Abstract
The development of T cells within the thymus is largely dependent on intact cortical and medullary epithelial cells. However, it has been reported that positive selection of natural killer antigen 1.1+ (NK1.1+) T cell antigen receptor (TCR)-α/β+ thymocytes recently identified among CD4+8− and CD4−8− subpopulations is attributable to major histocompatibility complex class Ib ligands expressed on bone marrow (BM)-derived components in the thymus. In the present study, we investigated generation of NK1.1+ TCR-α/β+ cells in the thymus of the aly/aly mouse which lacks lymph nodes and Peyer’s patches and shows abnormalities of thymic and splenic structure. We found that the proportion of the NK1.1+ TCR-α/β+ thymocytes was extremely low in these mice as compared with aly/+ and normal C57BL/6 mice. Thymic reconstitution by BM cells from aly/+ mice that possess a normal population of NK1.1+ TCR-α/β+ cell population did not restore the NK1.1+ TCR-α/β+ cell population in the thymus of lethally irradiated aly/aly mouse. When deoxyguanosine-treated fetal thymi from (B6 × B10.G)F1 mice were transplanted to aly/aly mice that had been thymectomized and reconstituted with BM cells of aly/aly mice, normal proportions of the NK1.1+ TCR-α/β+ thymocytes were present in the thymus grafts. These findings demonstrate that the development of NK1.1+ TCR-α/β+ thymocytes is accomplished under the influence not only of BM-derived components, but also of irradiation-resistant or deoxyguanosine-resistant components and an intact microenvironment of the thymus.
Keywords: thymus, natural killer T cell, bone marrow chimera, thymic chimera, aly/aly mouse
Mouse natural killer antigen 1.1+ (NK1.1+) T cell antigen receptor (TCR)-α/β+ thymocytes constitute a unique subset among CD4−8− and CD4+8− populations (1–3). This small population of thymocytes expresses low levels of extremely deviated α and β chains of TCR. However, the NK1.1+ thymocytes show characteristics of more numerous T cells in that they exhibit patterns of several surface markers usually seen in memory cells [CD44+, MEL-14low, interleukin 2 receptor (IL-2R) β+, etc.], prominent production of the cytokines (IL-4 and interferon γ) (4–6), Fas-mediated cytotoxicity without priming stimulation or priming toward either syngeneic or allogeneic CD4+8+ thymocytes (7), and considerable lymphokine activated killer activity (8, 9). The deviated TCR expression includes in some cases a self-antigen-reactive repertoire which suggests that the NK1.1+ thymocyte population has undergone an intrathymic selection different from that of the major NK1.1− thymocyte population. Indeed, recently it has been shown that the NK1.1+ thymocytes are not positively selected under the influence of thymic epithelial cells but rather positively selected by the presence of CD4+8+ thymocytes that express nonpolymorphic major histocompatibility complex class I-like molecules, CD1 (2, 10–12). In these prior studies using β2-microglobulin −/−, RAG −/−, TCR-β −/−, and/or TCR-α −/− mice, it has been shown that the population size of NK1.1+ thymocytes is determined by precursor cell type of the bone marrow (BM). It appeared from these findings that cells constituting the thymic structure exerted no significant influence on the selection of NK1.1+ thymocytes.
Recently, a new mutation designated aly (alymphoplasia) was found in a colony of C57BL/6 (B6) mice (13). This autosomal recessive mutation causes a systemic absence of lymph nodes and Peyer’s patches, and structural abnormalities of thymus and spleen (14). The homozygous aly/aly mouse develops a unique type of immunodeficient state (low antibody production to both T-independent and T-dependent antigens, prolonged survival of allogeneic skin grafts, and weak delayed type hypersensitivity response) in spite of presence of mature T and B cells that exhibit normal reactivity in vitro. In the present study, we investigated the NK1.1+ thymocyte population in aly/aly mice. We show herein that generation of NK1.1+ thymocytes is profoundly depressed in the aly/aly mice irrespective of the origin of the BM precursors. No defect was observed among the precursor cells of NK1.1+ TCR-α/β+ thymocytes from aly/aly mice.
MATERIALS AND METHODS
Mice.
ALY/Nsc Jcl-aly/aly (aly/aly) and aly/+ mice were obtained from the Clea Japan Animal (Tokyo). B6 mice were obtained from Shizuoka Laboratory Animal (Hamamatsu, Shizuoka Prefecture, Japan). B6.PL-Thy 1a/Cy (B6.PL-Thy 1.1) mice were obtained from The Jackson Laboratory. Ten- to 15-week-old female mice were used throughout the study. The (B6 × B10.G)F1 mice were bred in the animal facility at Hokkaido University.
Antibodies and Reagents.
Fluorescein isothiocyanate (FITC)–anti-TCR-α/β (H57–594), phycoerythrin–anti-NK1.1 (PK136), and biotin–anti-NK1.1 mAbs were obtained from PharMingen. Streptavidin-FITC was obtained from Biomeda (Foster City, CA). Anti-Thy 1.1 and Thy 1.2 mAbs were obtained from Serotec and FITC-anti-mouse immunoglobulin (Ig) from Organon Teknika–Cappel. Anti-Dq mAb was obtained from the Meiji Institute of Health (Tokyo).
