Abstract
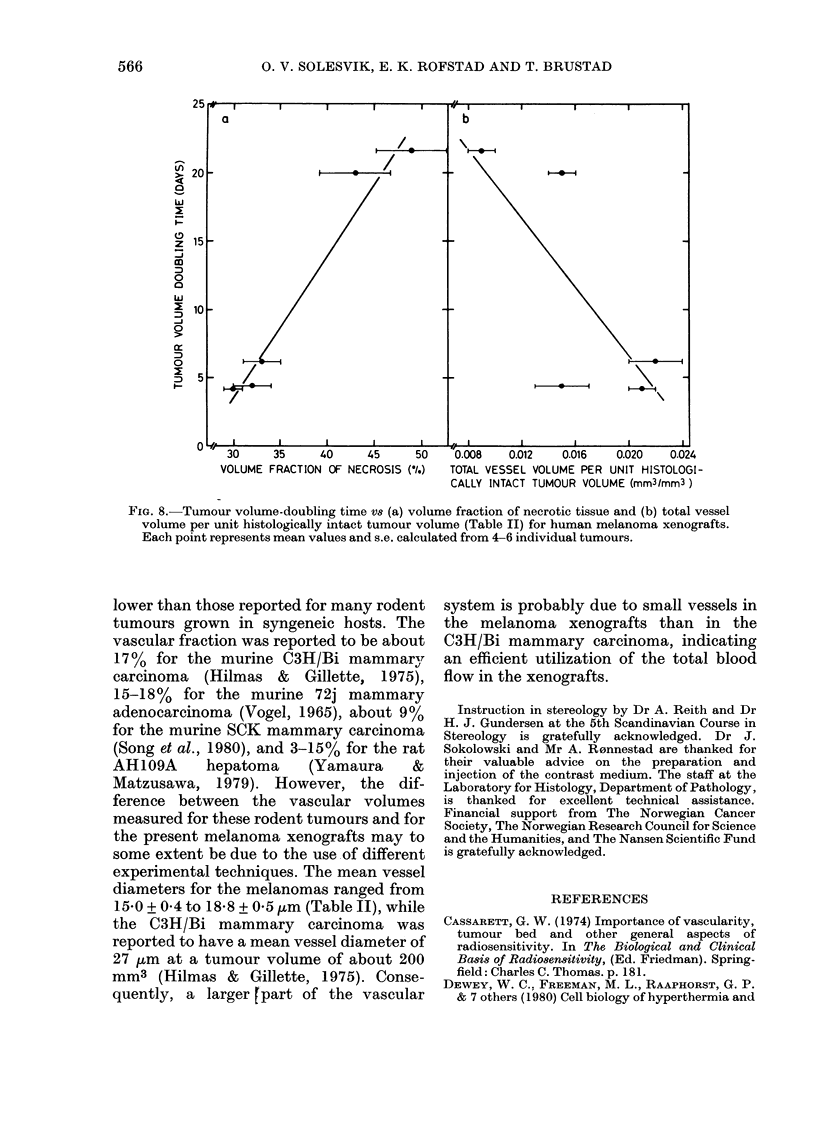
The vascular structure of 5 human malignant melanomas grown in athymic nude mice was characterized. The vessels were filled with a radio-opaque medium administered via the abdominal aorta of the mice. X-ray images, obtained from 720 micrometers-thick tumour sections, provided qualitative information on the vascular structure of the tumours. Histograms for vessel length, surface, and volume as a function of vessel diameter were obtained by stereological analysis of 2 micrometers-thick sections. The volume fraction of necrotic tissue in the tumours was also determined by stereological analysis. The 5 melanomas exhibited individual, characteristic vascular structures as well as individual, characteristic necrotic fractions. The total vessel length ranged from 32 +/- 2 to 80 +/- 4 mm, the total vessel surface from 1.6 +/- 0.1 to 3.8 +/- 0.2 mm2, and the total vessel volume from 0.009 +/- 0.001 to 0.022 +/- 0.002 mm3--all values per mm3 histologically intact tumour tissue. The necrotic fractions ranged from 30 +/- 1 to 49 +/- 4%, and tended to be higher in the poorly than in the well-vascularized melanomas. The volume doubling times ranged from 4.2 to 21.6 days. Melanomas with short volume-doubling times had lower necrotic fractions and tended to be better vascularized than those with long volume-doubling times.
