Abstract
We have developed a rapid, continuous method for monitoring the effectiveness of several antibacterial agents in real time, noninvasively, by using a recently described mouse model of chronic biofilm infection (J. L. Kadurugamuwa et al., Infect. Immun. 71:882-890, 2003), which relies on biophotonic imaging of bioluminescent bacteria. To facilitate real-time monitoring of infection, we used a Staphylococcus aureus isolate that was made bioluminescent by inserting a modified lux operon into the bacterial chromosome. This bioluminescent reporter bacterium was used to study the antimicrobial effects of several antibiotics belonging to different molecular families. Treatment with rifampin, tobramycin, and ciprofloxacin was started 7 days after subcutaneous implantation of catheters precolonized with 104 CFU of S. aureus. Three different doses of antibiotics were administered twice a day for 4 consecutive days. The number of metabolically active bacteria in untreated mice and the tobramycin- and ciprofloxacin-treated groups remained relatively unchanged over the 4-week observation period, indicating poor efficacies for tobramycin and ciprofloxacin. A rapid dose-dependent decline in metabolic activity in rifampin-treated groups was observed, with almost a 90% reduction after two doses and nearly undetectable levels after three doses. The disappearance of light emission correlated with colony counts. After the final treatment, cell numbers rebounded as a function of concentration in a time-dependent manner. The staphylococci isolated from the catheters of mice treated with rifampin were uniformly resistant to rifampin but retained their in vitro susceptibilities to tobramycin and ciprofloxacin. Since the metabolic activities of viable cells and a postantibiotic effect could be detected directly on the support matrix nondestructively and noninvasively, the methodology is specifically appealing for investigating the effects of antibiotics on biofilms in vivo. Moreover, our study points to the possible use of biophotonic imaging for the detection of the development of resistance to therapeutic agents during treatment of chronic infections in vivo.
Modern medical practice involves the implantation of an increasing number of diverse prosthetic devices. Microbial adhesion and biofilm formation on these devices are common occurrences and represent serious medical problems, as these infections are often difficult to resolve because of their increased resistance to host defense mechanisms and antimicrobial therapy (9, 21, 32). Despite the use of drugs that are highly active in standard in vitro susceptibility tests, the response to therapy may be disappointing and biofilm infections are often reestablished soon after treatment, which then leads to chronic infections. Frequently, the affected devices must be removed to fully eliminate the infection. Removal of these devices, for example, heart valves, joint prostheses, and central nervous system shunts, has serious implications (8, 25). In order to combat biofilm-associated infections, novel, effective drugs and new approaches to screening for the effectiveness of candidate compounds in vivo are essential to accelerate their development.
Attempts have been made to predict the outcomes of in vivo antibiotic treatments associated with bacterial biofilm infections with the help of in vitro methods. However, the standard in vitro susceptibility tests are not predictive of the therapeutic outcomes of device-related infections (3, 44). One major problem faced in biofilm research in vivo, especially those studies concerning antibiotic treatment, has been the lack of a nondestructive, reproducible, longitudinal monitoring system, which would permit the assessment of antibiotic activity in the same animal throughout the duration of the study. Furthermore, to test the effectiveness of the treatment regimen, animals must be killed at each sampling point and the foreign body must be removed to estimate the pathogen burden within the biofilm. Such procedures can yield large animal-to-animal variations and the consumption of large numbers of experimental animals.
Fortunately, due to recent advances in imaging technology and bioluminescent reporter systems, researchers can now monitor bacterial infections noninvasively and nondestructively at various sites within the host animal (5-7, 10, 11, 19, 34). Recently, Kadurugamuwa et al. (19) demonstrated such a method for monitoring device-related chronic biofilm infection in a mouse model in real time through noninvasive imaging of bioluminescent strains of two of the most common biofilm-forming pathogens, namely, Staphylococcus aureus and Pseudomonas aeruginosa. Here we extended that study to investigate whether biophotonic imaging could be used to evaluate the therapeutic efficacies of antimicrobial agents in the in vivo model. Additionally, the utility of the model was assessed for its ability to monitor noninvasively the postantibiotic effects and regrowth of biofilm bacteria in live animals through bioluminescent imaging.
(Parts of the present study were presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, 2002 [J. Kadurugamuwa et al., Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-270, 2002].)
MATERIALS AND METHODS
Bacterial strain.