BM and Thymic Chimeras.
BM transplantation was performed as described (15). BM chimeras prepared by injecting BM cells obtained from aly/aly mice into irradiated (10 Gy) aly/+ mice will be referred to as [aly/aly → aly/+]. Other chimeras established with different combinations will be expressed according to this nomenclature. Thymic chimeras were prepared as described (16). In brief, recipient aly/aly or aly/+ mice were thymectomized (ATx) at 5 weeks of age and rested for 12 weeks followed by lethal irradiation and infusion of aly/aly BM cells. Three days after BM transplantation, these [aly/aly → ATx.aly/aly] mice were grafted under the kidney capsule with 5–6 thymic lobes from (B6 × B10.G)F1 fetuses, or fetuses obtained by mating aly/aly mice. Fetal thymic lobes for grafts were isolated from donor mice at day 14–15 of gestation, and cultured with medium containing 1.35 mM 2-deoxyguanosine for 7 days (17). BM and thymic chimeras were given oxytetracycline hydrochloride solution (terramycin; Taitoh–Pfizer, Tokyo) for 3 weeks after reconstitution and the surgical procedures and then maintained under specific pathogen-free conditions for more than 10–12 weeks before analysis. All protocols of animal experiments were approved by the Hokkaido University Committee on Animal Experimentation.
Fluorescence-Activated Cell Sorter (FACS) Analysis.
FACS analysis was carried out as described (3, 7). Each mAb was used after blocking the Fc receptor with 2.4G2 mAb. To detect NK1.1+ T cells, thymocytes, splenocytes, and BM cells were stained with FITC–anti TCR-α/β mAb and phycoerythrin–anti-NK1.1 mAb. In some experiments, thymocytes were stained with anti-Thy 1.1, Thy 1.2 mAb or anti-Dq mAb followed by FITC–anti-mouse Ig to determine the origin of the cells. Propidium iodide red fluorescence dye (Sigma) was added to the cells immediately before analysis; propidium iodide stained, presumably nonviable cells, were excluded from analysis. The stained cells were analyzed by FACScan (Becton Dickinson).
IL-2 Activation.
Splenocytes from aly/aly or aly/+ mice were passed over nylon wool columns and 1 × 106 cells were cultured with 1000 units/ml of purified human recombinant IL-2 provided by Shionogi Pharmaceutical (Osaka) in 200 μl of RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and 5 × 10−5M 2-mercaptoethanol (8, 17). The plates were incubated for 7 days at 37°C in humidified incubator 5% CO2/95% air.
Immunohistology.
Immunohistological examination was performed as described (18, 19). Thymi from mice were embedded in OCT compound (Miles) and frozen in liquid nitrogen. Sections were allowed to react with primary mAbs overnight at 4°C and then stained with avidin–biotin complex kit (Vector Laboratories) and counterstained with methyl green. The primary mAbs used for immunohistological study include ER-TR4 (specific for cortical epithelial cells of the thymus), ER-TR5 (specific for medullary and subcapsular epithelial cells of the thymus), ER-TR7 (specific for reticular-fibroblasts), and ER-TR9 (specific for marginal zone macrophages) (20, 21). These mAbs were kindly provided by Willem van Ewijk (Erasmus University, Rotterdam, The Netherlands). For hematoxylin/eosin staining, thymi were fixed with phosphate-buffered formalin and embedded in paraffin, and sections were prepared and stained according to usual procedures.
Statistical Analyses.
Statistical analyses were carried out using Student’s t test. P values > 0.05 were considered not significant.
RESULTS
Developmental Defects of NK1.1+ TCR-α/β+ T cells in the Thymus of aly/aly Mouse.
