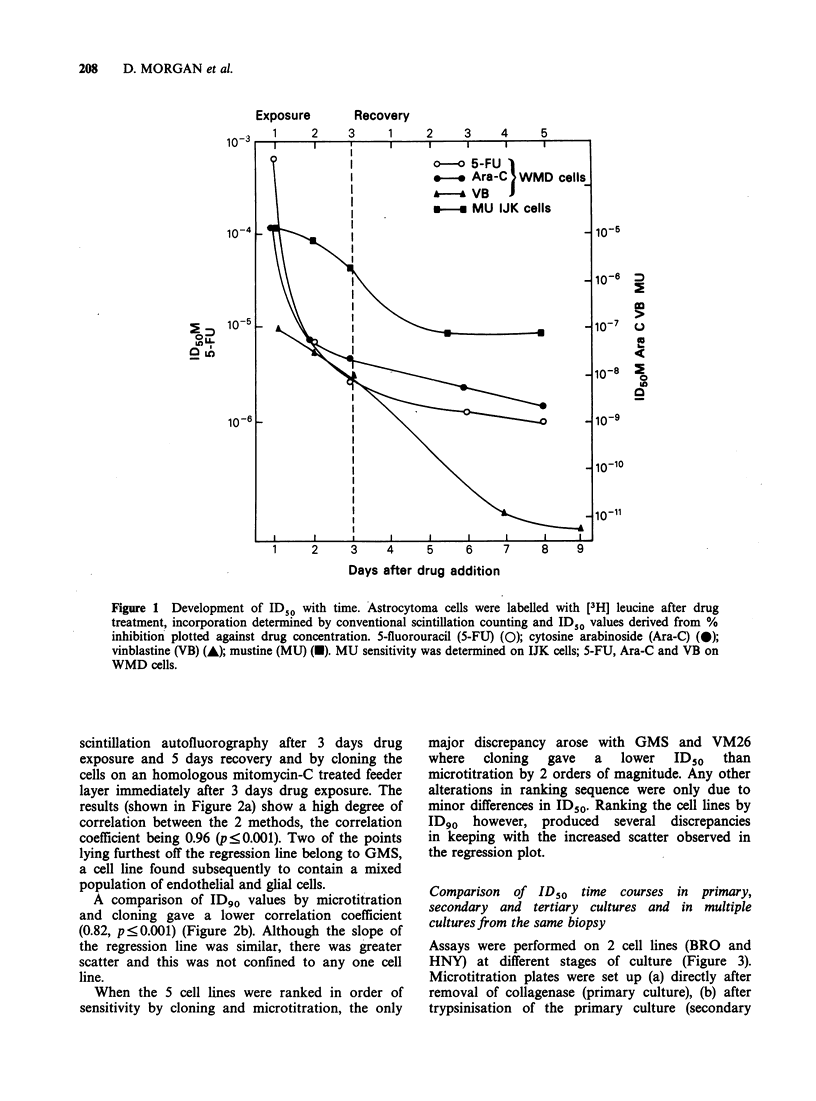
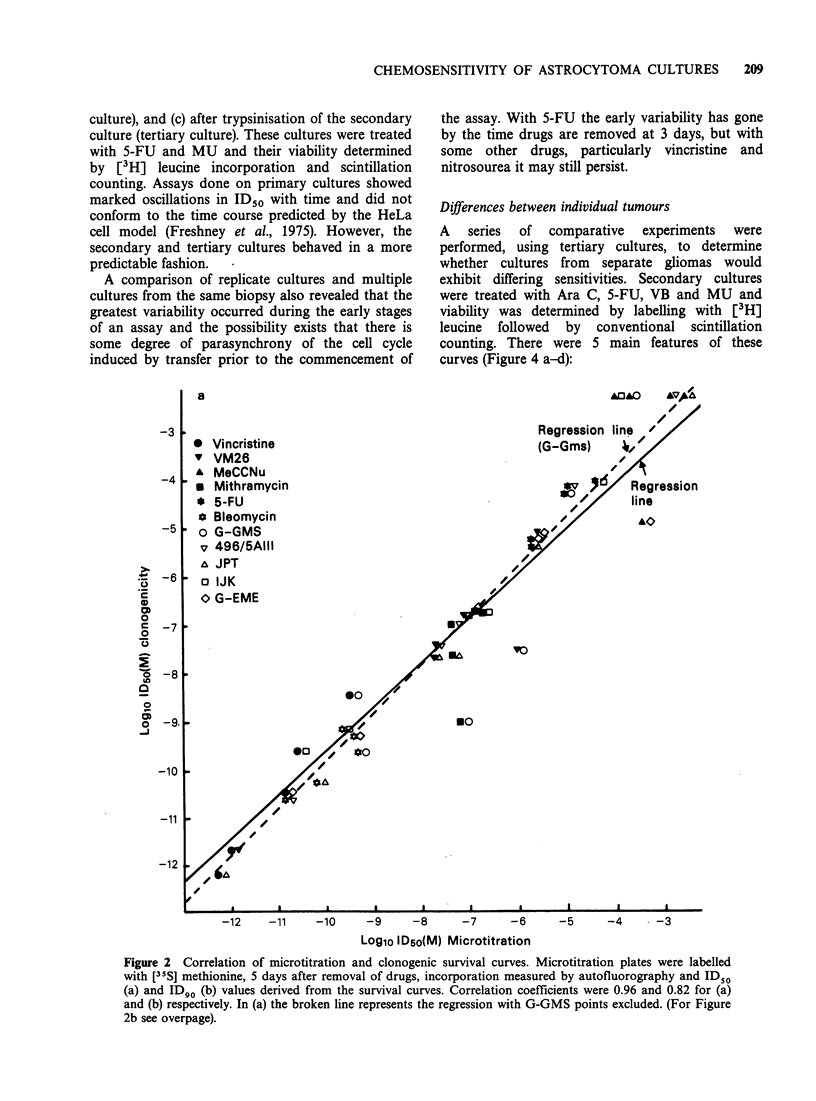
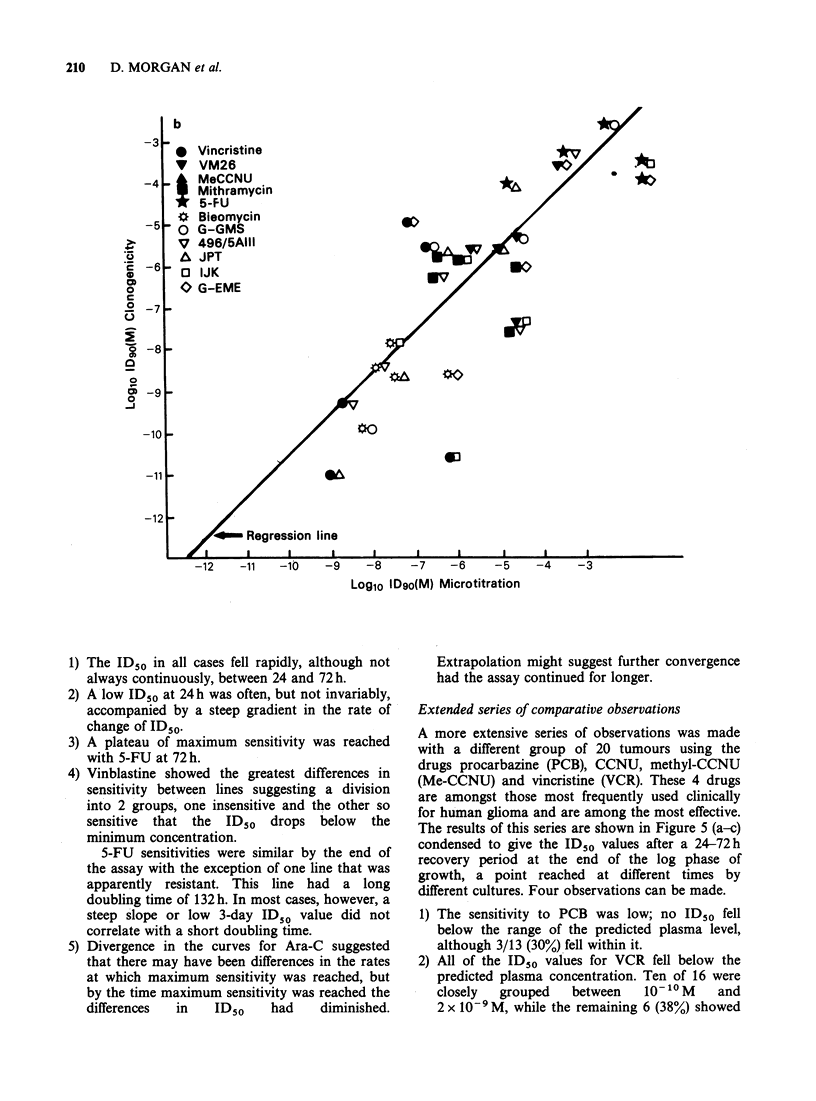
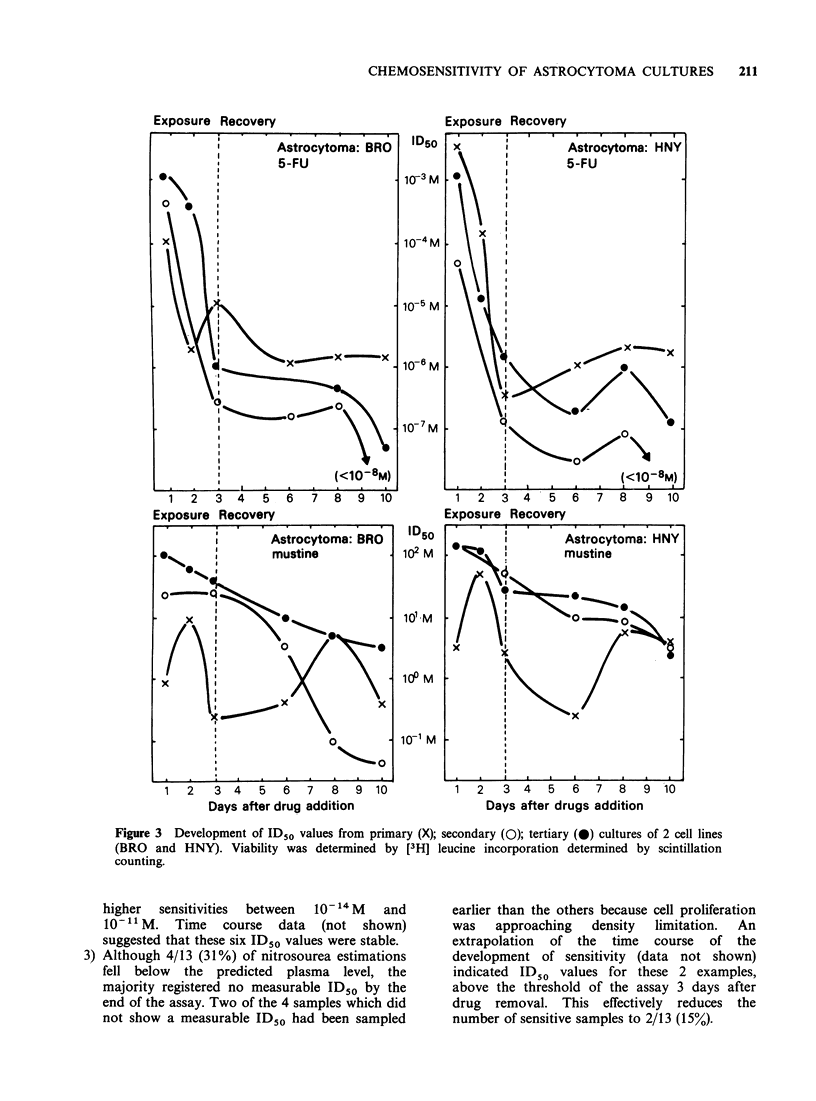
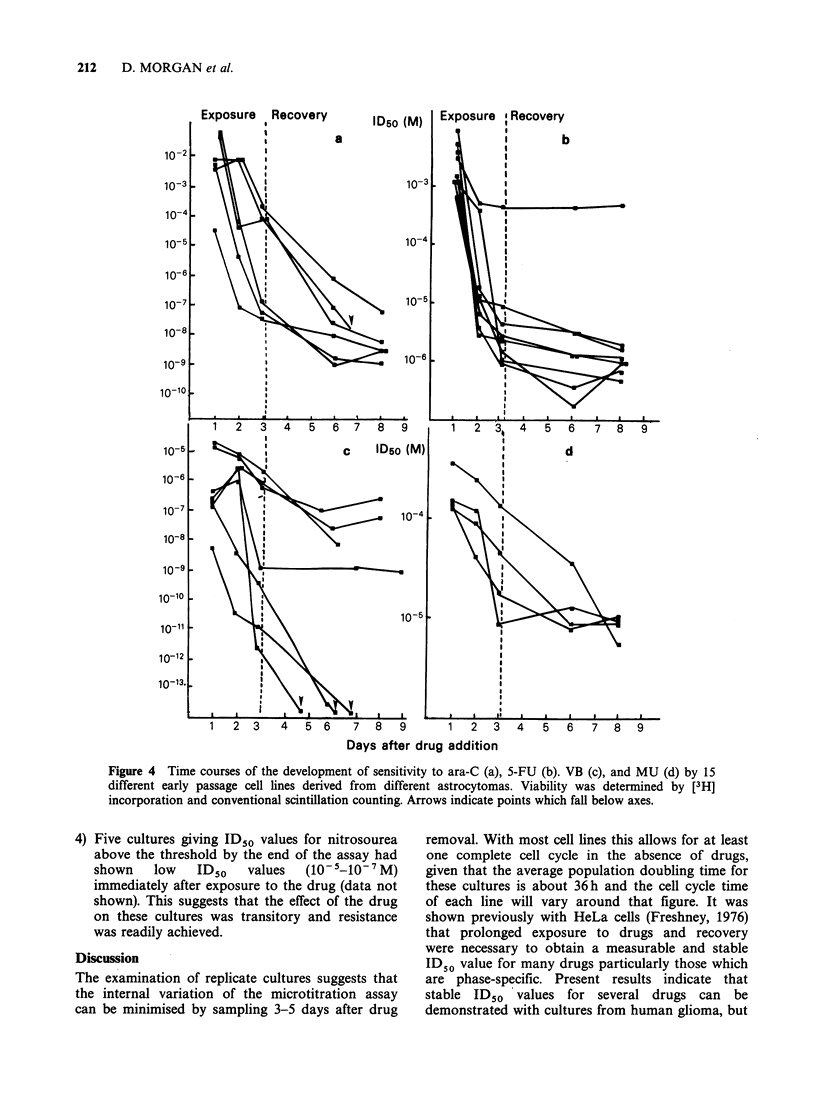
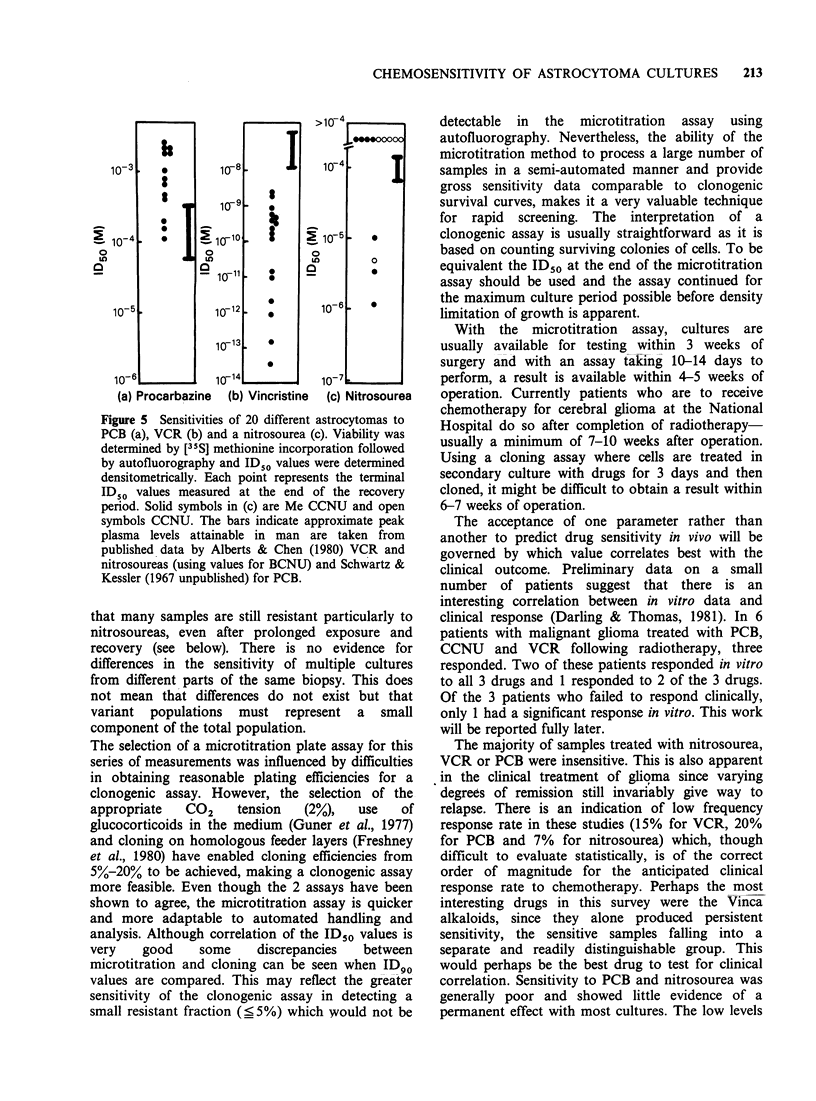
Abstract
A method has been developed for measuring the drug sensitivity of human gliomas in short-term culture, using scintillation counting or autofluorography. Cell cultures prepared from malignant astrocytomas were treated with anticancer drugs whilst in exponential growth in microtitration plates. After drug treatment and a recovery period, residual viability was measured by [3H] leucine incorporation followed by scintillation counting or by [35S] methionine incorporation and autofluorography in situ. In 