb plasmid, and the plasmids had apparently identical endonuclease restriction patterns. PCR resulted in a faint PCR product when qacG-specific primers were used, indicating the possible existence of qacG or a closely related gene in these seven isolates. Furthermore, originally QAC-resistant isolates became susceptible following plasmid curing, strongly suggesting that the resistance was associated with the 2.65-kb plasmid in three different staphylococci species. Species determination, confirmed by 16S rRNA sequencing, identified three S. intermedius isolates (isolates 119-r, 139-r, and 139-s), five S. aureus isolates (isolates 120-r, 140-r, 140-s, 538-r, 184-r), and one S. simulans isolate (isolate 138-r) (Table 1).
Nucleotide sequence analysis of plasmid pNVH01.
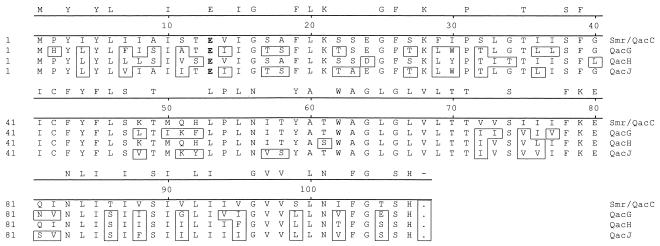
The nucleotide sequence of the S. aureus (isolate 120-r) QAC resistance plasmid, designated pNVH01, is presented in Fig. 1. The novel 2,650-bp plasmid contains two open reading frames (ORFs), designated repNVH01 and qacJ. The smaller ORF (nucleotides [nt] 1428 to 1748) encodes a putative protein (designated QacJ) of 107 amino acids (aa). An alternative start codon in qacJ is present at nt 1419 to 1421, potentially encoding a 110-aa protein. The larger ORF transcribed in the opposite direction (nt 100 to 1104) and encoded a putative protein of 334 aa with extensive similarities to previously described replication proteins of rolling-circle replicating plasmids. The −35 and −10 regions were difficult to point out due to the low degree of homology to previously suggested consensus promoters of gram-positive organisms (5). Figure 2 shows the alignment between the QacJ protein and other known staphylococcal proteins of the SMR family. A search for homology and analysis with the BLAST server revealed certain degrees of identity to known proteins belonging to the SMR family: Smr (72.5%), QacG (82.6%), and QacH (73.4%). Similarities in both coding and noncoding regions were also evident at the nucleotide level, with the highest degree of resemblance (93%) being to qacG-containing plasmid pST94 (8).
FIG. 1.
Nucleotide sequence of pNVH01 (GenBank accession no. AJ512814) containing two ORFs. The smaller ORF (qacJ; nt 1428 to 1748) encodes a putative protein (QacJ) of 107 aa. An alternative transcription start codon is present at nt 1419 to 1421 and potentially encodes a 110-aa protein. The larger ORF transcribed in the opposite direction (nt 100 to 1104) encodes a putative protein of 334 aa. The putative ribosome binding site (RBS) and the +ori sequence (including the potential replication nick site indicated by a vertical arrow) are underlined. The conserved single-stranded origion (SSOA; previously known as palA) is boxed. Direct repeat sequences are in boldface.
FIG. 2.
Alignment of different staphylococcal QAC resistance-related proteins. The amino acids that differ from those in Smr are boxed. The completely conserved amino acids in all four proteins are denoted on the line above the alignment. The different SMR proteins consist of four TMSs, TMS1 to TMS4, comprising amino acid residues 3 to 20, 30 to 47, 58 to 74, and 86 to 103, respectively. The only charged TMS amino acid residue is highly conserved glutamate-13 (in boldface).
Complete nucleotide sequencing of qacJ-harboring resistance plasmids in five other isolates belonging to three different staphylococcus species was performed. The plasmids in isolates 139-r and 120-r (Fig. 1) were found to be identical. One nucleotide substitution was observed in each plasmid from the following three isolates: 119-r (nt 1590), 140-r (nt 2374), and 138-r (nt 400). Three nucleotide substitutions (nt 1021, 1119, and 1135) and one nucleotide deletion (nt 1373) were observed in the plasmid from isolate 538-r. Three of the seven nucleotide variations were located within the protein-coding regions, and one of these gave rise to an amino acid substitution (nt 1590 in isolate 119-r) in the qacJ sequence: ATC (Ile) to CTC (Leu). Two mutations (at nt 400 and 1021) were silent: GCA to GCT and CGC to CGG, respectively. The control sequencing of the qacJ gene and promoter construct revealed one mutation (A to C) at position −283 of the qacJ gene. This mutation is not located in the coding region or the basic promoter and is therefore considered of no relevance for gene expression.