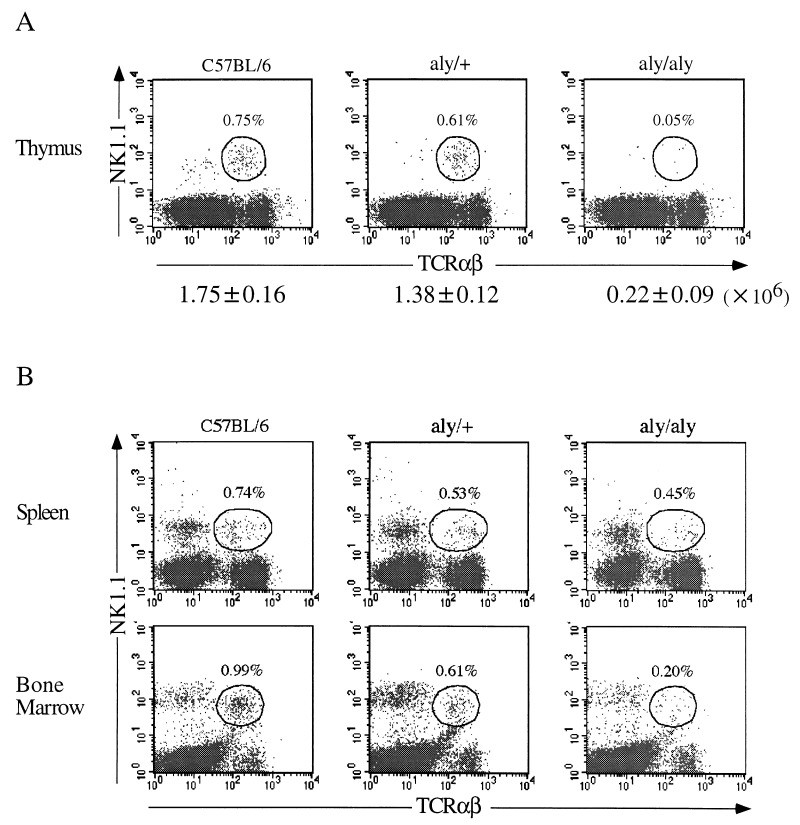
In normal B6 mice, NK1.1+ TCR-α/β+ T cells account for approximately 1.0% of the whole thymocyte population and for 10–20% of mature HSAlow thymocytes (1–3). When thymocytes from aly/aly mice and aly/+ mice were analyzed and compared, no difference was noted in the population size of four major subpopulations of the thymus (CD4−8−, CD4+8+, CD4+8−, and CD4−8+). By contrast, the proportion of NK1.1+ TCR-α/β+ cells among the whole thymocyte population of aly/aly mice was almost 10-fold lower than that of aly/+ mice (Fig. 1A). The actual number of NK1.1+ TCR-α/β+ thymocytes in the thymus of aly/aly mice (2.2 ± 0.9 × 105) was extremely low as compared with that of B6 mice (1.75 ± 0.16 × 106) (P < 0.01). By contrast, the number of NK1.1+ TCR-α/β+ cells in the thymus of aly/+ mice (1.38 ± 0.12 × 106) was almost the same as that of B6 mice.
Figure 1.
Proportion of NK1.1+ TCR-α/β+ cells in lymphoid tissues from aly/aly, aly/+ and B6 mice. Representative FACS profiles of NK1.1+ TCR-α/β+ (average ± SD) cells in the thymus (A) and spleen and BM (B) from three mice are shown. The actual numbers of NK1.1+ TCR-α/β+ thymocytes are shown below A.
We then analyzed the population size of NK1.1+ TCR-α/β+ T cells in spleen of aly/aly mice and compared the values obtained with those of the aly/+ and B6 mice. A slight decrease was detected in the proportion of NK1.1+ TCR-α/β+ cells in aly/aly mice as compared with those in aly/+ and B6 mice (Fig. 1B). Nevertheless, the number of NK1.1+ TCR-α/β+ T cells present in the spleen of aly/aly mice (3.65 ± 0.22 × 105) was significantly lower than that of aly/+ (8.81 ± 1.29 × 105) or B6 mice (9.64 ± 1.31 × 105) (P < 0.01). However, similar levels of NK1.1 expression were induced on T cell-enriched splenocytes of aly/aly and aly/+ mice after culture in the presence of recombinant IL-2 (data not shown).
When the NK1.1+ TCR-α/β+ T cell population in BM was compared among aly/aly, aly/+, and B6 mice, an intermediate pattern between that of thymus or spleen was observed. A significantly low proportion and slightly low proportion were detected in aly/aly mice and in aly/+ mice, respectively, compared with that in B6 mice (Fig. 1B). The present findings indicate that abnormalities of the lymphoid structure are associated with a deficiency that involves the development of the NK1.1+ TCR-α/β+ cells in aly/aly mice.
Generation of NK1.1+ TCR-α/β+ Thymocytes Is Determined by the Thymic Structure But Not by BM Precursor Cells in Allogeneic BM Chimeras.
It has been reported that NK1.1+ TCR-α/β+ T cells are positively selected by major histocompatibility complex class Ib molecules expressed on BM-derived cells in the thymus (10, 12, 22). Thus, it seemed that the generation of NK1.1+ TCR-α/β+ thymocytes might be determined by precursor cell type and not by the thymic epithelial cells.