5 glioma cell lines tested against 6 drugs, the microtitration method correlated well with monolayer cloning. Although replicate samples of the same tumour showed little variation in chemosensitivity, there was marked variation between the chemosensitivities of cultures derived from the tumours of different patients. However, as variability between replicates was apparent during drug exposure or shortly after, it is important to allow the assay to run as long as possible after drug removal. It is hoped that this assay may provide the basis of a method for the prediction of in vivo chemosensitivity or the screening of potential chemotherapeutic drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts D. S., Chen H. S. Tabular summary of pharmacokinetic parameters relevant to in vitro drug assay. Prog Clin Biol Res. 1980;48:351–359. [PubMed] [Google Scholar]
- Barranco S. C., Novak J. K., Humphrey R. M. Response of mammalian cells following treatment with bleomycin and 1,3-bis(2-chloroethyl)-1-nitrosourea during plateau phase. Cancer Res. 1973 Apr;33(4):691–694. [PubMed] [Google Scholar]
- Freshney R. I., Morgan D. Radioisotopic quantitation in microtitration plates by an autofluorographic method. Cell Biol Int Rep. 1978 Jul;2(4):375–380. doi: 10.1016/0309-1651(78)90023-1. [DOI] [PubMed] [Google Scholar]
- Freshney R. I., Paul J., Kane I. M. Assay of anti-cancer drugs in tissue culture: conditions affecting their ability to incorporate 3H-leucine after drug treatment. Br J Cancer. 