The bacterial strain used in this study was S. aureus ATCC 12600. This strain was engineered for bioluminescence by inserting a modified complete lux operon, as described previously (19), and was designated S. aureus Xen 29.
Antibiotics.
Selected model antibiotics belonging to different molecular families and having different mechanisms of action were tested for their effects against biofilms. The antibiotics selected for treatment of in vivo biofilm infections were rifampin, a transcriptional inhibitor; ciprofloxacin, a topoisomerase inhibitor; and tobramycin, an aminoglycoside which acts as both a translational inhibitor and a surface perturbating agent (18, 30).
Catheter-associated biofilms.
Biofilms of S. aureus were developed on a 14-gauge Teflon intravenous catheter (Abbocath-T; Burns Vet Supply, Vancouver, Wash.), as described previously (19).
Antibiotic susceptibility test. (i) MIC assays.
MICs were determined by the broth dilution method with an inoculum of 108 CFU of S. aureus per ml in Mueller-Hinton broth (MHB) by the procedures recommended by the National Committee for Clinical Laboratory Standards (29).
(ii) Biofilm susceptibility testing.
The biofilms that formed on 1-cm catheter segments were transferred to tubes containing dilutions of the specified antibiotics in MHB. Following 18 h of incubation at 37°C in the presence of an antimicrobial agent, the catheters were rinsed twice in MHB and the viability and metabolic activity of the biofilm were determined by measurement of the viable counts and reading of the bioluminescence with an IVIS Imaging System (Xenogen Corp., Alameda, Calif.). The minimal biofilm eradication concentration was defined as the lowest concentration of antibiotic that reduced ≥99.9% of the biofilm cell numbers with respect to the cell numbers for the untreated controls (4, 37).
Resistance to antimicrobial agents.
The bacteria recovered from the catheters were screened for the emergence of resistance to antimicrobial agents during therapy by plating the bacteria onto MHB containing each antibiotic at the MIC. Plates were examined for growth after 24 h of incubation at 37°C. Bacteria that showed increased resistance to antibiotics were further tested by the broth dilution method to determine the MIC.
Experimental model of infection and monitoring of response to therapy.
The experimental infection in a murine model was established as described previously (19, 35). Seven days after implantation of a 1-cm segment of a precolonized catheter carrying 104 CFU, groups of mice were treated with rifampin, ciprofloxacin, and tobramycin. Three doses of each antibiotic (30, 20, and 10 mg/kg of body weight) were tested by administering tobramycin (subcutaneously) or rifampin and ciprofloxacin (intraperitoneally) in 0.1 ml of saline. The antibiotics were given every 12 h for 4 consecutive days (total of eight doses). The concentrations of the antibiotics and the treatment regimens were selected on the basis of information available in comparable studies in the literature (13, 16, 26, 28, 43). Since the concentrations of antibiotics used in the present study were similar to or greater than those used in previous studies, we expected that the drug concentrations in serum were well above the MIC for S. aureus. A separate group of animals served as an untreated infection control group that was treated with saline. A sterile catheter was implanted in another extra group of animals, which served as a negative control group. The mice were maintained in an anesthetized state with 1.5% isoflurane gas by using an IVIS manifold placed inside the imaging chamber and were imaged for a maximum of 5 min at various time points following inoculation by using an IVIS Imaging System. Total photon emissions from defined regions of interest within the images of each mouse were quantified by using the Living Image software package (Xenogen Corp.). The photon signals from the catheter were quantified from the dorsal image of each mouse. During the treatment and after the final imaging time point, mice were euthanized, and the infected catheter was surgically removed for enumeration of bacteria by both bioluminescence imaging and the conventional viable count method. The bioluminescence of the bacteria recovered at the end of the experimental period was compared with that of the inoculating strain.
Extraction and quantification of bacteria from the catheter biofilm assay. (i) In vitro.
Two to three catheters were removed from the incubating tubes according to each experimental objective. The catheters were rinsed in fresh Trypticase soy broth and imaged to quantify the bioluminescence signal. The catheters were then transferred to a separate tube containing 1 ml of Trypticase soy broth. The tubes were placed in an ultrasonic bath (38.5 to 40.5 kHz; Van Waters & Rogers, San Francisco, Calif.) and sonicated for 5 to 10 min, followed by votexing for 1 min to remove the biofilm bacteria from the support surface. To assess the catheters for the complete removal of the biofilm bacteria, the catheters were imaged at different time intervals and the loss of a bioluminescence signal from the catheter was used to define the complete biofilm removal protocol. The bacteria that were removed from the catheter were diluted, plated on Trypticase soy agar, and incubated at 37°C for colony counting.