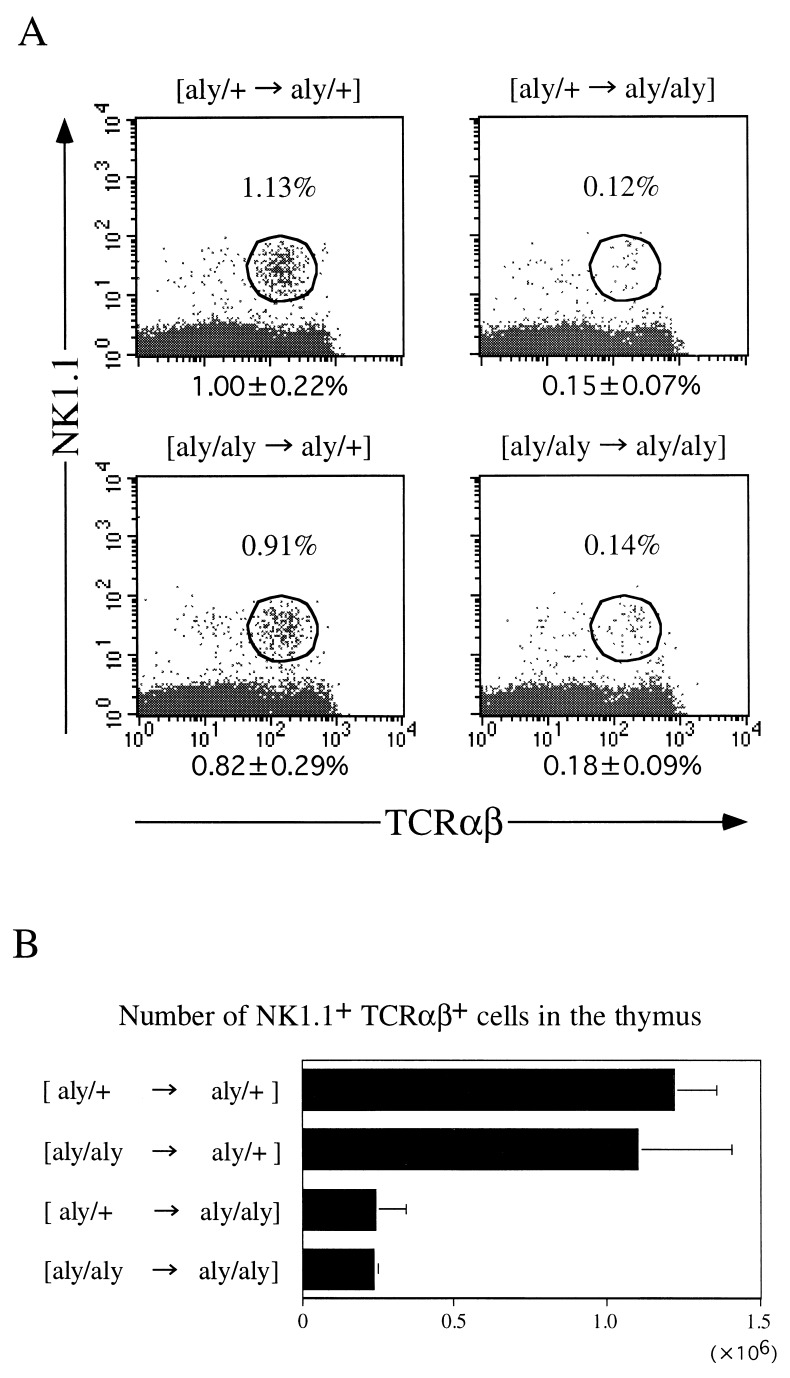
To examine whether the developmental arrest of NK1.1+ TCR-α/β+ thymocytes in aly/aly mice is attributable to the type of precursor cells or to abnormalities of thymic structure as a major basis for the extremely small size of NK1.1+ TCR-α/β+ cell population, we performed reciprocal BM transplantation between aly/aly and aly/+ mice. Fig. 2A shows a representative profile of FACS analysis of thymocytes from four kinds of chimeras and the average proportions (±SD) as calculated from three separate experiments. Fig. 2B summarizes average numbers (±SD) of thymocytes of the chimeras. It is seen in this figure that NK1.1+ TCR-α/β+ thymocytes are not efficiently generated when aly/aly mice are recipients ([aly/+ → aly/aly], [aly/aly → aly/aly] chimeras). On the other hand, a considerable population of NK1.1+ TCR-α/β+ cells was present in the thymi of [aly/aly → aly/+] chimeras as well as [aly/+ → aly/+] chimeras.
Figure 2.
Proportion of NK1.1+ TCR-α/β+ thymocytes in BM chimeras. Four kinds of BM chimeras prepared by reciprocal BM transplantation between aly/aly and aly/+ mice were analyzed for the population size of NK1.1+ TCR-α/β+ thymocytes (A). (B) Data for the average ± SD (number) of three to five chimeric mice.