1975 Jan;31(1):89–99. doi: 10.1038/bjc.1975.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney R. I., Sherry A., Hassanzadah M., Freshney M., Crilly P., Morgan D. Control of cell proliferation in human glioma by glucocorticoids. Br J Cancer. 1980 Jun;41(6):857–866. doi: 10.1038/bjc.1980.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guner M., Freshney R. I., Morgan D., Freshney M. G., Thomas D. G., Graham D. I. Effects of dexamethasone and betamethasone on in vitro cultures from human astrocytoma. Br J Cancer. 1977 Apr;35(4):439–447. doi: 10.1038/bjc.1977.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knock F. E., Galt R. M., Oester Y. T., Sylvester R. In vitro estimate of sensitivity of individual human tumors to antitumor agents. Oncology. 1974;30(1):1–22. doi: 10.1159/000224937. [DOI] [PubMed] [Google Scholar]
- Limburg H., Heckmann U. Chemotherapy in the treatment of advanced pelvic malignant disease with special reference to ovarian cancer. J Obstet Gynaecol Br Commonw. 1968 Dec;75(12):1246–1255. doi: 10.1111/j.1471-0528.1968.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Nissen E., Tanneberger S., Projan A., Morack G., Peek U. Recent results of in vitro drug prediction in human tumour chemotherapy. Arch Geschwulstforsch. 1978;48(7):667–672. [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W., Soehnlen B., Durie B. G., Alberts D. S., Moon T. E. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978 Jun 15;298(24):1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]