(ii) In vivo.
During the evaluation period and after the final imaging time point, the mice were humanely killed by euthanasia, and the catheters were gently removed from the subcutaneous tissue by making an incision in the skin approximately 2 cm from the implant wound. The harvested catheters were imaged, and the biofilm bacteria were detached from the catheter material as described above and enumerated by conventional colony count assay. Catheters from the control mice were included in each experiment to assess the adequacies of the aseptic and surgical techniques.
RESULTS
Antimicrobial susceptibility studies.
The MICs of rifampin, ciprofloxacin, and tobramycin for strain Xen 29 were 0.016, 4, and 4 mg/ml, respectively. The corresponding minimal biofilm eradication concentrations of these agents were 0.128, 32, and 64 mg/ml, respectively.
Response to treatment.
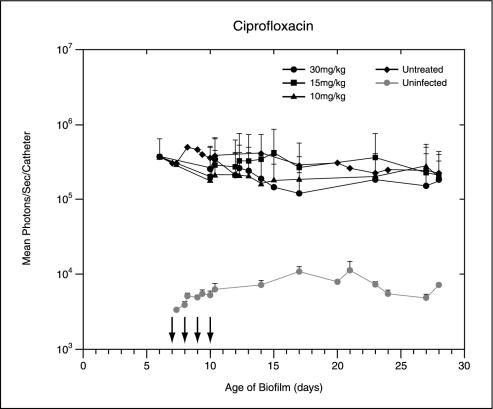
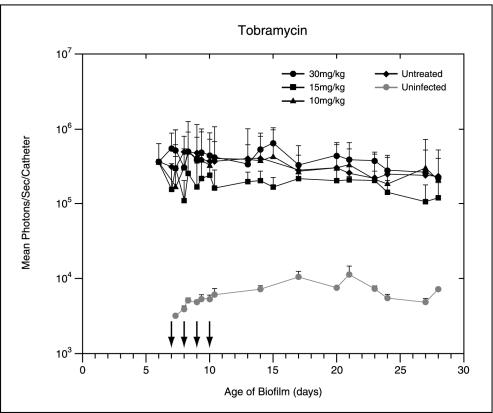
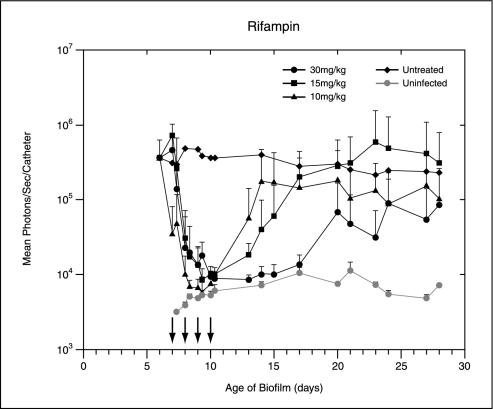
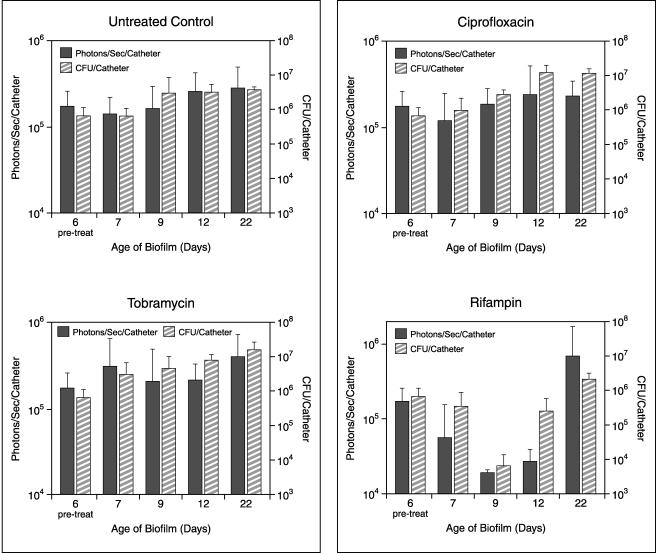
Following implantation of the catheters precolonized with 104 CFU of Xen 29 per catheter, the bioluminescence measurements increased exponentially over 24 h. It reached approximately 105 photons/s/catheter and remained moderately stable until the termination of the experiment, thus allowing the efficacies of the antibiotic treatments to be effectively assessed. Treatment with rifampin, ciprofloxacin, and tobramycin was started 7 days after implantation. The total photon emission from infected sites was quantified by using Living Image software, with the cumulative results shown in Fig. 1. Real-time in vivo biophotonic images of representative animals from the control group and the groups of animals treated with the highest dose of each antibiotic are shown in Fig. 2. Compared with the bioluminescence for the control group, the bioluminescences for mice treated with tobramycin or ciprofloxacin at concentrations up to 30 mg/kg showed no apparent differences throughout the study (Fig. 1 and 2). In contrast, a rapid dose-dependent decline in metabolic activity in the rifampin-treated group was observed by day 1, with an