Susceptibility testing.
The MICs of BC ranged from 3.5 to 4.5 μg/ml for resistant wild-type strains and between 1.0 and 1.5 μg/ml for sensitive and cured strains. The MICs of CTAB ranged from 7.5 to 10.5 μg/ml for resistant wild-type strains and from 2.0 to 4.0 μg/ml for sensitive and cured strains. The MICs were reproducible within a range of 1.0 μg/ml. Despite several attempts, we did not manage to cure pNVH01 from the S. simulans (138-r) and S. intermedius (119-r) isolates. The MICs of the QACs for the various RN4220 recombinants are presented in Table 2. S. aureus isolate 538-r was resistant to benzylpenicillin, because of β-lactamase production, and to streptomycin, with MICs of >256 and 96 μg/ml, respectively. All isolates showed moderate resistance to sulfadiazine (MICs > 128 μg/ml), and the S. intermedius and S. simulans isolates were moderately resistant to trimethoprim (MICs > 4 μg/ml). All nine isolates were susceptible to chloramphenicol, enrofloxacin, gentamicin, tetracycline, cephalothin, clindamycin, erythromycin, fusidic acid, and oxacillin.
TABLE 2.
MICsa of BC and CTAB for S. aureus RN4220 recombinants
| Isolate | MIC (μg/ml)
|
|
|---|---|---|
| BC | CTAB | |
| RN4220 smr | ≤3.5 | ≤15.5 |
| RN4220 qacG | ≤5.0 | ≤15.5 |
| RN4220 qacH | ≤3.5 | ≤8.0 |
| RN4220 qacJ | ≤6.0 | ≤16.0 |
| RN4220(pSK265)b | ≤1.0 | ≤2.5 |
MICs were reproducible within a maximum range of 1.0 μg/ml.
Negative control.
Molecular epidemiology.
The sources and PFGE types of the seven isolates that contained qacJ and the two QAC-susceptible isolates are shown in Table 3. Among the S. aureus isolates, identical or nearly identical PFGE fingerprints were obtained for both epidemiologically related isolates (horses A and B) and unrelated isolates (horse D). Plasmid pNVH01 was present in S. aureus and S. intermedius strains with different PFGE fingerprints. These results suggest recent intra- and interspecies horizontal plasmid transfer, in addition to the clonal spread of qacJ.
TABLE 3.
PFGE types (SmaI) of nine staphylococcal isolates, with or without the qacJ gene, recovered from four horses on three different equine operations
| Operation | Horse | Isolate | Species | qacJ | PFGE type |
|---|---|---|---|---|---|
| 1 | A | 120-r | S. aureus | + | SA-1aa |
| 119-r | S. intermedius | + | SI-1 | ||
| 1 | B | 140-r | S. aureus | + | SA-1b |
| 140-s | S. aureus | − | SA-1b | ||
| 139-r | S. intermedius | + | SI-2ab | ||
| 139-s | S. intermedius | − | SI-2b | ||
| 138-r | S. simulans | + | SS-1 | ||
| 2 | C | 538-r | S. aureus | + | SA-2 |
| 3 | D | 184-r | S. aureus | + | SA-1b |
Closely related to SA-1b (three-fragment difference).
Closely related to SI-2b (two-fragment difference).
DISCUSSION
A novel staphylococcal plasmid-borne gene, designated qacJ, encoding efflux-mediated resistance to QACs was identified and sequenced. The qacJ gene was located on a plasmid of 2,650 bp in S. aureus, S. intermedius, and S. simulans. The nucleotide sequence of pNVH01 from the first isolate collected, S. aureus strain 120-r, is shown in Fig. 1. The sequence similarities between the putative QacJ protein and known staphylococcal proteins shown in Fig. 2 suggest that QacJ is a new member of the SMR family.
The discovery of yet another member of the SMR family supports the view that there is a high degree of freedom concerning amino acid substitutions in SMR proteins. The only obvious restriction is conservation of hydrophobic amino acids within the four transmembrane-spanning segments (TMSs). Protein sequence analysis of more than 60 members of the SMR family has resulted in a proposed consensus for the TMSs (TMS1 to TMS4) (17). The study revealed that the glutamate at position 13 or 14 is the only conserved amino acid throughout all sequences. Interestingly, the Cys-42 deviation from the proposed SMR family consensus sequence is conserved in all staphylococcal SMR proteins and has been shown to be of importance for proper SMR protein function (20). Not surprisingly, both the conserved Glu-13 and the Cys-42 deviation are present in the putative QacJ protein.
