Abstract
gp130 is a common signal-transducing receptor component for the interleukin 6 family of cytokines functioning in, for example, immune, hematopoietic, and nervous systems. In this study, to investigate the physiological functions of gp130 and to determine the pathological consequences of impaired gp130 signals, we have generated transgenic mice expressing a cytoplasmically truncated form of mouse gp130. Expression of this form of gp130 in lymphocytes significantly suppressed interleukin 6-induced tyrosine phosphorylation of endogenous gp130 and a downstream signaling molecule, signal transducer and activator of transcription 3, indicating that this form has a dominant negative function. In spite of the impaired gp130 signals, the development of lymphocytes in the transgenic mice appeared normal in terms of surface marker phenotypes. These mice, however, exhibited severe defects in antigen-specific antibody production of most immunoglobulin isotypes other than IgM after immunization with 2,4-dinitrophenol-conjugated ovalbumin. These results demonstrate in vivo that gp130 is essential for antigen-specific antibody production.
Keywords: cytokine receptor, Ig production
Interleukin (IL) 6 is a multifunctional cytokine that plays important roles in host defense. Its biological activities include growth promotion, growth inhibition, specific gene expression, and induction of differentiation, depending on the target cells (1). These biological activities are mediated by the specific receptor complex that consists of two functionally different subunits: the ligand-binding IL-6 receptor (IL-6R) chain and the non-ligand-binding signal transducer, gp130 (2–5). The latter is now known as a common signal-transducing receptor component for IL-6, ciliary neurotrophic factor, leukemia inhibitory factor, oncostatin M, interleukin 11, and cardiotrophin-1, which explains the functional overlap of these cytokines (6–10). After binding to their specific receptor chains, all these ligands induce homo- or heterodimerization of gp130 (11, 12). This initiates cytoplasmic signaling cascades by activating associated protein kinases in the Janus kinases family and a latent cytoplasmic transcriptional factor, signal transducer and activator of transcription 3 (STAT3) (13–16).
gp130 is ubiquitously expressed in all organs examined (17). In contrast, the expression of the ligand-binding receptor chains is somewhat restricted. Even tissues that do not express a receptor for IL-6 or other gp130-stimulatory cytokines express gp130, suggesting that gp130 might be able to function as a signal transducer for unknown cytokines (18). Although mice lacking IL-6, leukemia inhibitory factor, or ciliary neurotrophic factor have been generated (19–22), they manifest considerably less severe phenotypes than would be expected from their previously reported pleiotropic functions determined in vitro. This is probably because the functions of one cytokine can be compensated for by other gp130-stimulatory cytokines that have overlapping biological functions. Recently, to determine the biological roles of gp130 in vivo, we generated mice lacking gp130 by gene targeting and showed that they were embryonically lethal (23). During embryogenesis, gp130-deficient embryos had multiple defects (e.g., in the development of ventricular myocardium and hematopoietic progenitors). These multiple defects represented the diverse activities of gp130. However, the detailed physiological functions of gp130 are not considered to have been fully elucidated, especially in tissues that develop in late embryogenesis or after birth, since complete disruption of gp130 molecules by gene targeting causes embryonic lethality as described above. These findings therefore have led to the necessity of generating viable mice where the functions of gp130 are impaired, which should be useful for the better understanding of the roles of gp130 and for getting a clue to find out a disease for which abnormality in gp130 is responsible.
In this study, we have created transgenic mice overexpressing a dominant-negative form of gp130 and focused on the immunological analyses. These mice showed severe impairment of antigen-specific antibody production, although the development of lymphocytes appeared virtually normal.
MATERIALS AND METHODS
Transgene Construction and Production of Transgenic Mice.
A truncated mouse gp130 cDNA was generated by a site-directed mutagenesis kit (CLONTECH) converting Cys-702 to a stop codon (TGA). The generated mutant gp130 cDNA contains the complete extracellular and transmembrane regions, and a 63-aa intracellular juxtamembrane portion. The wild-type (WT) as well as the truncated mouse gp130 cDNA was inserted into the unique XhoI site of pCAGGS, which carries the chicken β-actin gene promoter (24). The vectors were linearlized by SalI and NotI enzymes and microinjected into the pronuclei of fertilized C57BL/6 mouse eggs. Mice were obtained as described elsewhere (25).
Immunoblot Analysis and Antibodies.