Full text
PDF


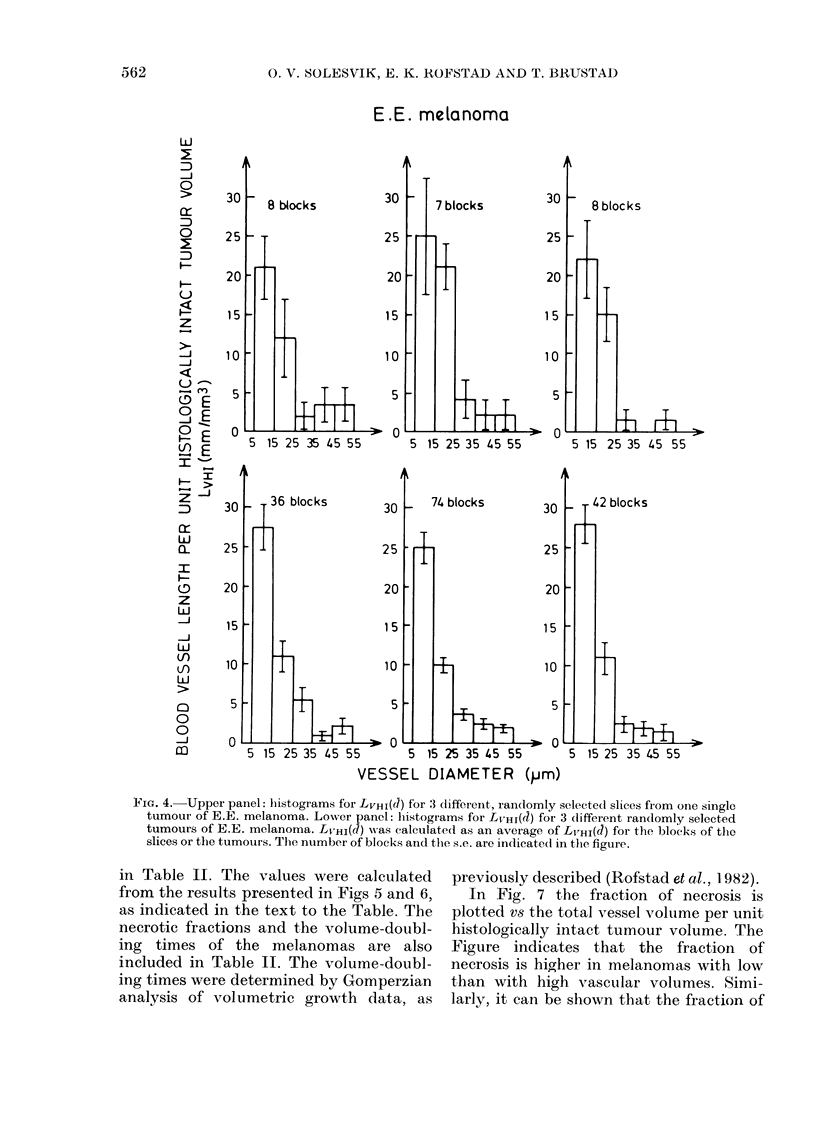
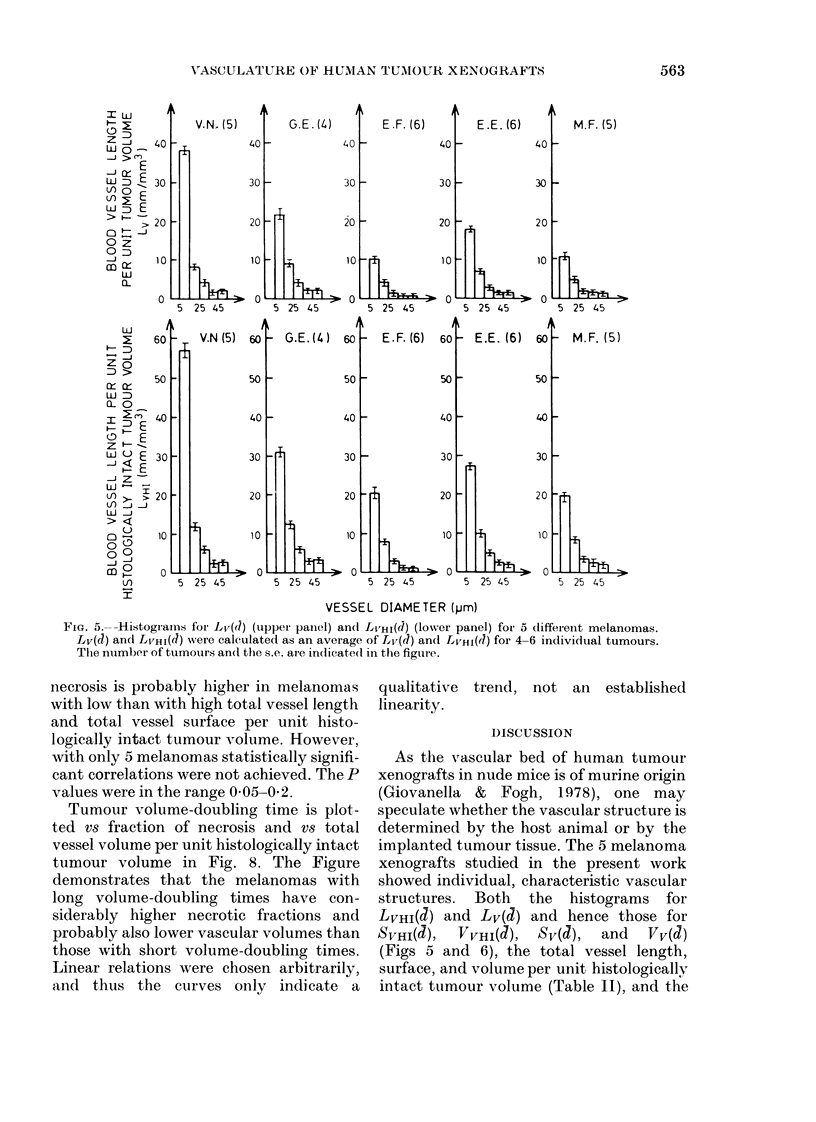
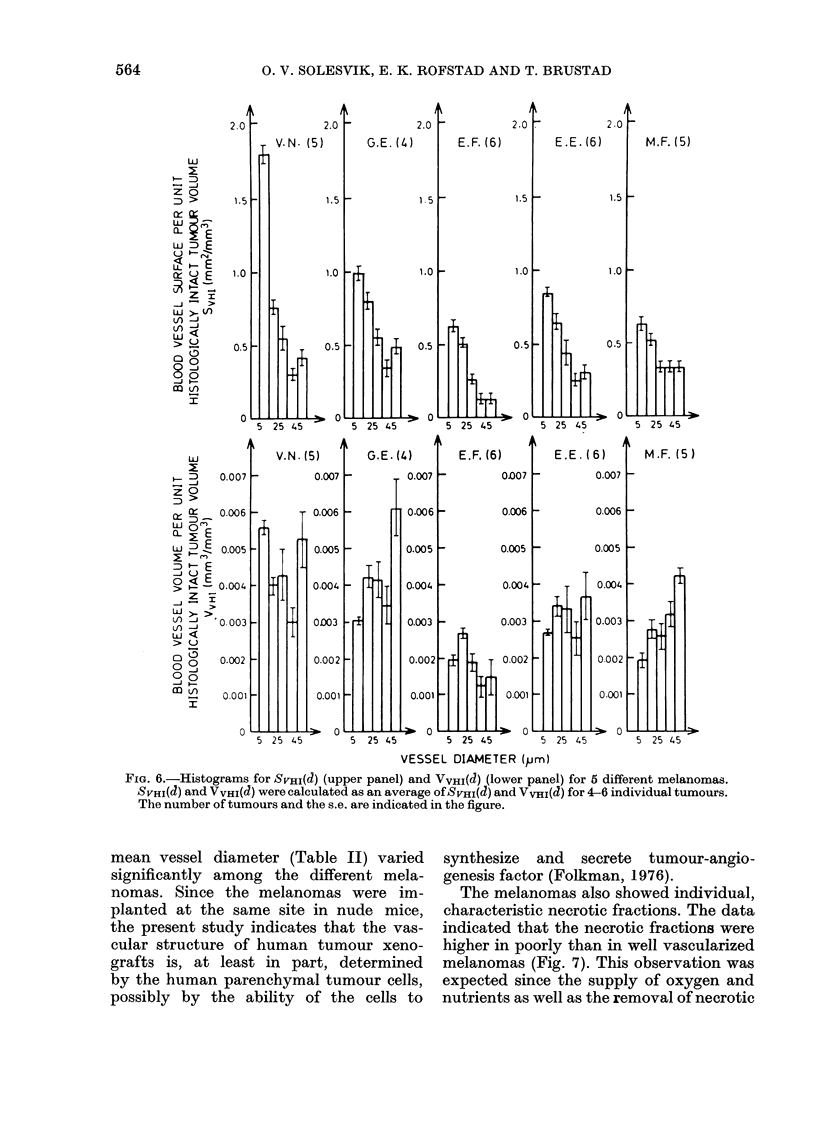
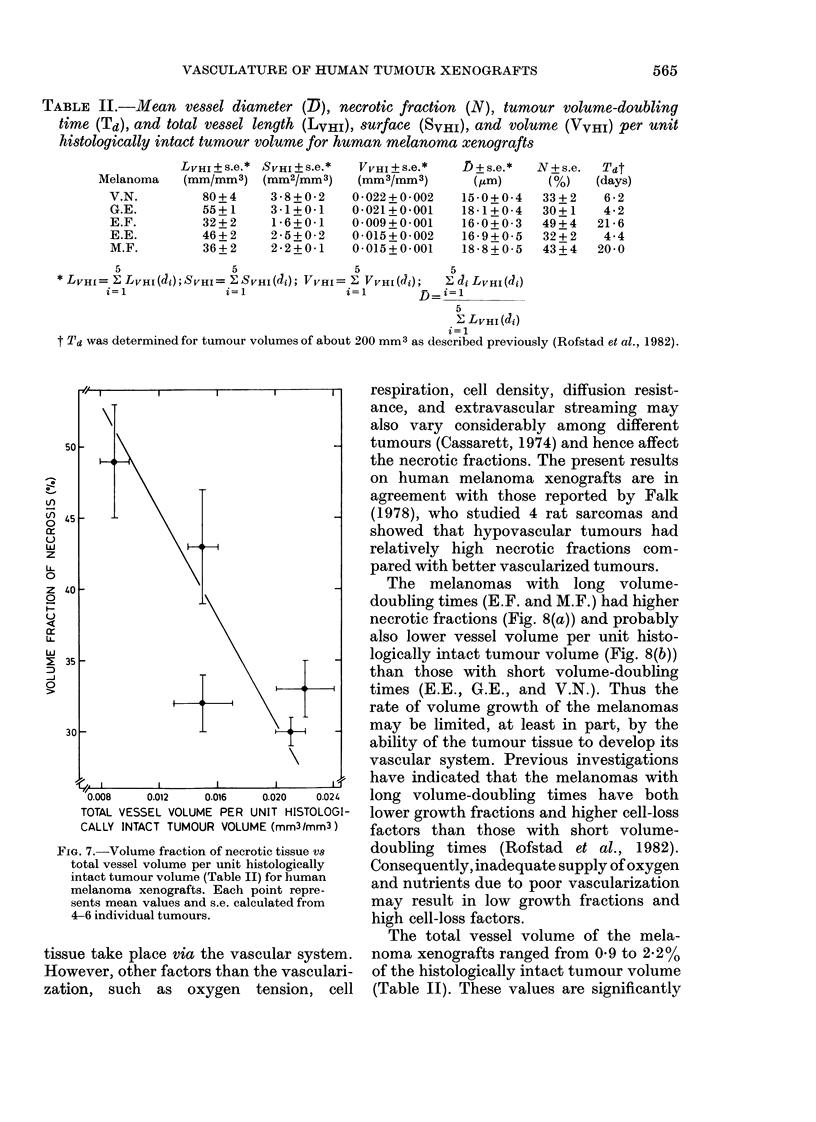

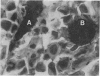
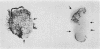
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Falk P. Patterns of vasculature in two pairs of related fibrosarcomas in the rat and their relation to tumour responses to single large doses of radiation. Eur J Cancer. 1978 Mar;14(3):237–250. doi: 10.1016/0014-2964(78)90187-1. [DOI] [PubMed] [Google Scholar]
- Field S. B., Bleehen N. M. Hyperthermia in the treatment of cancer. Cancer Treat Rev. 1979 Jun;6(2):63–94. doi: 10.1016/s0305-7372(79)80043-2. [DOI] [PubMed] [Google Scholar]