The gene promoter studies followed by MIC determinations suggest that QacJ confers an increased level of resistance to BC compared with the level of resistance conferred by Smr, i.e., MICs of 6.0 and 3.5 μg/ml, respectively, but these two proteins mediate nearly identical levels of resistance to CTAB (Table 2). These results indicate an amino acid substitution affecting the substrate specificity rather than a transcriptional change caused by a mutation in the qacJ promoter. Such a promoter mutation would most likely have led to a proportional increase or decrease in resistance to both BC and CTAB. Differences in resistance phenotypes have been observed among otherwise isogenic strains expressing similar proteins, QacG, QacH, or Smr. The compact size and the number of genetic and phenotypic variants within this homogeneous group of efflux proteins should make these proteins suitable for further functional analyses.
Small staphylococcal plasmids containing genes encoding proteins of the SMR family are structurally very similar, with highly conserved gene orientations and localizations. These plasmids are built up of sequence cassettes typical for plasmids of the pC194 family of rolling-circle replicating plasmids (18, 24). The various cassette-like regions interspersed with other stretches of sequences most often show extensive homology to regions present in both small and large plasmids. We observed complete conservation in regions reported to take part in plasmid replication and maintenance. This is the case for the single-stranded origin SSOA (previously termed palA), involved in lagging-strand replication of rolling-circle replicating plasmids and the putative +ori sequence (Fig. 1). The SSOA region is located just downstream of the QAC resistance determinants in all reported plasmids that harbor smr, qacG, or qacH; and this was also observed in pNVH01.
A longer stretch in which the sequences were nearly identical (96% identity) was observed between nt 1105 and 1300 of pNVH01 and the equivalent sequence of pST94 (putative replication gene promoter region). A sequence identity similar to that of pST94 extended from nt 2463 to 1 and the replication gene. In the downstream region (nt 1301 to the initiation codon qacJ at nt 1428) comprising the qacJ promoter region, no relatedness to other plasmids was observed. Interestingly, both the direct repeats (nt 1276 to 1284 plus nt 1287 to 95 and nt 2282 to 2301 plus nt 2329 to 2348) and inverted repeats (nt 1293 to 1301 plus nt 1312 to 1320 and nt 2419 to 2428 plus nt 2435 to 2444) are located close to the junctions between the homologous and nonhomologous sequences. The possible role of repeat structures in plasmid recombination is unknown, although they may play a role in the integration and excision of cassettes typical for members of the pC194 plasmid family. The presence of regions with sequences with extensive identity to the sequences of other staphylococcal plasmids, including both small and large plasmids, suggests frequent recombination between various plasmids and lateral DNA transfer, at least within the genus Staphylococcus. These events may explain the many variants of small QAC resistance plasmids that have been reported (2, 7, 8, 10, 13, 15).
Our findings suggest that the spread of pNVH01 (containing qacJ) is due to both the dissemination of a pNVH01-containing S. aureus clone and plasmid transfer within and between various equine staphylococcal species (Table 3). The contagious nature of certain S. aureus strains is well documented in both veterinary and human medicine (26, 28, 33). There are strong indications of host specificity among S. aureus clones (16), but rare cases of human-to-horse transmission of staphylococcal infections have been reported (25). Our results suggest direct or indirect contact between animals as likely factors for the spread of QAC-resistant S. aureus clones between and within equine operations.
Interestingly, nucleotide sequencing showed a nearly identical pNVH01 plasmid in three different staphylococcal species (S. aureus, S. intermedius, and S. simulans). Comparisons revealed a maximum of 2 nucleotide differences among five isolates (including three different staphylococcal species) from the same operation, substantiating the assumption that the pNVH01 plasmids harbored by these isolates were of a recent common origin. The sequence of pNVH01 from the remaining isolate, recovered from another operation, differed at four or five positions from the sequences of each of the other isolates. Isolates with and without pNVH01 were observed among particular S. aureus and S. intermedius clones, indicating that the plasmid is rather easily acquired or lost. We tried to cure pNVH01 from various isolates. The plasmid was not eliminated from the S. intermedius and S. simulans isolates; however, the plasmid was easily cured from the S. aureus isolates. This indicates that the stability of maintenance of small plasmids may vary between different strains or species. Although pNVH01 does not contain any genetic elements enabling self-transmission, the plasmid apparently has a potential for dispersion. The mechanism of transfer of these small plasmids is not known. An in vitro study found that the sub-MIC of CTAB significantly enhanced the transduction of pWG613 in S. aureus in the recovery medium (21). This increase in transduction efficiency might be explained in part by an effect of the membrane-active agent that leads to increased access to receptor sites for transduction (21). The prevalence of staphylococci harboring qacJ and the mechanisms for transfer of this gene within and between staphylococcal species should be further investigated.