Splenocytes were solubilized with lysis buffer containing 0.5% Nonidet P-40, 10 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 1 mM Na2VO3, and 5 μg/ml aprotinin. The lysates were immunoprecipitated with anti-mouse gp130 (mgp130) mAb and protein A-Sepharose (Pharmacia). Then, immune complexes were analyzed by SDS/PAGE and subsequent immunoblotting with anti-mgp130 polyclonal Ab or antiphosphotyrosine mAb using the Amersham ECL (enhanced chemiluminescence) system. mAb (RX435; rat IgG2a) and polyclonal rabbit antiserum against mouse gp130, which specifically recognize mgp130, were established in this laboratory. Rabbit antiserum against STAT3 was also described elsewhere (15). Antiphosphotyrosine mAb (4G10) was purchased from Upstate Biotechnology.
Flow Cytometry and Antibodies.
Flow cytometric analysis of single-cell suspension from thymus and spleen was performed using a FACScan (Becton Dickinson). The antibodies used for staining were biotinylated anti-IgM, fluorescein isothiocyanate (FITC)-anti-B220 (RA3–6B2), phycoerythrin (PE)-conjugated anti-Thy-1 (30-H12), FITC-anti-CD8 (53–6.7, PharMingen), and PE-conjugated anti-CD4 (RM-4–5, PharMingen).
Assay for IL-6-Aided Anamnestic Antibody Response to Sheep Erythrocytes (SRBCs).
SRBCs were i.v. injected (1 × 108 cells per mouse). Three days later, SRBC-primed splenocytes (5 × 105 cells per well) were cultured with SRBCs (1 × 105 cells per well) in the presence or absence of IL-6 in 96-well flat-bottomed plates in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, 100 μg/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 5 × 10−5 M 2-mercaptoethanol (hereafter referred to as complete medium). After 3 days in culture, cells were harvested, washed twice with Hanks’ medium, and resuspended in 100 μl of the same medium, and then direct plaque-forming cell assays were performed as described elsewhere (26).
Thymocyte Proliferation Assay.
Fresh thymocytes were seeded at a density of 5 × 105 cells per well in 0.2 ml of complete culture medium as described above. Phytohemagglutinin and IL-1 were purchased from Difco and Genzyme, respectively. For proliferation assays, thymocytes were incubated at 37°C for 3 days, and 1 μCi (1 Ci = 37 GBq) of [3H]thymidine in 20 μl of medium was added to each well 16 hr before harvest.
Immunization of Mice with 2,4-Dinitrophenol-Conjugated Ovalbumin (DNP-OVA).
Six-week-old mice were immunized i.p. with 100 μg of DNP-OVA in complete Freund’s adjuvant on day 0 and boosted at day 21. Animals were bled before immunization and at day 7, 14, and 28. DNP-specific antibody titers of sera were measured as described below.
ELISA for Antigen-Specific Immunoglobulins.
To quantify DNP-specific antibodies, serum samples were added to the 96-well flat-bottomed microtiter plates coated with DNP-conjugated bovine serum albumin in PBS, and bound antibodies were detected using alkaline phosphatase-labeled, isotype-specific antibodies (Southern Biotechnology Associates).
Studies at each institution were performed according to its guidelines for animal use and care.
RESULTS
Generation of Transgenic Mice Expressing Truncated or WT gp130.
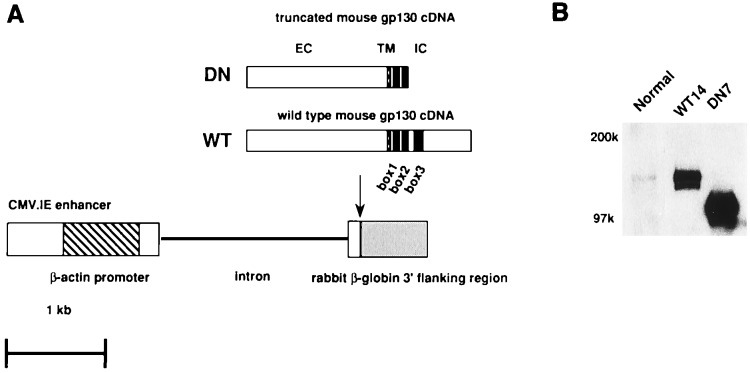
We prepared two gp130 constructs for generating transgenic mice as shown in Fig. 1A. One was for expressing a truncated form of gp130 containing only 63 aa with box1 and box2 motifs of the intracellular juxtamembrane region, in which the membrane distal part, containing the box3, was deleted. The deleted part in the C-terminal region that includes box3 is considered to play critical roles in gp130-mediated biological responses, such as proliferation of a mouse pro-B cell line, BAF-B03, differentiation of mouse myeloid leukemic M1 cells into macrophages, and acute phase protein production in hepatoma cell lines (27–29). This portion is known to be necessary for STAT3 activation (30), which has been proven to play a critical role in these biological responses. The other construct was to produce control transgenic mice expressing WT gp130. Offspring from eggs microinjected with these fragments were selected by PCR and Southern blot analysis. We established five founders (DN7, 8, 10, 17, and 18) carrying the truncated form of gp130 and three founders (WT10, 12 and 14) carrying the WT gp130. All the established founders expressed high levels of the transgenes under the control of cytomegalovirus enhancer/chicken β-actin promoter as assessed by Western blot analysis. Fig. 1B shows the expression of the transgenes in the representative lines, WT14 and DN7.