almost 90% reduction and nearly undetectable levels after three treatments. Continuing rifampin administration diminished the luminescence to nearly background levels in all groups. A reduction of almost 1.5 log units was observed after the final treatment. Once the signals were reduced, after the final treatment (day 10 postinfection) the signals remained undetectable for the next 3 to 5 days in the group treated with the highest dose of rifampin. Although attenuation of the signal from the biofilm was initially shown for mice treated with all doses of rifampin, relapse was observed in each animal at various later times, as judged by the intensity of the bioluminescent signal, indicating that the biofilms were not irreversibly damaged and could regrow (Fig. 1 and 2). This increase in the luminescence of the biofilm images correlated with the increase in viable cell numbers.
FIG. 1.
In vivo bioluminescence monitoring of S. aureus in the mouse model of biofilm infection during treatment with ciprofloxacin, tobramycin, or rifampin. The total number of photons detected over the infected catheter per second was determined by using an IVIS camera and was plotted with respect to time. Two catheters were implanted in each mouse, and each datum point is the mean ± standard deviation for two to three mice. Arrows indicate the days of antibiotic administration. The data are the averages of results from three separate experiments.
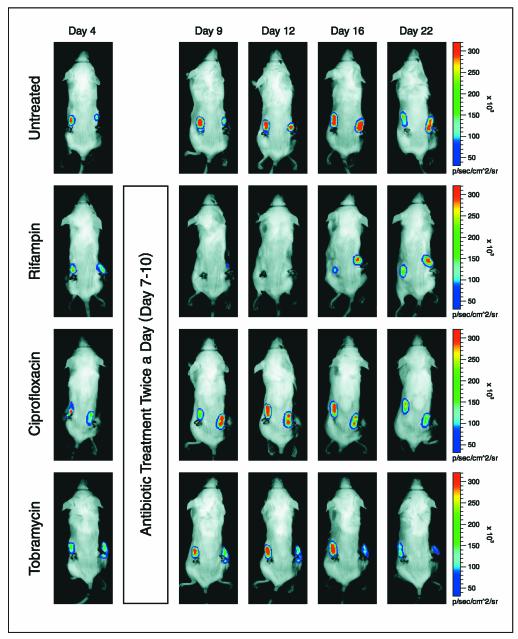
FIG. 2.
Real-time monitoring of the effects of antibiotics on S. aureus Xen 29 biofilm infection in mice. Seven days after implantation of precolonized catheters with 104 CFU of S. aureus, the mice were treated or not treated with antibiotics (twice a day for 4 consecutive days) and were imaged periodically over a 4-week period by using an IVIS camera. A representative animal from the group receiving the highest dose of antibiotic or the untreated control group is shown. The color bar indicates the signal intensity. p/sec/cm^2/sr, number of photons per second per square centimeter of catheter/steradian.
Relationship between bioluminescence measurement and conventional viable count.