Results from recent studies in Norway suggest that QAC resistance genes are common in human clinical staphylococci and that a direct linkage between resistance to QACs and resistance to penicillin occurs in clinical isolates of human and animal origin as well as in food-related staphylococci (1, 28, 29). No evidence of such linkage between QAC resistance and antibiotic resistance was found in our study. However, in human hospital environments, staphylococci resistant to BC have been shown to be more resistant to certain antibiotics than BC-sensitive isolates (28). Therefore, it would be of interest to investigate if long-term exposure to QAC-containing preparations could represent a driving force for the selection of bacterial strains resistant to antibiotics in horse environments as well as in other environments where QAC-based antiseptics and disinfectants are being extensively applied.
Acknowledgments
This study was partially funded by The Research Council of Norway grant 140723/110.
We thank Bjørg Kvitle, Department of Animal Health, National Veterinary Institute, Oslo, for performing bacteriological examinations and PFGE. We are also grateful to Tormod Mørk at the Department of Animal Health, National Veterinary Institute, and to Henning Sørum and scientific staff at the Department of Pharmacology, Microbiology, and Food Hygiene, The Norwegian School of Veterinary Science, for access to the laboratory facilities and for helpful discussions and constructive comments.
REFERENCES
- 1.Anthonisen, I.-L., M. Sunde, T. M. Steinum, M. S. Sidhu, and H. Sørum. 2002. Organization of the antiseptic resistance gene qacA and Tn552-related β-lactamase genes in multidrug-resistant Staphylococcus haemolyticus strains of animal and human origins. Antimicrob. Agents Chemother. 46:3606-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorland, J., M. Sunde, and S. Waage. 2001. Plasmid-borne smr gene causes resistance to quaternary ammonium compounds in bovine Staphylococcus aureus. J. Clin. Microbiol. 39:3999-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung, Y. J., and M. H. Saier, Jr. 2001. SMR-type multidrug resistance pumps. Curr. Opin. Drug. Discov. Dev. 4:237-245. [PubMed] [Google Scholar]
- 4.Dom, P., L. Devriese, F. Haesebrouck, M. Desmidt, and P. Deherdt. 1995. Prevalence of pathogenic bacteria and dermatophytes in skin disorders in Belgian horses. Vlaams Diergeneeskundig Tijdschr. 64:15-18. [Google Scholar]
- 5.Graves, M. C., and J. C. Rabinowitz. 1986. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for “extended” promoter elements in gram-positive organisms. J. Biol. Chem. 261:11409-11415. [PubMed] [Google Scholar]
- 6.Hanahan, D., J. Jessee, and F. R. Bloom. 1991. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 204:63-113. [DOI] [PubMed] [Google Scholar]
- 7.Heir, E., G. Sundheim, and A. L. Holck. 1998. The Staphylococcus qacH gene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiol. Lett. 163:49-56. [DOI] [PubMed] [Google Scholar]
- 8.Heir, E., G. Sundheim, and A. L. Holck. 1999. The qacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry. J. Appl. Microbiol. 86:378-388. [DOI] [PubMed] [Google Scholar]
- 9.Heir, E., G. Sundheim, and A. L. Holck. 1999. Identification and characterization of quaternary ammonium compound resistant staphylococci from the food industry. Int. J. Food Microbiol. 48:211-219. [DOI] [PubMed] [Google Scholar]
- 10.Im, S. H., S. J. Yoon, W. K. Kim, C. K. Shin, D. W. Lee, and K. H. Moon. 1996. Characterization of cryptic plasmid of multidrug-resistant Staphylococcus aureus SA2. J. Microbiol. Biotechnol. 6:145-146. [Google Scholar]
- 11.Jones, C. L., and S. A. Khan. 1986. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J. Bacteriol. 166:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreiswirth, B. N., S. Loftdahl, M. J. Betley, M. O'Reilly, P. M. Schleivert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature (London) 305:709-712. [DOI] [PubMed] [Google Scholar]
- 13.Leelaporn, A., N. Firth, I. T. Paulsen, A. Hettiaratchi, and R. A. Skurray. 1995. Multidrug resistance plasmid pSK108 from coagulase-negative staphylococci; relationship to Staphylococcus aureus qacC plasmids. Plasmid 34:62-67. [DOI] [PubMed] [Google Scholar]
- 14.Littlejohn, T. G., D. DiBerardino, L. J. Messerotti, S. J. Spiers, and R. A. Skurray. 1991. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene 101:59-66. [DOI] [PubMed] [Google Scholar]
- 15.McDonnel, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musser, J. M., and R. K. Selander. 1990. Genetic analysis of natural populations of Staphylococcus aureus, p. 59-67. In R. Novick and R. A. Skurray (ed.), Molecular biology of the staphylococci. VCH Publishers, New York, N.Y.