Figure 1.
Diagram of transgene constructs and expression of the transgenes. (A) Schematic structures of transgene constructs. Functional elements include the enhancer of the cytomegalovirus (open box), the promoter of the chicken β-actin (diagonally striped box), the coding sequence of the truncated or WT mouse gp130 cDNA including the ATG translation initiation codon and the TGA translation stop codon (black box), and rabbit β-globulin poly A (shaded box). The WT and truncated form of gp130 contain full (277 aa) or the membrane-proximal 63-aa part of the cytoplasmic region of gp130. The truncated form of gp130 did not have the membrane distal part containing box3. (B) Expression of truncated or WT gp130 in splenocytes. Nonidet P-40 lysates from splenocytes were immunoprecipitated with anti-mgp130 mAb (RX435) and immunoblotted with rabbit anti-mouse gp130 polyclonal Ab. NC, normal control mice; WT, transgenic mice expressing wild-type gp130; DN, transgenic mice expressing the truncated form of gp130.
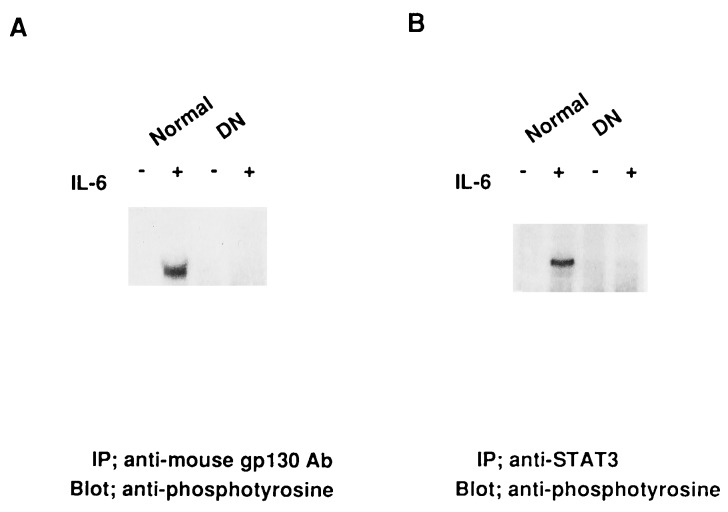
Tyrosine Phosphorylation of Endogenous gp130 and STAT3 Was Severely Impaired in Transgenic Mice Expressing the Truncated Form of gp130.
Tyrosine phosphorylation of gp130 is considered to be an initial critical step for gp130-mediated signal transduction (11). We examined the influence of overexpression of the truncated form of gp130. As shown in Fig. 2, the tyrosine phosphorylation of endogenous gp130 and STAT3 after stimulation with IL-6 was severely impaired at least under this stimulatory condition in splenocytes from transgenic mice expressing the truncated form of gp130. These results indicate that the truncated form of gp130 has a dominant-negative function over the endogenous gp130, which suppressed the tyrosine phosphorylation of a downstream molecule, STAT3. Hereafter, we refer to the transgenic mice expressing the truncated form of gp130 as DN transgenic mice.
Figure 2.
Impaired tyrosine phosphorylation of gp130 and STAT3 in transgenic mice expressing a truncated form of gp130. Splenocytes (1 × 107 cells) were stimulated with IL-6 (100 ng/ml) plus soluble form of IL-6R (200 ng/ml) for 10 min, and solubilized in Nonidet P-40 lysis buffer as described. The lysates were immunoprecipitated with RX435 or rabbit anti-STAT3 serum, then immunoblotted with antiphosphotyrosine (4G10).