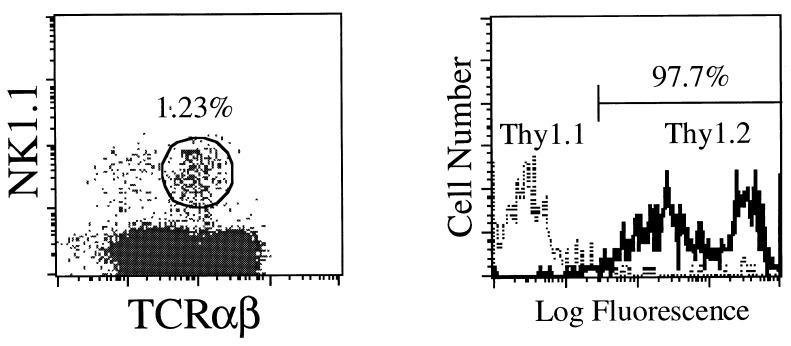
To exclude the possibility that the NK1.1+ TCR-α/β+ thymocytes in [aly/aly → aly/+] chimeras are derived from radio-resistant precursors of the recipient (aly/+) mice, we then analyzed the surface phenotype of thymocytes from [aly/aly → B6. PL-Thy 1.1] chimeras. Because appropriate markers were not available in the aly/aly and aly/+ combination, we chose B6.PL-Thy 1.1 mice. After 3 months, a normal proportion of NK1.1+ TCR-α/β+ cells was detected in the thymus of [aly/aly → B6.PL-Thy 1.1.] chimeras, and actually all of these NK1.1+ TCR-α/β+ cells were shown to be Thy 1.2+ (Fig. 3). These findings indicate that the population size of NK1.1+ TCR-α/β+ thymocytes derived from donor BM is determined by the recipient when allogeneic BM chimeras are studied. However, these results stand in marked contrast to prior reports (10, 12, 22) in which it has been concluded that positive selection of the NK1.1+ TCR-α/β+ thymocytes is induced by BM-derived components.
Figure 3.
Proportion of NK1.1+ TCR-α/β+ thymocytes in [aly/aly → B6.PL-Thy 1.1] BM chimeras. A representative profile of NK1.1+ TCR-α/β+ cells in whole thymocytes is shown from two separate experiments (Left). The origin of thymocytes was determined by anti-Thy 1 mAb (Right).
Histopathology of the Thymus.
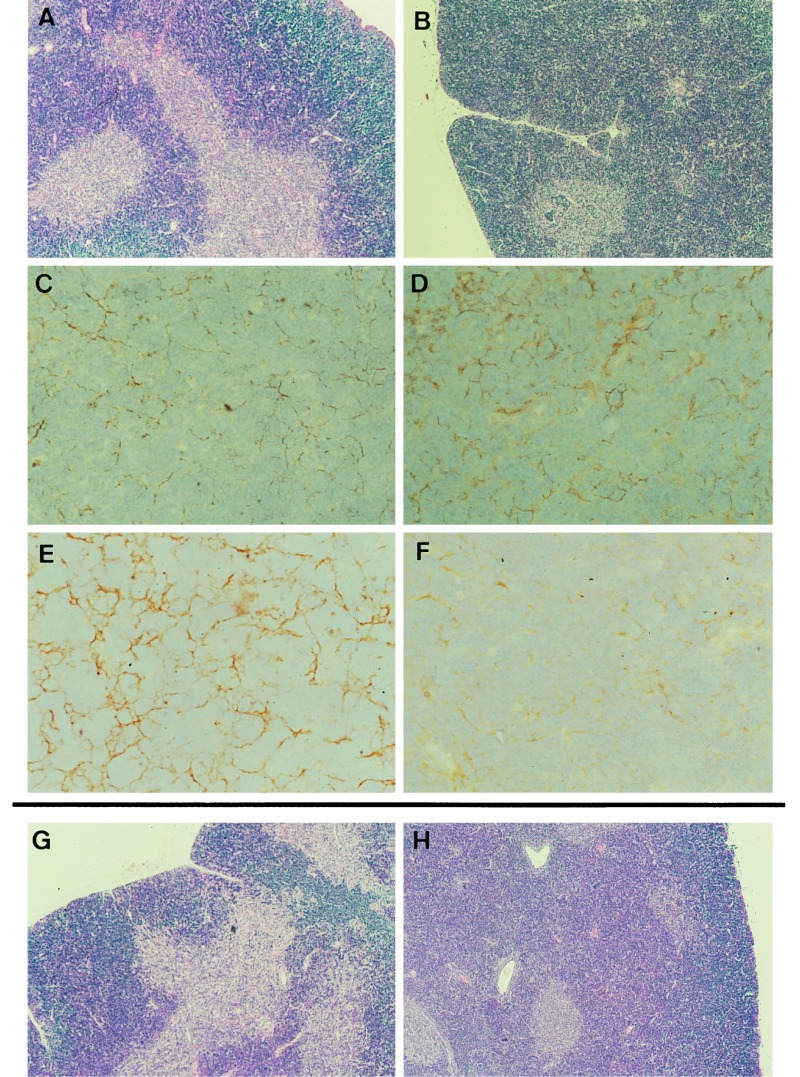
It has been reported that the thymus of aly/aly mice have an abnormal structure (13, 14). As is shown in Fig. 4B, the thymus of aly/aly mouse showed a poorly defined cortico-medullary junction and no clear boundary area. Further, the medullary area of thymus of aly/aly mouse was extremely small as compared with that of the thymus in aly/+ mice (Fig. 4A). When cortical epithelial cells and medullary epithelial cells were stained with ER-TR4 and ER-TR5, respectively, and compared between aly/aly and aly/+ mice, the small size of the medulla of the thymus of aly/aly mice was confirmed. Cortical epithelial cells of aly/aly thymus were heavily stained (Fig. 4D) as compared with those of aly/+ mice (Fig. 4C). On the other hand, ER-TR5-positive cells (medullary epithelial cells) were sparse in the aly/aly thymus (Fig. 4F). Furthermore, more abundant and thicker ER-TR7-positive bundles (reticular fibroblasts) were noted in the cortex of thymus of aly/aly mice as compared with those present in aly/+ mice (data not shown). It thus seems that the thymic structure of aly/aly mouse exhibits abnormalities in both the cortex and medulla.