- 17.Ninio, S., D. Rotem, and S. Schuldiner. 2001. Functional analysis of novel multidrug transporters from human pathogens. J. Biol. Chem. 276:48250-48256. [DOI] [PubMed] [Google Scholar]
- 18.Novick, R. P. 1989. Staphylococcal plasmids and their replication. Annu. Rev. Microbiol. 43:537-565. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen, I. T., M. H. Brown, T. G. Littlejohn, B. A. Mitchell, and R. A. Skurray. 1996. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc. Natl. Acad. Sci. USA 93:3630-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen, I. T., M. H. Brown, S. J. Dunstan, and R. A. Skurray. 1995. Molecular characterization of the staphylococcal multidrug resistance export protein QacC. J. Bacteriol. 177:2827-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce, H., S. Messager, and J.-Y. Maillard. 1999. Effect of biocides commonly used in the hospital environment on the transfer of antibiotic-resistance genes in Staphylococcus aureus. J. Hosp. Infect. 43:101-107. [DOI] [PubMed] [Google Scholar]
- 22.Russell, A. D. 1997. Plasmids and bacterial resistance to biocies. J. Appl. Microbiol. 83:155-165. [DOI] [PubMed] [Google Scholar]
- 23.Schenk, S., and R. A. Laddaga. 1986. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol. Lett. 94:133-138. [DOI] [PubMed] [Google Scholar]
- 24.Seery, L. T., N. C. Nolan, P. M. Sharp, and K. M. Devine. 1993. Comparative-analysis of the pC194 group of rolling circle plasmids. Plasmid 30:185-196. [DOI] [PubMed] [Google Scholar]
- 25.Seguin, J. C., R. D. Walker, J. P. Caron, W. E. Kloos, C. G. George, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 1999. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J. Clin. Microbiol. 37:1459-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu, A., J. Kawano, C. Yamamoto, O. Kakutani, T. Anzai, H. Saito, and M. Kamada. 1997. Genetic analysis of equine methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis. J. Vet. Med. Sci. 59:935-937. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu, A., J. Kawano, J. Ozaki, N. Sasaki, S. Kimura, S. Kamada Anzai, H. Saito, and H. Sato. 1991. Characteristics of Staphylococcus aureus isolated from lesions of horses. J. Vet. Med. Sci. 53:601-606. [DOI] [PubMed] [Google Scholar]
- 28.Sidhu, M. S., E. Heir, T. Leegaard, K. Wiger, and A. Holck. 2002. Frequency of disinfectant resistance genes and genetic linkage with β-lactamase transposon Tn552 among clinical staphylococci. Antimicrob. Agents Chemother. 46:2797-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidhu, M. S., E. Heir, H. Sørum, and A. Holck. 2001. Genetic linkage between resistance to quaternary ammonium compounds and β-lactam antibiotics in food-related Staphylococcus spp. Microb. Drug Resist. 7:363-371. [DOI] [PubMed] [Google Scholar]
- 30.Smyth, G. B. 1991. Bone abscess in the mandible of a quarter horse gelding. Cornell Vet. 81:239-243. [PubMed] [Google Scholar]
- 31.Sundheim, G., T. Hagtvedt, and R. Dainty. 1992. Resistance of meat associated staphylococci to a quaternary ammonium compound. Food Microbiol. 9:161-167. [Google Scholar]
- 32.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waage, S., J. Bjorland, D. A. Caugant, H. Oppegaard, T. Tollersrud, T. Mørk, and F. M. Aarestrup. 2002. Spread of Staphylococcus aureus resistant to penicillin and tetracycline within and between dairy herds. Epidemiol. Infect. 129:193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]