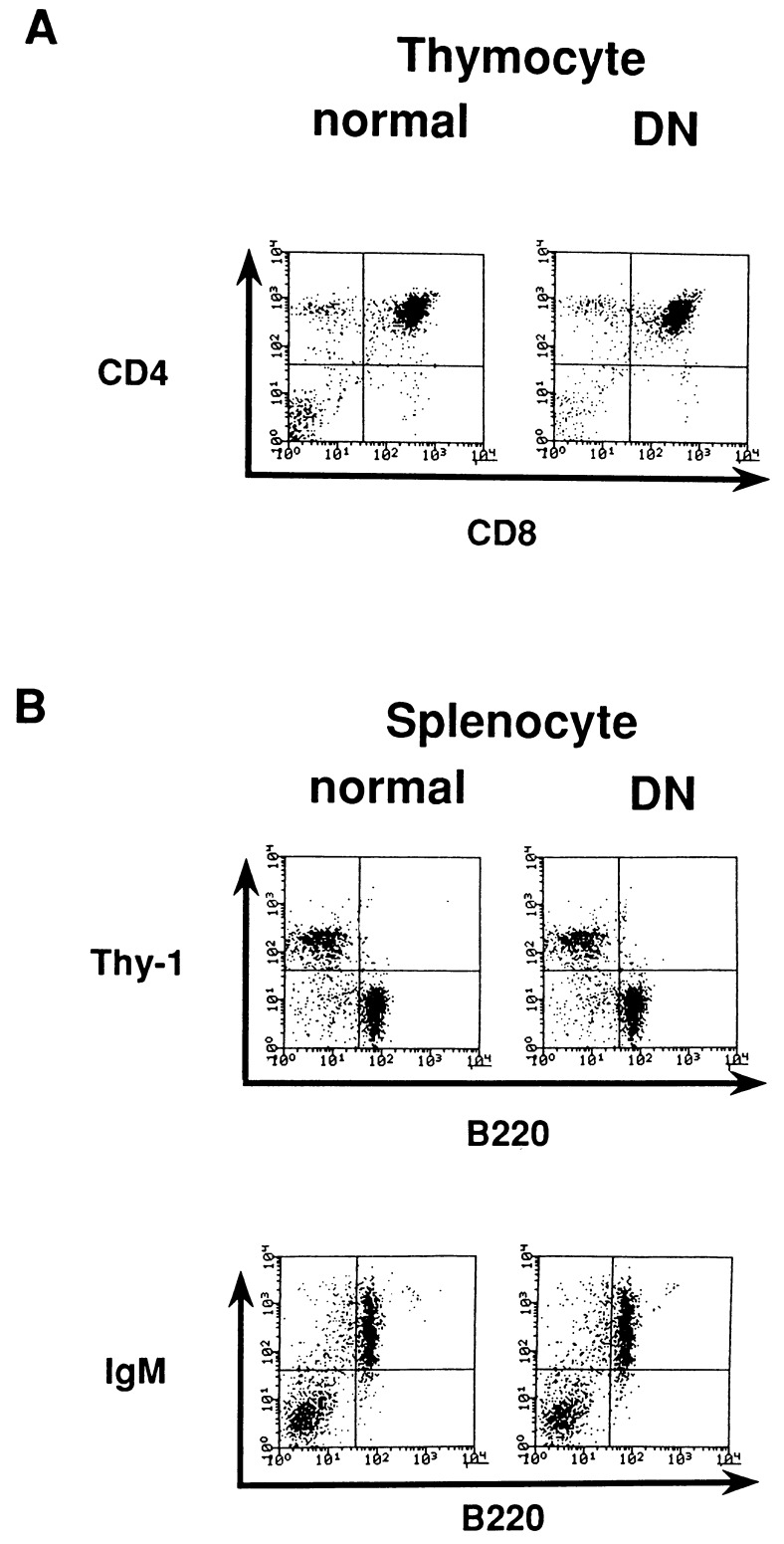
Development of Lymphocytes in DN Transgenic Mice.
The numbers of thymocytes and splenocytes were lower in the DN than in the WT (thymocytes, 70% ± 15; splenocytes, 75% ± 10, n = 11, compared with the WT transgenic mice). The extent of the reduction varied among mice. We then performed a flow cytometric analysis of splenocytes and thymocytes to determine whether or not the expression of the dominant-negative form of gp130 affected lymphocyte development. As shown in Fig. 3, no major differences in the subsets of splenocytes and thymocytes were detected in terms of surface marker phenotypes as examined for B220, IgM, Thy-1, CD4, and CD8. These results suggest that the development of lymphocytes are not largely dependent on the gp130 signals, although the impairment of gp130 signals somewhat affected the lymphocyte cell number.
