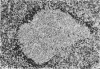
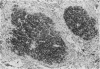
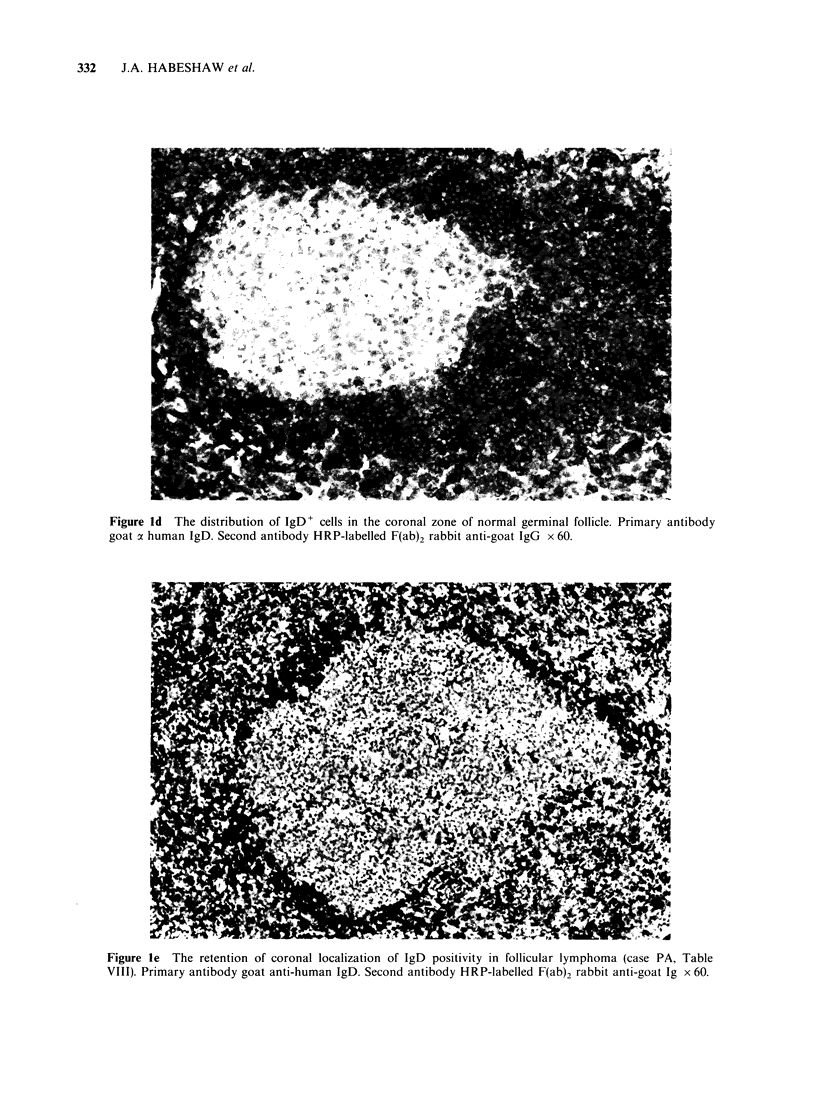
Abstract
Five samples of tonsil, 10 reactive lymph nodes and 65 consecutive cases of non-Hodgkin lymphoma (NHL) were evaluated in suspension phenotyping with the monoclonal antibodies alpha Leu-I, alpha Leu-2a, alpha Leu-3a, OKT1, OKT3, OKT4, OKT6, OKT8, W6/32, 26/114, DA-2, 2DI, J5, AN51 and OKT9 together with conventional surface marking by rosetting (E, Fc gamma, Fc mu, C3b, C3d) and staining for surface and cytoplasmic immunoglobulin (SIg, CyIg) heavy and light chain classes. The results confirm the reproductability and specificity of staining with monoclonal antibodies against T cells and T cells subsets. Evidence is presented for reactivity of alpha Leu-I antibody with SIg positive and Ia positive cells in some lymphomas (centroblastic centrocytic, lymphocytic and immunoblastic), and 2 cases showed evidence of marking with OKT3 on SIg positive cells in T cell predominant immunoblastic lymphoma. Lymphoblastic lymphomas of T cell type expressed the marker OKT6. On the basis of these results criteria for the diagnosis of T cell lymphoma are suggested. The monoclonal antibody J5, reactive with C-ALL antigen, showed variable positivity, occasionally strong in B cells in cases of centroblastic and centrocytic follicular lymphoma. Proportions of cells staining with the monoclonal antibody OKT9 showed a correlation between levels of cellular expression of transferrin (trf) receptor and the histological grade of malignancy, OKT9+ cells being elevated in high grade lymphomas, and in some cases of transforming lymphoma of low grade histological class. These results are discussed and indicate the advantage of employing a wide range of defined monoclonal reagents in the phenotypic evaluation of NHL.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C. Current concepts in immunology: Cell-surface markers in lymphoproliferative disease. N Engl J Med. 1981 Feb 5;304(6):331–336. doi: 10.1056/NEJM198102053040606. [DOI] [PubMed] [Google Scholar]
- Aisenberg A. C., Wilkes B. M. Unusual human lymphoma phenotype defined by monoclonal antibody. J Exp Med. 1980 Oct 1;152(4):1126–1131. doi: 10.1084/jem.152.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A., Boumsell L., Reinherz E. L., Nadler L. M., Ritz J., Coppin H., Richard Y., Valensi F., Dausset J., Flandrin G. Cell surface characterization of malignant T cells from lymphoblastic lymphoma using monoclonal antibodies: evidence for phenotypic differences between malignant T cells from patients with acute lymphoblastic leukemia and lymphoblastic lymphoma. Blood. 1981 Jun;57(6):1105–1110. [PubMed] [Google Scholar]