Figure 4.
Histopathology of the thymus from aly/aly and aly/+ and chimeric mice. Thymic sections obtained from aly/+ (A, C,and E), aly/aly (B, D, and F), [aly/aly → aly/+] (G), and [aly/+ → aly/aly] (H) mice were stained either with hematoxylin/eosin (HE) (A, B, G, and H), ER-TR4 (C and D) or ER-TR5 (E and F). [HE staining (A, B, G, and H) = ×40; immunohistochemical staining (C, D, E, and F) = ×400.]
The characteristics of thymic structure was basically unchanged following lethal irradiation plus reciprocal BM transplantation. The extremely small size of the medullary areas and poorly defined cortico-medullary junctions were observed in the thymus of [aly/+ → aly/aly] chimeras (Fig. 4H). Thus, infusion of normal BM cells into aly/aly mice did not restore toward normal from abnormal architecture of the medullary compartment of the thymus. This finding stands in marked contrast to prior reports that reconstitution of the severe combined immunodeficient (SCID) thymus with BM cells from normal mice resulted in the complete development of both cortices and medullas (23). It appears that the abnormalities or deficiencies in the medullary microenvironment of the thymus in aly/aly mice reside in intrinsic defects or deficiencies of epithelial cells.
On the other hand, normal structure was maintained in the thymus of [aly/aly → aly/+] chimeras (Fig. 4G). Thus, it appeared that the thymic structure had not been profoundly influenced by the type of cells derived from donor BM in these chimeras.
NK1.1+ TCR-α/β+ Thymocytes Are Generated in Normal Thymic Lobes Grafted in aly/aly Mice.
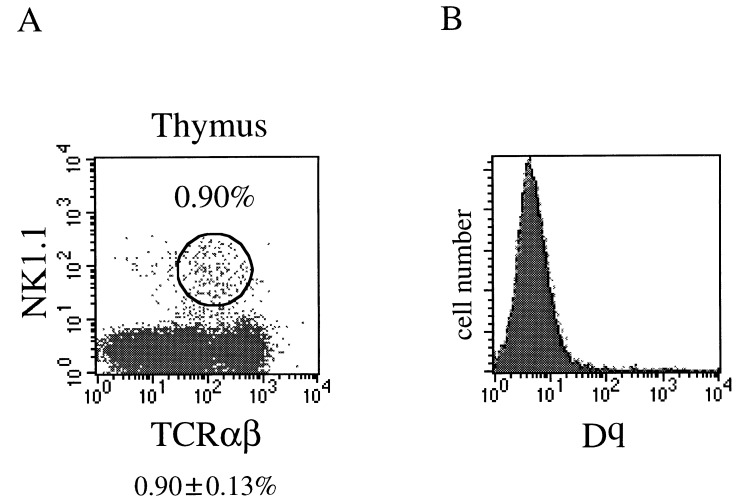
To examine the direct influence of thymus on the development of NK1.1+ TCR-α/β+ thymocytes, we then prepared thymic chimeras. After lethal irradiation, ATx.aly/aly mice were reconstituted with aly/aly BM cells and grafted with deoxyguanosine-treated (B6 × B10.G)F1 fetal thymi. In these thymic chimeras, components other than thymic epithelia were derived from aly/aly mouse. As shown in Fig. 5A, significant proportions of NK1.1+ TCR-α/β+ cells were generated in the thymic lobes (0.90 ± 0.13%) grafted in [aly/aly → ATx.aly/aly] chimeras. NK1.1+ TCR-α/β+ cells generated in these thymic chimeras were shown to be Dq negative (Fig. 5B). These findings indicate that these NK1.1+ TCR-α/β+ cells had been developed from host marrow and not from residual cells of the graft. It thus seems that thymi with normal structure are both necessary and sufficient to generate thymic NK1.1+ TCR-α/β+ cells. When aly/+ mice were reconstituted with aly/aly BM cells after lethal irradiation followed by grafting of deoxyguanosine-treated fetal thymi from aly/aly mice, these mice exhibited severe autoimmune-like or graft-versus-host-disease-like diseases (skin rash, enlargement of lymph nodes, and splenomegaly). The grafted thymi, however, were involuted and sufficient cells could not be recovered from these thymi for analyses. A similar observation was made with B6 nu/nu mice grafted with thymic lobes from aly/aly mice (N.K., K.I., K.O., R.A.G., and K.O., unpublished data). The mechanism or mechanisms underlying these pathological findings are now being investigated. At any rate, we have thus far been unable to analyze the thymic populations in the latter thymic chimeras.
Figure 5.