Figure 3.
Development of lymphocytes in DN transgenic mice. (A) Thymocytes from 4-week-old mice were stained with FITC-anti-CD8 and PE-anti-CD4. (B) Splenocytes freshly prepared from 6-week-old mice were stained with the following: FITC-anti-B220 and PE-conjugated anti-Thy-1; and FITC-anti-B220 and biotinylated anti-IgM + PE-conjugated avidin.
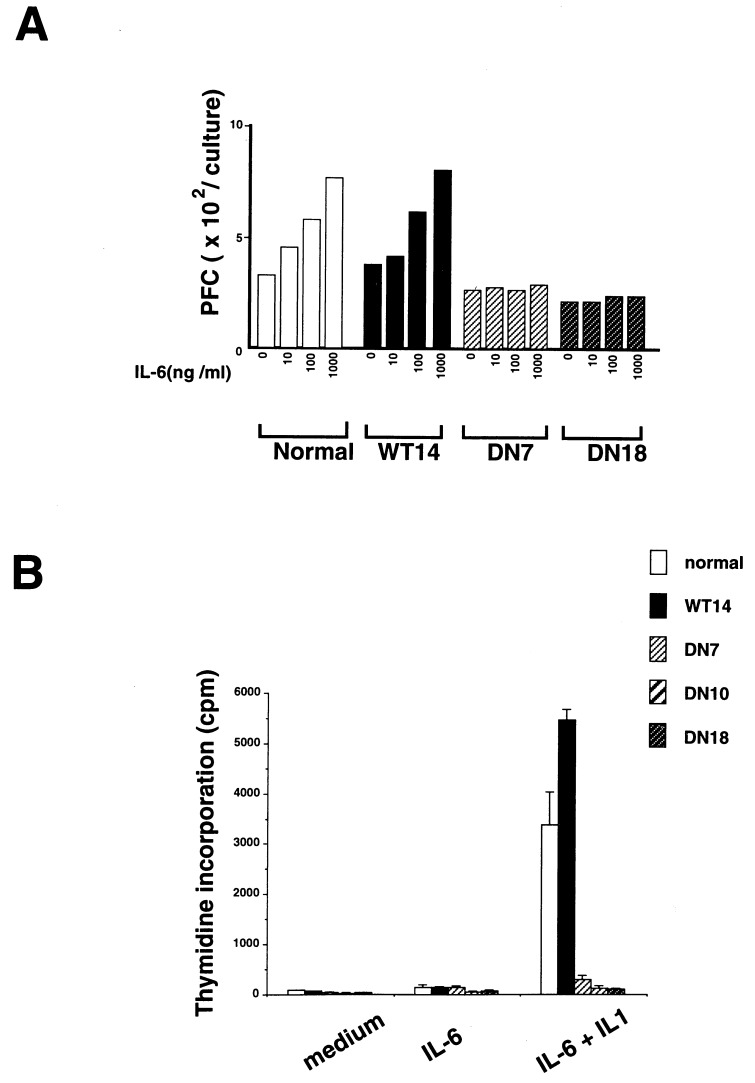
Lymphocytes from the Transgenic Mice Expressing the Dominant-Negative Form of gp130 Do Not Respond to IL-6.
IL-6 augments the production of anti-SRBC antibody production of IgM, IgG, and IgA isotypes in SRBC-primed mouse B cells in vitro (31). We first examined the influence of the dominant-negative form of gp130 in B cell responses in vitro. As shown in Fig. 4A, IL-6 did not induce any augmentation of anti-SRBC-specific antibody production in DN transgenic mice as assessed by direct PFC, although IL-6 enhanced the anamnestic responses in SRBC-primed B cells from both normal and WT transgenic mice. IL-6 also acts as a potent costimulant of thymocyte proliferation in association with IL-1 (32, 33). We examined the response of thymocytes from DN transgenic mice to IL-6 plus IL-1. Fig. 4B shows that the proliferation of thymocytes in response to IL-6 plus IL-1 was almost completely abrogated in the DN, but not in WT transgenic mice. In contrast, the responses of B cells to bacterial lipopolysaccharide and that of T cells to Con A were not severely affected in DN transgenic mice (data not shown). These results indicate that gp130-mediated signals were severely and selectively impaired in lymphocytes of DN transgenic mice.
Figure 4.