In order to determine whether bacterial death could explain the decline in light emission, groups of mice were killed during the course of the study to determine the number of CFU present in the biofilm colonizing the catheter material. A comparison of bioluminescence and CFU data for control mice and tobramycin- and ciprofloxacin-treated mice revealed that the number of CFU extracted from the catheters of the animals that had been killed closely paralleled the bioluminescence signal (Fig. 3). Treatment with rifampin for 1 day (day 7 postinfection) resulted in a rapid decrease in bioluminescence. Similarly, evaluation of antibacterial activity by the standard methodology of CFU determination also demonstrated a drop in the numbers of CFU. However, the magnitude of the reduction in the bioluminescent signal appeared to be greater than that obtained by CFU determination. This is probably because the antibiotic inhibited protein synthesis in the cell immediately but took longer to induce cell death to occur. Concomitant alterations in bioluminescence and the numbers of CFU were observed for subsequent time points. Compared to real-time bioluminescence monitoring, CFU determinations took considerably longer to demonstrate a response to treatment.
FIG. 3.
Growth and bioluminescence signals of S. aureus in the mouse model of biofilm infection during treatment with phosphate-buffered saline (untreated control group), tobramycin, ciprofloxacin, or rifampin. Viable counts are reported as the number of CFU per catheter, and bioluminescence is represented as the number of photons per second per catheter determined with an IVIS camera. Each datum point is the mean ± standard error for three to four catheters. Bioluminescence was determined at each time point immediately prior to determination of the viable cell count by harvesting the catheter.
No spontaneous healing of implant infections occurred in the untreated group, even after a prolonged (40 days) observation period (data not shown). Each bacterium from all colonies harvested from the in vivo biofilm infections expressed luciferase activity that was comparable to that of the original inoculum. These findings demonstrate that the engineered strain is stable in vivo and indicate that the reduction in apparent growth determined by luminescence is due to a reduction in metabolic activity and not to a loss of the lux insertion. The infection remained localized to the catheter.
Emergence of resistance during therapy.
The potential emergence of resistant mutants during therapy of chronic biofilm infection was determined for staphylococci recovered from the catheters in all groups. No evidence of resistance to tobramycin or ciprofloxacin was observed when the biofilm bacteria were cultivated on plates containing the respective antibiotics. In contrast, a large proportion of the bacteria recovered from the biofilms after rifampin treatment were resistant to greater than 2.5 mg of rifampin per ml. Compared with the MIC for the strain recovered from untreated animals, the MIC for rifampin-resistant isolates showed an approximately >64-fold increase.
DISCUSSION
We have demonstrated the use of biophotonic imaging to evaluate the efficacies of antimicrobial agents against a subcutaneous catheter-associated S. aureus biofilm infection in mice. To facilitate real-time monitoring of infection, we used bacteria that expressed a modified lux operon from Photorhabdus luminescens (10) and a low-light imaging system. In this manner, the bioluminescent light generated within the pathogen can be used to monitor the progression of infection and the efficacy of antibiotic treatment noninvasively in animals in real time. Images of the mice in each group revealed spatial distribution and temporal patterns of biofilm development that represented a full range of responses to therapy, including disappearance, relapse, and apparent unimpeded growth. By this methodology we have demonstrated that not only antibacterial properties but also the course and the relapse of the infection can be monitored optically in vivo in real time in living animals. In addition, the approach resulted in a considerable savings in time, since results were obtained in minutes rather than days, as with conventional methods.
Bioluminescent bacteria have previously been used successfully for the measurement of antibacterial activity in vitro (14, 22, 33, 36, 38, 40, 41) and for the measurement of activity against acute in vivo infections (5, 6, 10, 11, 16, 34). The model presented here permits for the first time longitudinal monitoring of chronic biofilm infections and the response to therapy. Prior to the introduction of this novel methodology, the response to therapy for device-related infections in animal models had been monitored mainly by CFU determinations. These methods suffer from the disadvantage of requiring large numbers of animals, in addition to more labor, more time, difficulties in extraction of the bacteria from the support matrix, and sampling limitations (6, 20). The guinea pig tissue cage model of Zimmerli et al. (46) allows repeated sampling of fluid for bacterial enumeration and for determination of the pharmacodynamics of antibiotics or the specifics of the local host response. However, this method provides the number of sessile bacteria within aspirated tissue cage fluid surrounding the foreign body but not the number of biofilm bacteria. Moreover, this method, like other conventional ex vivo methods, does not provide data in real time. By the method described here, it was possible to evaluate antibacterial effects and the regrowth of biofilm bacteria in living animals without the limitations associated with death as an end point for sampling. In addition, our methodology is not subject to the problems of plate contamination or antibiotic carryover effect that can bias the results for treated groups.
