Abstract
The in vitro activities of human β-defensin 3 (hBD-3) alone or combined with lysozyme, metronidazole, amoxicillin, and chlorhexidine were investigated with the oral bacteria Streptococcus mutans, Streptococcus sanguinis, Streptococcus sobrinus, Lactobacillus acidophilus, Actinobacillus actinomycetemcomitans, and Porphyromonas gingivalis. hBD-3 showed bactericidal activity against all of the bacterial species tested. The bactericidal effect was enhanced when the peptide was used in combination with the antimicrobial agents mentioned above.
Human defensins are small cationic, cysteine-rich peptides with a wide range of antimicrobial activities (9). They include four α- and four β-defensins, which can be distinguished according to the localization of the cysteine residues and their participation in three disulfide linkages (9). Human β-defensin 3 (hBD-3) has recently been isolated from human psoriatic scales (3, 5) and is widely expressed in skin, placenta, tongue, and other oral tissues (2, 3, 5, 6). hBD-3 is active against a number of human pathogens, including multiresistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium (5).
In addition to their potential antimicrobial role in vivo, defensins are currently the objects of extensive studies to assess their possible use as a new class of antibiotics. Possible problems in stability, delivery, and pathogen targeting may arise in the use of defensins as antimicrobial agents, but they have clear advantages over traditional antibiotics, including a broad activity range, quick bacterial killing by the physical disruption of cell membranes, activity against antibiotic-resistant pathogens, and relative difficulty in selection of resistant mutants in vitro (4).
The aims of this study were (i) to evaluate the antibacterial activity of hBD-3 in vitro against various oral bacteria involved in cariogenesis or in periodontal diseases; and (ii) to investigate over time the in vitro bactericidal activity of hBD-3 in association with some antibiotics (amoxicillin and metronidazole), an oral disinfectant (chlorhexidine), or with lysozyme, an antimicrobial protein that is naturally present in saliva.
The antimicrobial activity of hBD-3 was evaluated by a liquid microdilution assay, modified for cationic peptides (5, 8), against the following bacterial strains: Porphyromonas gingivalis ATCC 49417; Actinobacillus actinomycetemcomitans ATCC 43717; Streptococcus mutans serotype c, reference strain T282 (1); Streptococcus sobrinus serotype g, reference strain TH21 (1); and clinical isolates of Streptococcus sanguinis (strain VC01) and Lactobacillus acidophilus (strain VY01).
P. gingivalis was grown in an anaerobic atmosphere at 37°C in tryptone soy broth (TSB) (Oxoid, Basingstoke, England) with 0.1% yeast extract, 5 μg of hemin (Sigma, St. Louis, Mo.) per ml, 10 μg of menadione (Sigma) per ml, and 0.05% l-Cys HCl (Sigma). A. actinomycetemcomitans, S. mutans, S. sobrinus, S. sanguinis, and L. acidophilus were grown in TSB with 0.5% yeast extract at 37°C or 34°C (A. actinomycetemcomitans). Synthetic hBD-3 (Sigma Genosys) was diluted in 0.1% acetic acid to obtain a stock solution of 1 mg/ml.
Exponentially growing bacteria were resuspended in 10 mM sodium phosphate buffer (pH 7.4) to reach a density of 5 × 107 CFU/ml. Ten microliters of each bacterial suspension was exposed for 1.5 h under the appropriate culture condition to different concentrations of hBD-3 in 100 μl of 10 mM sodium phosphate buffer (pH 7.4) with or without 1% (vol/vol) TSB. Because preliminary data had demonstrated a two- to fourfold decrease in hBD-3 activity in the presence of 1% TSB, we investigated whether the bactericidal assay could be performed in the absence of medium. The lack of TSB in the assay buffer caused a significant reduction in the numbers of S. sobrinus, S. sanguinis, L. acidophilus, and P. gingivalis CFU even in the absence of hBD-3, while it did not affect the vitality of A. actinomycetemcomitans and S. mutans. Therefore, bactericidal assays without TSB were carried out only with the latter two species. At the end of the incubation period, 10-fold dilutions of each sample were performed in TSB, and 0.2 ml of each dilution was plated in duplicate onto the appropriate agar media. The number of CFU was determined after incubation of the plates for 3 or 6 (P. gingivalis) days under the culture conditions described above. Bactericidal activity was defined as a reduction in viable bacteria of ≥3 log10 CFU/ml after 1.5 h of incubation.