Impairment of IL-6 effects on lymphocytes from the transgenic mice expressing a dominant negative form of gp130. (A) Impairment of IL-6-induced antibody production in B cells from DN transgenic mice. Three days after immunization with SRBCs, spleen cells were cultured with SRBCs in the absence or presence of IL-6 for 3 days. SRBC-specific direct plaque-forming cell assay were counted using spleen cell suspensions as the source of antibody-secreting cells. Representative results of three separate experiments are shown. (B) Impaired costimulant effects of IL-6 on thymocytes from DN transgenic mice. Thymocytes from 4-week-old normal, WT, and DN mice were incubated at a density of 5 × 105 cells per well in the presence of PHA at 5 μg/ml for 3 days. The cultures were pulsed with [3H]thymidine during the last 16 hr of incubation.
Antibody Responses of the DN Transgenic Mice.
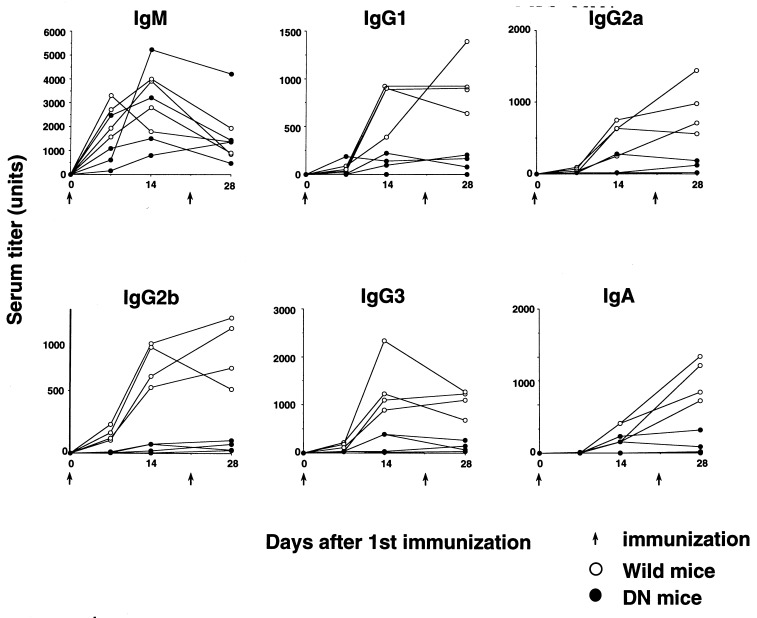
IL-6 is considered a critical molecule for the terminal differentiation of B cells into Ig-secreting cells (1). To examine the dominant-negative effects of the truncated gp130 on antibody production in vivo, DN and WT transgenic mice were immunized with a thymus-dependent antigen, DNP-OVA, and we determined the titers of DNP-specific antibodies. As shown in Fig. 5, primary and secondary anti-DNP antibody production of most Ig isotypes other than IgM was severely impaired in DN transgenic mice. As for IgM, anti-DNP IgM responses varied greatly and showed no significant differences. These results indicate that antigen-specific antibody production of the isotypes other than IgM is largely dependent on gp130-mediated signals.
Figure 5.
Antibody responses to DNP-OVA. WT (○) and DN (•) transgenic mice (6 weeks old) were injected i.p. with DNP-OVA in complete Freund’s adjuvant to induce IgM, IgG, and IgA responses; boosted 21 days later; then bled at indicated times. Levels of anti-DNP antibodies were determined using isotype-specific ELISA. All plots were means of duplicate data. We defined 1/1000 of titers against DNP of WT-sera from 28 days after immunization as arbitrary units.
DISCUSSION
In this study, transgenic mice expressing a dominant-negative form of gp130 were generated to investigate the pathological consequences of impaired gp130 signals in immune responses in adult mice. These analyses were impossible in gp130-deficient mice generated by gene targeting because of embryonic lethality (23).