Proportion of NK1.1+ TCR-α/β+ cells in grafted thymi. Adult thymectomized aly/aly mice were reconstituted with aly/aly BM cells followed by grafting of (B6 × B10.G)F1 fetal thymi, and the proportion of the NK1.1+ TCR-α/β+ cells in the thymi was analyzed (A). Average ± SD (%) of three chimeras was indicated at the bottom. The origin of thymocytes was determined with anti-Dq mAb (B).
DISCUSSION
NK1.1+ TCR-α/β+ cells comprise 0.5–1.0% of thymocytes (1–3). It has been demonstrated that the NK1.1+ TCR-α/β+ cell population has undergone intrathymic selection that differs from that of the major NK1.1− thymocyte population. Thus, the NK1.1+ TCR-α/β+ thymocytes appear to be positively selected in the presence of CD4+8+ thymocytes that express CD1 molecules (2, 10–12). It has been generally considered that cells constituting the thymic structure exert no significant influence on the generation of NK1.1+ thymocytes.
In the present study, we found that the thymic structure plays an important role in generation of NK1.1+ thymocytes. In aly/aly mice which are characterized by abnormalities in the thymic structure, significantly low proportions of NK1.1+ TCR-α/β+ thymocytes as compared with those of aly/+ mice were demonstrated. Thymic reconstitution with cells from BM of aly/+ mice did not influence the small population size of NK1.1+ TCR-α/β+ thymocytes in aly/aly mice. On the other hand, when the thymus of aly/+ mice was reconstituted with BM cells from NK1.1+ thymocyte deficient aly/aly mouse, a normal population of NK1.1+ TCR-α/β+ thymocytes was produced. In addition, thymic lobes of (B6 × B10.G)F1 mice grafted under the kidney capsule of [aly/aly → ATx.aly/aly] chimeras, a normal population of NK1.1+ TCR-α/β+ thymocytes was developed from BM cells of aly/aly mice. Thus, CD1 expression on aly/aly cells, which is an essential requisite for positive selection of NK1.1+ TCR-α/β+ cells (1, 2, 11, 12), appeared to be intact. The aly mutation was mapped to chromosome 11 which is different from that of CD1 (chromosome 3) (24). These findings justify the conclusion that the development of NK1.1+ TCR-α/β+ thymocytes is accomplished under the influence of both BM-derived components and epithelial components which, together, provide an intact microenvironment within the thymus.
The population size and differentiation process of NK1.1− major thymocytes is determined by the structural components of the thymus (15, 16, 25–29), although it has been shown that macrophages and dendritic cells derived from BM precursors were partially involved in functional maturation of thymocytes (30). The present findings demonstrated once again that the developmental arrest of NK1.1+ TCR-α/β+ thymocytes in aly/aly mouse is associated with structural abnormalities of the thymus that are peculiar to the aly/aly mouse. Prior studies (1, 2, 10, 12) demonstrated no significant role of thymic structure on positive selection of NK1.1+ TCR-α/β+ T cells. We reasoned that it might be difficult to detect the functional significance of the thymic structural components in the absence of particular experimental animals such as aly/aly mice.
One question left unanswered in the present study is that the NK1.1+ TCR-α/β+ cell population in the spleen of aly/aly mouse is smaller than that in the aly/+ mouse. The differences, however, were not as impressive as those found in the thymus and BM. The spleen of aly/aly mouse also showed certain structural abnormalities (13, 14). Thus, the abnormal structure of the spleen might exert a less significant influence on the reduced generation of the NK1.1+ TCR-α/β+ cell population than is contributed by the thymus of aly/aly mice. Alternatively, the NK1.1+ TCR-α/β+ cells may be generated in tissues other than the thymus and more readily migrate into the spleen than into the thymus. It has been reported that only very limited populations of mature T cells enter the thymus (19, 31). However, we consider it likely that the intrathymic NK1.1+ TCR-α/β+ cells and extrathymic NK1.1+ TCR-α/β+ cells follow different developmental pathways. Indeed, several characteristics (surface phenotype, usage of TCR-α chain, etc.) have been reported to be different between intrathymic and extrathymic NK1.1+ TCR-α/β+ cells (1, 2, 32, 33). At any rate, it was shown that aly/aly mice treated with anti-CD3 mAb produced significantly low levels of IL-4 as compared with aly/+ or B6 mice treated by the same protocol (unpublished observation). Thus, aly/aly mice exhibited not only a reduced population size of NK1.1+ TCR-α/β+ cells, but also depressed functions of NK1.1+ TCR-α/β+ T cells (34). These issues should be pursued in future investigations.
Acknowledgments
We thank Ms. Michiyo Konishi for manuscript preparation, Ms. Tazim Verjee for manuscript preparation and editing, and Ms. Senri Sudoh for excellent technical assistance. This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Culture and Sports, Japan, grants from the Institute of Biological Science, Mitsui Pharmaceuticals, Inc., East Tomakomai Hospital, the Nishimura Aging Fund, and the National Institute of Aging (Grant 05628–12 to R.A.G.).