It is accepted that eradication of a foreign body-related infection generally requires long courses of combination antibiotic therapy (1, 2, 12, 13, 21, 23, 27, 31, 32, 37, 39, 47). However, in this study the main intent of the evaluation was the application of an in vivo imaging technology to monitor the effects of different antibiotics on bacterial growth and the recurrence of infection rather than the clinical response per se. We therefore opted to treat the mice singly with a relatively short course of antibiotics. In this way we could demonstrate the ability to monitor the regrowth of the biofilm noninvasively in real time in the same animal following repeated treatments. Unlike other methods, removal of the infected device was not necessary to enumerate its pathogen burden. The ability to monitor the postantibiotic effect and detect the in vivo development of survivors resistant to a given therapeutic agent during treatment in living individual animals under various therapeutic regimens provided both temporal and spatial information. As reported previously (19), catheter segments with preformed biofilms containing 104 CFU/catheter produced a chronic infection in 100% of the mice into which the catheters were implanted without causing spread of the infection beyond the implant, delayed death, or spontaneous resolution. In this respect, the catheter model simulates human infections, in the sense that no spontaneous cure occurs. In addition, the potential for a relapse of infection following treatment mimics the clinical condition. This is therefore an acceptable model for evaluating an antibiotic's ability to alter an otherwise defined outcome.
The ability to rapidly assess microorganism viability is important in the evaluation of susceptibility to antimicrobial compounds. The light emission from the biofilm paralleled the number of CFU and reflected the metabolic status of the biofilm cells. Bacterial bioluminescence depends on a complex cellular biochemistry, in which a large number of genes (five structural genes, luxABCDE) and proteins are involved in a highly controlled manner (24). Consequently, light production indicates that the genetic and biochemical apparatuses of the cell are intact. The variation in color intensity of the biofilm represents the relative metabolic activity at a given location of the biofilm, indicating the physiological heterogeneity within the biofilm, as shown previously (17). We found that rifampin was effective, causing ∼1.0 to 1.5 log reductions in the number of CFU and the bioluminescence signal within the catheter. This proves that the catheter-associated biofilm in mice responded to the antibacterial agents, and the response could be detected noninvasively by the methodology described here. Tobramycin and ciprofloxacin did not reduce the bioluminescence or bacterial counts during treatment and did not cure the infection. This relative resistance of the biofilm to tobramycin and ciprofloxacin is in accordance with previous observations in the literature (15, 27, 44). Our results are consistent with those of previous studies demonstrating the significant bactericidal effect of rifampin against staphylococcal biofilm infections (1, 2, 12, 13, 27, 31, 37, 42, 44-47). In addition, our in vivo results confirm the high risk of the rapid emergence of a rifampin-resistant population, as reported previously, which can result in clinical failures when rifampin is used alone (12, 23, 31, 44, 47).
At present, convenient methods that allow the detection of bacterial resistance to antibiotics that develops during treatment of in vivo biofilm infections are not available. By our methodology, the efficacies of antibacterial agents against biofilm infections could be rapidly evaluated in vivo at multiple times during the course of disease, allowing single animals to be monitored over time and removing intersample variability. Thus, far fewer animal subjects are required to obtain statistically meaningful results. Moreover, because the studies can be performed with minimal invasiveness, the stress on the animals can be reduced dramatically. We have demonstrated that the imaging technology simplifies the evaluation of biofilm viability in an experimental infection and accelerates the in vivo analysis of the efficacies of drugs against biofilm infections. We believe that the model presented here offers spatiotemporal information about biofilm formation and drug treatment not available from other conventional methods. In addition, this technology permits a more convenient and rapid means of assessing the in vivo potentials of novel antimicrobial compounds, susceptibility testing, resistance development, and compound screening at an early stage in drug development.
Acknowledgments
We thank E. Reynolds and C. Dalesio (Graphics and Communications, Xenogen Corp.) for assistance with the drawings.