Development of the lymphoid compartment in DN transgenic mice was not seriously affected by the impaired gp130 signals in terms of surface marker phenotypes, although the average total cell numbers of thymocytes and splenocytes were somewhat reduced (by 30% ± 15 and 25% ± 10, n = 11, respectively). The in vitro responses of B cells to bacterial lipopolysaccharide and of T cells to Con A in DN transgenic mice were almost normal (data not shown), indicating that the development of lymphocytes are virtually normal under the condition where gp130 signals were severely impaired. However, after antigen immunization, DN transgenic mice showed severe impairment of antigen-specific antibody production of most Ig isotypes except for IgM. Thus, these results indicate that antigen-specific antibody production is significantly dependent on the gp130 signals. Determination of serum antibody levels in 6- to 8-week-old mice did not reveal significant differences between normal and DN transgenic mice (data not shown). In addition, we observed germinal center formation in spite of the severe defects in antigen-specific antibody production (A.K., S.M., T.K., T.T., and T.K., unpublished data). Taken together, the mechanism for impaired antigen-specific antibody responses in DN transgenic mice seems to be different from that in CD40 or CD40L-null mice, which have defects of germinal center formation accompanied by loss of class switching and antigen-specific antibody production (34–36). Thus, gp130-mediated signals might be critically involved in final differentiation stages of B cells for expanding antigen-specific antibody production. Among gp130-stimulatory cytokines, mice lacking IL-6 show several-fold reduction in IgG titers to infection with vesicular stomatitis virus, indicating that the antigen-specific IgG immune response is somewhat impaired in the absence of IL-6 (22). It is difficult to make a simple comparison between these mice and DN transgenic mice because of the difference in antigens for immunization. However, there seems to be more severe impairment of antigen-specific antibody production in DN transgenic mice than in IL-6-deficient mice. Because gp130 is a common signal transducer of the IL-6 family of cytokines, the compensatory mechanism could not function in DN transgenic mice, resulting in more severe defects.
Under the control of cytomegalovirus enhancer/chicken β-actin promoter, the dominant-negative form of gp130 was strongly expressed (Fig. 1B), resulting in impaired tyrosine phosphorylation of endogenous gp130 and STAT3 (Fig. 2), which are critical for gp130-mediated signals. Recent studies showed that STAT3 played a critical role in the proliferation of BAF-B03 cells and in the differentiation process of M1 cells into macrophages (28, 29). Furthermore, the eliminated part of gp130 also evokes other critical cytoplasmic kinase cascades such as SHP-2-p42/p44MAPK pathway (29), which was also shown to play a critical role in the proliferation of BAF-B03 cells. Hence, these critical signaling pathways would be severely impaired in DN transgenic mice, resulting in loss of function. In a separate experiment, we generated mutant mice in which endogenous gp130 was replaced by the truncated form of gp130. According to our examination, these mice showed the same phenotypes, including embryonic lethality, as gp130-null mice (A.K., S.M., T.K., T.T., and T.K., unpublished data), indicating that the eliminated part of gp130 plays a critical role in gp130-mediated biological responses.
We previously reported that mice deficient in gp130 showed embryonic lethality, indicating that gp130 plays critical roles in embryogenesis (23). In contrast, DN transgenic mice presented here survived after birth. This may be because there could be a low level of leaked signals from endogenous gp130, which may be sufficient for fetal development. Required amounts of gp130 signals under naive conditions may be different from those under stress conditions (e.g., antigen challenges). Although low levels of gp130 signals may be sufficient for embryonic development, they were insufficient to respond to antigen challenges. In DN transgenic mice, the expression of the dominant-negative form of gp130 was relatively weak during embryogenesis or in certain critical tissues for maintaining pregnancy, such as placenta (data not shown), which may explain the survival of embryos.
In general, gp130-stimulatory cytokines are thought to play a critical role under stress conditions such as inflammation and nerve regeneration (1, 5, 37). Thus, it is important to examine responses of DN transgenic mice under various stress conditions to elucidate pathological significance of gp130. Therefore, the transgenic mice expressing the dominant-negative form of gp130 are useful models for the in vivo analysis of gp130, especially after some types of stress such as liver-toxic reagents. Because gp130 is expressed in all organs examined (17), the dominant-negative form of gp130 is ubiquitously expressed under the control of chicken β-actin promoter. Thus, these mice will be of value to determine the biological roles of gp130 in a broad range of tissues.
Acknowledgments
We thank Ms. Kubota for her excellent secretarial assistance. We also thank Dr. J.-I. Miyazaki for kindly providing us with the pCAGGS construct. This work was supported by grants from the Ministry of Education of Japan and the Human Frontier Science Program.