- Folkman J. The vascularization of tumors. Sci Am. 1976 May;234(5):58-64, 70-3. doi: 10.1038/scientificamerican0576-58. [DOI] [PubMed] [Google Scholar]
- Hilmas D. E., Gillette E. L. Microvasculature of C3H/Bi mouse mammary tumors after x-irradiation. Radiat Res. 1975 Jan;61(1):128–143. [PubMed] [Google Scholar]
- Jørgen H., Gundersen G. Estimation of tubule or cylinder LV, SV and VV on thick sections. J Microsc. 1979 Dec;117(3):333–345. doi: 10.1111/j.1365-2818.1979.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Kennedy K. A., Teicher B. A., Rockwell S., Sartorelli A. C. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol. 1980 Jan 1;29(1):1–8. doi: 10.1016/0006-2952(80)90235-x. [DOI] [PubMed] [Google Scholar]
- POWERS W. E., TOLMACH L. J. A multicomponent x-ray survival curve for mouse lymphosarcoma cells irradiated in vivo. Nature. 1963 Feb 16;197:710–711. doi: 10.1038/197710b0. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., Brustad T. Radiation response in vitro of cells from five human malignant melanoma xenografts. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Dec;40(6):677–680. doi: 10.1080/09553008114551671. [DOI] [PubMed] [Google Scholar]
- Song C. W., Kang M. S., Rhee J. G., Levitt S. H. Vascular damage and delayed cell death in tumours after hyperthermia. Br J Cancer. 1980 Feb;41(2):309–312. doi: 10.1038/bjc.1980.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel G. G., Peckham M. J. Human tumour xenografts: a critical appraisal. Br J Cancer Suppl. 1980 Apr;4:133–141. [PMC free article] [PubMed] [Google Scholar]
- Steel G. G. The growth and therapeutic response of human tumours in immune deficient mice. Bull Cancer. 1978;65(4):465–472. [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972 Jul;45(535):515–524. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- VOGEL A. W. INTRATUMORAL VASCULAR CHANGES WITH INCREASED SIZE OF A MAMMARY ADENOCARCINOMA: NEW METHOD AND RESULTS. J Natl Cancer Inst. 1965 May;34:571–578. [PubMed] [Google Scholar]
- Yamaura H., Matsuzawa T. Tumor regrowth after irradiation; an experimental approach. Int J Radiat Biol Relat Stud Phys Chem Med. 1979 Mar;35(3):201–219. doi: 10.1080/09553007914550241. [DOI] [PubMed] [Google Scholar]