REFERENCES
- 1.Bayer, A. S., and K. Lam. 1985. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J. Infect. Dis. 151:157-165. [DOI] [PubMed] [Google Scholar]
- 2.Blaser, J., P. Vergeres, A. F. Widmer, and W. Zimmerli. 1995. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob. Agents Chemother. 39:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, M. R., and P. Williams. 1985. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu. Rev. Microbiol. 39:527-556. [DOI] [PubMed] [Google Scholar]
- 4.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contag, C. H., P. R. Contag, J. I. Mullins, S. D. Spilman, D. K. Stevenson, and D. A. Benaron. 1995. Photonic detection of bacterial pathogens in living hosts. Mol. Microbiol. 18:593-603. [DOI] [PubMed] [Google Scholar]
- 6.Contag, C. H., S. D. Spilman, P. R. Contag, M. Oshiro, B. Eames, P. Dennery, D. K. Stevenson, and D. A. Benaron. 1997. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem. Photobiol. 66:523-531. [DOI] [PubMed] [Google Scholar]
- 7.Contag, P. R., I. N. Olomu, D. K. Stevenson, and C. H. Contag. 1998. Bioluminescent indicators in living mammals. Nat. Med. 4:245-247. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 9.Davies, D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114-122. [DOI] [PubMed] [Google Scholar]
- 10.Francis, K. P., D. Joh, C. Bellinger-Kawahara, M. J. Hawkinson, T. F. Purchio, and P. R. Contag. 2000. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect. Immun. 68:3594-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis, K. P., J. Yu, C. Bellinger-Kawahara, D. Joh, M. J. Hawkinson, G. Xiao, T. F. Purchio, M. G. Caparon, M. Lipsitch, and P. R. Contag. 2001. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect. Immun. 69:3350-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagnon, R. F., A. D. Harris, J. Prentis, and G. K. Richards. 1989. The effects of heparin on rifampin activity against Staphylococcus epidermidis biofilms. Adv. Perit. Dial. 5:138-142. [PubMed] [Google Scholar]
- 13.Gagnon, R. F., G. K. Richards, and R. Subang. 1992. Experimental Staphylococcus epidermidis implant infection in the mouse. Kinetics of rifampin and vancomycin action. Am. Soc. Artif. Intern. Organs J. 38:M596-M599. [DOI] [PubMed] [Google Scholar]
- 14.Gracia, E., A. Fernandez, P. Conchello, J. L. Alabart, M. Perez, and B. Amorena. 1999. In vitro development of Staphylococcus aureus biofilms using slime-producing variants and ATP-bioluminescence for automated bacterial quantification. Luminescence 14:23-31. [DOI] [PubMed] [Google Scholar]
- 15.Gracia, E., A. Lacleriga, M. Monzon, J. Leiva, C. Oteiza, and B. Amorena. 1998. Application of a rat osteomyelitis model to compare in vivo and in vitro the antibiotic efficacy against bacteria with high capacity to form biofilms. J. Surg. Res. 79:146-153. [DOI] [PubMed] [Google Scholar]
- 16.Hickey, M. J., T. M. Arain, R. M. Shawar, D. J. Humble, M. H. Langhorne, J. N. Morgenroth, and C. K. Stover. 1996. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob. Agents Chemother. 40:400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, C., F. Yu, G. McFeters, and P. Stewart. 1995. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl. Environ. Microbiol. 61:2252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadurugamuwa, J. L., A. J. Clarke, and T. J. Beveridge. 1993. Surface action of gentamicin on Pseudomonas aeruginosa. J. Bacteriol. 175:5798-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadurugamuwa, J. L., L. Sin, E. Albert, J. Yu, K. Francis, M. DeBoer, M. Rubin, C. Bellinger-Kawahara, T. R. Parr, Jr., and P. R. Contag. 2003. Direct continuous method for monitoring biofilm infection in a mouse model. Infect. Immun. 71:882-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, S. H., and A. Camilli. 2000. Novel approaches to monitor bacterial gene expression in infected tissue and host. Curr. Opin. Microbiol. 3:97-101. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loimaranta, V., J. Tenovuo, L. Koivisto, and M. Karp. 1998. Generation of bioluminescent Streptococcus mutans and its usage in rapid analysis of the efficacy of antimicrobial compounds. Antimicrob. Agents Chemother. 42:1906-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucet, J. C., M. Herrmann, P. Rohner, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1990. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 34:2312-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marincs, F. 2000. On-line monitoring of growth of Escherichia coli in batch cultures by bioluminescence. Appl. Microbiol. Biotechnol. 53:536-541. [DOI] [PubMed] [Google Scholar]