ABBREVIATIONS
- IL
interleukin
- DNP-OVA
2,4-dinitrophenol-conjugated ovalbumin
- STAT3
signal transducer and activator of transcription 3
- mAb
monoclonal antibody
- WT
wild type
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- SRBC
sheep erythrocyte
References
- 1.Kishimoto T, Akira S, Taga T. Science. 1992;258:593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 2.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 3.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 4.Taga T, Kishimoto T. FASEB J. 1992;6:3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto T, Taga T, Akira S. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 6.Gearing D P, Comeau M R, Friend D J, Gimpel S D, Thut C J, McGourty J, Brasher K K, King J A, Gillis S, Mosley B, Ziegler S F, Cosman D. Science. 1992;255:1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 7.Ip N Y, Nye S H, Boulton T G, Davis S, Taga T, Li Y, Birren S J, Yasukawa K, Kishimoto T, Anderson D J, Stahl N, Yancopoulos G D. Cell. 1992;69:1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- 8.Taga T, Narazaki M, Yasukawa K, Saito M, Miki D, Hamaguchi M, Davis S, Shoyab M, Yancopoulos G D, Kishimoto T. Proc Natl Acad Sci USA. 1992;89:10998–11001. doi: 10.1073/pnas.89.22.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin T, Taga T, Tsang M L-S, Yasukawa K, Kishimoto T, Yang Y-C. J Immunol. 1993;151:2555–2561. [PubMed] [Google Scholar]
- 10.Pennica D, Shaw K J, Swanson T A, Moore M W, Shelton D L, Zioncheck K A, Rosenthal A, Taga T, Paoni N F, Wood W I. J Biol Chem. 1995;270:10915–10922. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- 11.Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T. Science. 1993;260:1808–1810. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Aldrich T H, Stahl N, Pan L, Taga T, Kishimoto T, Ip N Y, Yancopoulos G D. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 13.Narazaki M, Witthuhn B M, Yoshida K, Silvennoinen O, Yasukawa K, Ihle J N, Kishimoto T, Taga T. Proc Natl Acad Sci USA. 1994;91:2285–2289. doi: 10.1073/pnas.91.6.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahl N, Boulton T G, Farruggella T, Ip N Y, Davis S, Witthuhn B A, Quelle F W, Silvennoinen Q, Barbieri G, Pellegrini S, Ihle J N, Yancopoulos G D. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Nishio Y, Inoue M, Wang X-J, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 16.Darnell J E, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 17.Saito M, Yoshida K, Hibi M, Taga T, Kishimoto T. J Immunol. 1992;148:4066–4071. [PubMed] [Google Scholar]
- 18.Yoshida K, Chambers I, Nichols J, Smith A G, Saito M, Yasukawa K, Shoyab M, Taga T, Kishimoto T. Mech Dev. 1994;45:163–171. doi: 10.1016/0925-4773(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 19.Stewart C L, Kaspar P, Brunet L J, Bhatt H, Gadi I, Kontgen F, Abbondanzo S J. Nature (London) 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 20.Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Nature (London) 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 21.Escary J-L, Perreau J, Dumenil D, Ezine S, Brulet P. Nature (London) 1993;363:361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- 22.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. Nature (London) 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, Taga T, Saito S, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamaguchi T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang X-J, Mori C, Shiota K, Yoshida N, Kishimoto T. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 25.Nishimoto H, Kikutani H, Yamamura K, Kishimoto T. Nature (London) 1987;328:432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- 26.Jerne N K, Nordin A A. Science. 1963;140:405–408. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- 27.Baumann H, Symes A J, Comeau M R, Morella K K, Wang Y, Friend D, Ziegler S F, Fink J S, Gearing D P. Mol Cell Biol. 1994;14:138–146. doi: 10.1128/mcb.14.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, Akira S. Proc Natl Acad Sci USA. 1996;93:3963–3966. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 30.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Yancopoulos G D. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 31.Takatsuki F, Okano A, Suzuki C, Chieda R, Takahara Y, Hirano T, Kishimoto T, Hmuro J, Akiyama Y. J Immunol. 1988;141:3072–3077. [PubMed] [Google Scholar]
- 32.Garman R D, Jacobs K A, Clark S C, Raulet D H. Proc Natl Acad Sci USA. 1987;84:7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le J, Fredrickson G, Reis L F, Diamantstein T, Hirano T, Kishimoto T, Vilcek J. Proc Natl Acad Sci USA. 1988;85:8643–8647. doi: 10.1073/pnas.85.22.8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castigli E, Alt F W, Davidson L, Bottaro A, Mizoguchi E, Bhan A K, Geha R S. Proc Natl Acad Sci USA. 1994;91:12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Foy T M, Laman J D, Elliot E A, Dunn J J, Waldschmidt T J, Elsemore J, Noelle R J, Flavell R A. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 37.Hirota H, Kiyama H, Kishimoto T, Taga T. J Exp Med. 1996;183:2627–2634. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]