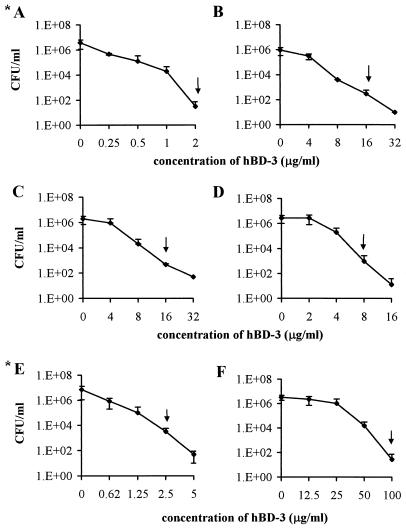
hBD-3 demonstrated a broad spectrum of bactericidal activity towards all the bacterial species tested. The minimal concentrations of peptide that induced a reduction of at least 3 log10 after 1.5 h of incubation were as follows: S. mutans, 2 μg/ml; S. sobrinus and S. sanguinis, 16 μg/ml; L. acidophilus, 8 μg/ml; A. actinomycetemcomitans, 2.5 μg/ml; and P. gingivalis, 100 μg/ml (Fig. 1).
FIG. 1.
Bactericidal activity of hBD-3 against S. mutans (A), S. sobrinus (B), S. sanguinis (C), L. acidophilus (D), A. actinomycetemcomitans (E), and P. gingivalis (F). Arrows indicate the minimal peptide concentration able to induce a reduction in the number of CFU of at least 3 log10 after 1.5 h of incubation. *, bactericidal assays carried out without 1% TSB in the assay volume.
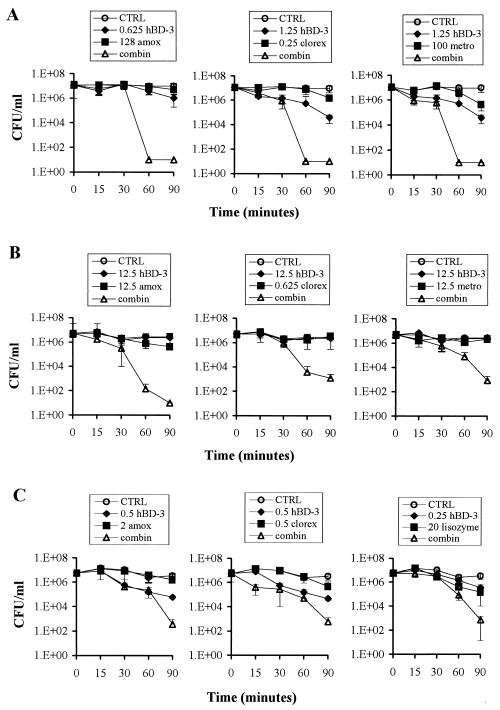
We selected A. actinomycetemcomitans, P. gingivalis, and S. mutans to evaluate the bactericidal effect of hBD-3 when used in combination with lysozyme, amoxicillin, metronidazole, or chlorhexidine (all agents from Sigma). The peptide and antimicrobial agent combinations were tested in the bactericidal assay described above by a checkerboard method in order to identify those combinations able to cause an enhanced bactericidal effect, which was defined as a ≥2-log10 decrease in CFU per milliliter between the combination and its most active constituent. The combinations able to exhibit this enhanced bactericidal effect at the lowest concentration of antibacterial agent were selected to carry out time-kill assays. As illustrated in Fig. 2A, combinations of nonbactericidal concentrations of hBD-3 and amoxicillin, chlorhexidine, and metronidazole killed A. actinomycetemcomitans after only 1 h of incubation. Similar results were obtained when hBD-3 was tested in combination with amoxicillin and chlorhexidine against P. gingivalis (Fig. 2B).
FIG. 2.
Time-kill curves of hBD-3 in combination with the indicated antimicrobial agents against A. actinomycetemcomitans (A), P. gingivalis (B), and S. mutans (C). The concentrations of hBD-3 and the antimicrobial agents reported in the figure are expressed in micrograms per milliliter. Bacteria incubated in the absence of both hBD-3 and antimicrobial agents represent controls (CTRL). clorex, chlorhexidine; amox, amoxicillin; metro, metronidazole; combin, combination of hBD-3 with the antimicrobial agent.
In contrast, an enhanced bactericidal effect was observed, but not before 1.5 h of incubation of the peptide in combination with metronidazole against P. gingivalis (Fig. 2B) and with amoxicillin, chlorhexidine, and lysozyme against S. mutans (Fig. 2C).