- 25.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 26.Montero, A., J. Ariza, X. Corbella, A. Domenech, C. Cabellos, J. Ayats, F. Tubau, C. Ardanuy, and F. Gudiol. 2002. Efficacy of colistin versus β-lactams, aminoglycosides, and rifampin as monotherapy in a mouse model of pneumonia caused by multiresistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 46:1946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monzon, M., F. Garcia-Alvarez, A. Lacleriga, E. Gracia, J. Leiva, C. Oteiza, and B. Amorena. 2001. A simple infection model using pre-colonized implants to reproduce rat chronic Staphylococcus aureus osteomyelitis and study antibiotic treatment. J. Orthoped. Res. 19:820-826. [DOI] [PubMed] [Google Scholar]
- 28.Mouton, J. W., M. L. van Ogtrop, D. Andes, and W. A. Craig. 1999. Use of pharmacodynamic indices to predict efficacy of combination therapy in vivo. Antimicrob. Agents Chemother. 43:2473-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Neu, H. C. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 31.Norden, C. W., and E. Keleti. 1980. Treatment of experimental staphylococcal osteomyelitis with rifampin and trimethoprim, alone and in combination. Antimicrob. Agents Chemother. 17:591-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Toole, G. A. 2002. Microbiology: a resistance switch. Nature 416:695-696. [DOI] [PubMed] [Google Scholar]
- 33.Parveen, A., G. Smith, V. Salisbury, and S. M. Nelson. 2001. Biofilm culture of Pseudomonas aeruginosa expressing lux genes as a model to study susceptibility to antimicrobials. FEMS Microbiol. Lett. 199:115-118. [DOI] [PubMed] [Google Scholar]
- 34.Rocchetta, H. L., C. J. Boylan, J. W. Foley, P. W. Iversen, D. L. LeTourneau, C. L. McMillian, P. R. Contag, D. E. Jenkins, and T. R. Parr, Jr. 2001. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob. Agents Chemother 45:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salisbury, V., A. Pfoestl, H. Wiesinger-Mayr, R. Lewis, K. E. Bowker, and A. P. MacGowan. 1999. Use of a clinical Escherichia coli isolate expressing lux genes to study the antimicrobial pharmacodynamics of moxifloxacin. J. Antimicrob. Chemother. 43:829-832. [DOI] [PubMed] [Google Scholar]
- 37.Schwank, S., Z. Rajacic, W. Zimmerli, and J. Blaser. 1998. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob. Agents Chemother. 42:895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon, L., C. Fremaux, Y. Cenatiempo, and J. M. Berjeaud. 2001. Luminescent method for the detection of antibacterial activities. Appl. Microbiol. Biotechnol. 57:757-763. [DOI] [PubMed] [Google Scholar]
- 39.Svensson, E., H. Hanberger, M. Nilsson, and L. E. Nilsson. 1997. Factors affecting development of rifampicin resistance in biofilm-producing Staphylococcus epidermidis. J. Antimicrob. Chemother. 39:817-820. [DOI] [PubMed] [Google Scholar]
- 40.Tenhami, M., K. Hakkila, and M. Karp. 2001. Measurement of effects of antibiotics in bioluminescent Staphylococcus aureus RN4220. Antimicrob. Agents Chemother. 45:3456-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulitzur, S. 1986. Determination of antibiotic activities with the aid of luminous bacteria. Methods Enzymol. 133:275-284. [DOI] [PubMed] [Google Scholar]
- 42.Van Wijngaerden, E., W. E. Peetermans, J. Vandersmissen, S. Van Lierde, H. Bobbaers, and J. Van Eldere. 1999. Foreign body infection: a new rat model for prophylaxis and treatment. J. Antimicrob. Chemother. 44:669-674. [DOI] [PubMed] [Google Scholar]
- 43.Vogelman, B., S. Gudmundsson, J. Turnidge, J. Leggett, and W. A. Craig. 1988. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J. Infect. Dis. 157:287-298. [DOI] [PubMed] [Google Scholar]
- 44.Widmer, A. F., R. Frei, Z. Rajacic, and W. Zimmerli. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96-102. [DOI] [PubMed] [Google Scholar]
- 45.Zak, O., W. M. Scheld, and M. A. Sande. 1983. Rifampin in experimental endocarditis due to Staphylococcus aureus in rabbits. Rev Infect. Dis. 5(Suppl. 3):S481-S490. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerli, W., F. A. Waldvogel, P. Vaudaux, and U. E. Nydegger. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J. Infect. Dis. 146:487-497. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, P. E. Ochsner, et al. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]