ABBREVIATIONS
- NK1.1
natural killer antigen 1.1
- TCR
T cell antigen receptor
- BM
bone marrow
- ATx
adult thymectomized
- IL
interleukin
- B6
C57BL/6
- FITC
fluorescein isothiocyanate
- FACS
fluorescence-activated cell sorter
References
- 1.Bendelac A. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald H R. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arase H, Arase N, Ogasawara K, Good R A, Onoé K. Proc Natl Acad Sci USA. 1992;89:6506–6510. doi: 10.1073/pnas.89.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase H, Arase N, Nakagawa K, Good R A, Onoé K. Eur J Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul W E. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arase H, Arase N, Kobayashi Y, Nishimura Y, Yonehara S, Onoé K. J Exp Med. 1994;180:423–432. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arase H, Arase-Fukushi N, Good R A, Onoé K. J Immunol. 1993;151:546–555. [PubMed] [Google Scholar]
- 9.Ballas Z K, Rasmussen W. J Immunol. 1987;139:3542–3549. [PubMed] [Google Scholar]
- 10.Coles M C, Raulet D H. J Exp Med. 1994;180:395–399. doi: 10.1084/jem.180.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendelac A, Lantz O, Quimby M E, Yewdell J W, Bennink J R, Brutkiewicz R R. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 12.Bendelac A. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 14.Koike R, Nishimura T, Yasumizu R, Tanaka H, Hataba Y, Watanabe T, Miyawaki S, Miyasaka M. Eur J Immunol. 1996;26:669–675. doi: 10.1002/eji.1830260324. [DOI] [PubMed] [Google Scholar]
- 15.Iwabuchi K, Negishi I, Arase H, Iwabuchi C, Ogasawara K, Good R A, Onoé K. Proc Natl Acad Sci USA. 1989;86:5089–5093. doi: 10.1073/pnas.86.13.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negishi I, Ogasawara K, Iwabuchi K, Wang B, Good R A, Onoé K. Cell Immunol. 1991;136:185–193. doi: 10.1016/0008-8749(91)90393-p. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson E J, Franchi L L, Kingston R, Owen J J T. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 18.Hatakeyama S, Iwabuchi K, Ato M, Iwabuchi C, Kajino K, Takami K, Katoh M, Ogasawara K, Good R A, Onoé K. Microbiol Immunol. 1996;40:223–231. doi: 10.1111/j.1348-0421.1996.tb03338.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukushi N, Arase H, Wang B, Ogasawara K, Gotohda T, Good R A, Onoé K. Proc Natl Acad Sci USA. 1990;87:6301–6305. doi: 10.1073/pnas.87.16.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Vliet E, Jenkinson E J, Kingston R, Owen J J T, Van Ewijk W. Eur J Immunol. 1985;15:675–681. doi: 10.1002/eji.1830150707. [DOI] [PubMed] [Google Scholar]
- 21.Van Ewijk W, Shores E W, Singer A. Immunol Today. 1994;15:214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 22.Ohteki T, MacDonald H R. J Exp Med. 1994;180:699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shores E W, Van Ewijk W, Singer A. Eur J Immunol. 1991;21:1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 24.Kuramoto T, Mashimo T, Koike R, Miyawaki S, Yamada T, Miyasaka M, Serikawa T. Int Immunol. 1994;6:991–994. doi: 10.1093/intimm/6.7.991. [DOI] [PubMed] [Google Scholar]
- 25.Sprent J, Lo D, Gao E-K, Ron Y. Immunol Rev. 1988;101:173–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 26.Marrack P, Lo D, Brinster R, Palmiter R, Burkly L, Flauell R H, Kappler J. Cell. 1988;53:627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- 27.Van Ewijk W. Annu Rev Immunol. 1991;9:591–615. doi: 10.1146/annurev.iy.09.040191.003111. [DOI] [PubMed] [Google Scholar]
- 28.Katsume C, Fernandes G, Iwabuchi K, Ogasawara K, Gotohda T, Good R A, Onoé K. Microbiol Immunol. 1989;33:313–328. doi: 10.1111/j.1348-0421.1989.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 29.Iwabuchi C, Iwabuchi K, Kobayashi S, Ogasawara K, Negishi I, Wang B, Wambua P P, Arase H, Fukushi N, Itoh Y, Gotohda T, Good R A, Onoé K. J Immunol. 1991;146:26–34. [PubMed] [Google Scholar]
- 30.Wambua P P, Iwabuchi K, Iwabuchi C, Ogasawara K, Itoh Y, Arase H, Kajiwara M, Gotohda T, Kajino K, Good R A, Onoé K. Microbiol Immunol. 1994;38:879–890. doi: 10.1111/j.1348-0421.1994.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 31.Agus D B, Surh C D, Sprent J. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budd R C, Mixter P F. Immunol Today. 1995;16:428–431. doi: 10.1016/0167-5699(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 33.Adachi Y, Koseki H, Zijlstra M, Taniguchi M. Proc Natl Acad Sci USA. 1995;92:1200–1204. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimoto T, Bendelac A, Hu-Li J, Paul W E. Proc Natl Acad Sci USA. 1995;92:11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]