To date, only β-defensin 2 has been tested and has shown activity against oral bacteria (7). Among the α-defensins, only human neutrophil peptides 1 (hNP-1), 2, and 3 have been assayed against A. actinomycetemcomitans, P. gingivalis, and S. mutans (8). The results showed that all three bacterial species were naturally resistant to these α-defensins, while they were susceptible to similar modified forms of hNP-1 in which two hydrophobic amino acids at the N terminus and two basic residues at the C terminus had been inserted (8). Interestingly, a similar amino acid profile has been reported for hBD-3, which presents three nonpolar amino acid residues at the N terminus and four basic residues at the C terminus (3, 5). This observation further supports the hypothesis that the N- and C-terminal residues in defensin peptides are the crucial determinants of their microbicidal activity (8). The different susceptibilities of the oral bacteria to hBD-3 observed in the present study do not seem to be due to structural differences between gram-positive and gram-negative bacteria. Rather, these findings could be due to slight differences among different species in the charges of molecules on the bacterial surfaces, which could facilitate the first step of defensin-bacterium interaction by electrostatic and ionic strength. The low susceptibility of P. gingivalis to hBD-3 could be due to its known ability to secrete proteases (10).
The results of the present study demonstrate an enhanced bactericidal effect of hBD-3 with several antibacterial compounds exerting different modes of action. It can be argued that drugs causing a breakdown of the bacterial cell wall (amoxicillin and lysozyme) or damaging the outer membrane (chlorhexidine) might facilitate the access of hBD-3 to the cytoplasmic membrane, which represents the main site of action of the peptide (4). On the other hand, increased permeability of the outer and/or inner membranes caused by the peptide could facilitate the entry of antibiotics with an intracytoplasmic target (metronidazole and chlorhexidine).
In conclusion, our results demonstrate that hBD-3 is active against oral bacteria and exerts an enhanced bactericidal effect when used in combination with other antimicrobial agents, suggesting a future use of the peptide in association with clinically used drugs in the local therapy of oral infections.
Acknowledgments
This work was supported by grants from “Progetti M.I.U.R.” prot. 2002067349_001, Rome (Italy).
REFERENCES
- 1.Batoni, G., F. Marchetti, F. Ota, E. Ghelardi, S. Barnini, S. Inoue, C. Uchiyama, K. Hirota, Y. Minato, M. R. Giuca, M. Gabriele, M. Campa, and S. Senesi. 1993. First characterisation in Italy of clinical isolates of mutans streptococci by using specific monoclonal antibodies. Eur. J. Epidemiol. 9:483-488. [DOI] [PubMed] [Google Scholar]
- 2.Dunsche, A., Y. Acil, H. Dommisch, R. Siebert, J. M. Schroder, and S. Jepsen. 2002. The novel human beta-defensin-3 is widely expressed in oral tissues. Eur. J. Oral Sci. 109:121-124. [DOI] [PubMed] [Google Scholar]
- 3.Garcia, J. R. C., F. Jaumann, S. Schultz, A. Krause, J. Rodriguez-Jimenez, U. Forssmann, K. Adermann, E. Kluver, C. Vogelmeier, W. G. Forssmann, and R. Bals. 2001. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 306: 257-264. [DOI] [PubMed]
- 4.Hancock, R. E. W. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 5.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 6.Hong Peng, J., B. C. Schuttle, A. Schudy, R. Linzmeier, J. M. Guthmiller, G. K. Johnson, B. F. Tack, J. P. Mitros, A. Rosenthal, T. Ganz, and P. B. McCray, Jr. 2001. Discovery of new human β-defensin using a genomics-based approach. Gene 263:211-218. [DOI] [PubMed] [Google Scholar]
- 7.Mineshiba, F., S. Takashiba, J. Mineshiba, K. Matsuura, S. Kokeguchi, and Y. Murayama. 2003. Antibacterial activity of synthetic human β defensin-2 against periodontal bacteria. J. Int. Acad. Periodontol. 5:35-40. [PubMed] [Google Scholar]
- 8.Raj, P. A., K. J. Antonyraj, and T. Karunakaran. 2000. Large-scale synthesis and functional elements for the antimicrobial activity of defensins. Biochem. J. 347:633-641. [PMC free article] [PubMed] [Google Scholar]
- 9.Ryley, H. C. 2001. Human antimicrobial peptides. Rev. Med. Microbiol. 12:177-186. [Google Scholar]
- 10.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]