- Beverley P. C., Linch D., Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980 Sep 25;287(5780):332–333. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield C. D., Gajl-Peczalska K. J., Frizzera G., Kersey J. H., Goldman A. I. Clinical utility of lymphocyte surface markers combined with the Lukes-Collins histologic classification in adult lymphoma. N Engl J Med. 1979 Sep 6;301(10):512–518. doi: 10.1056/NEJM197909063011002. [DOI] [PubMed] [Google Scholar]
- Boumsell L., Coppin H., Pham D., Raynal B., Lemerle J., Dausset J., Bernard A. An antigen shared by a human T cell subset and B cell chronic lymphocytic leukemic cells. Distribution on normal and malignant lymphoid cells. J Exp Med. 1980 Jul 1;152(1):229–234. doi: 10.1084/jem.152.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia P. F. Characterization of the blast cell in childhood non-Hodgkin's lymphoproliferative malignancies. Semin Oncol. 1977 Sep;4(3):287–296. [PubMed] [Google Scholar]
- Dorreen M. S., Habeshaw J. A., Wrigley P. F., Lister T. A. Distribution of T-lymphocyte subsets in Hodgkin's disease characterized by monoclonal antibodies. Br J Cancer. 1982 Apr;45(4):491–499. doi: 10.1038/bjc.1982.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Lazarus H., Penta A. C., Schlossman S. F. Two functionally distinct subpopulations of human T cells that collaborate in the generation of cytotoxic cells responsible for cell-mediated lympholysis. J Immunol. 1978 Apr;120(4):1423–1428. [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Bloomfield C. D., Coccia P. F., Sosin H., Brunning R. D., Kersey J. H. B and T cell lymphomas. Analysis of blood and lymph nodes in 87 patients. Am J Med. 1975 Nov;59(5):674–685. doi: 10.1016/0002-9343(75)90228-4. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Rao J., Hariri G., Verbi W., Catovsky D., Kung P., Goldstein G. Phenotypic heterogeneity and cellular origins of T cell malignancies. Leuk Res. 1981;5(4-5):281–299. doi: 10.1016/0145-2126(81)90001-1. [DOI] [PubMed] [Google Scholar]
- Habeshaw J. A. Heterogeneity of lymphoblastic malignancies in children. J Cancer Res Clin Oncol. 1981;101(1):23–27. doi: 10.1007/BF00405060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeshaw J. A., Macaulay R. A., Stuart A. E. Correlation of surface receptors with histological appearance in 29 cases of non-Hodgkin lymphoma. Br J Cancer. 1977 Jun;35(6):858–867. doi: 10.1038/bjc.1977.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre C. A., Minden M. D., Howatson A. F., McCulloch E. A. Colony formation by normal and malignant human B-lymphocytes. Br J Cancer. 1980 Sep;42(3):430–437. doi: 10.1038/bjc.1980.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. S., Braylan R. C., Nanba K., Frank M. M., Berard C. W. Functional markers: a new perspective on malignant lymphomas. Cancer Treat Rep. 1977 Sep;61(6):953–962. [PubMed] [Google Scholar]
- Jaffe E. S., Shevach E. M., Frank M. M., Berard C. W., Green I. Nodular lymphoma--evidence for origin from follicular B lymphocytes. N Engl J Med. 1974 Apr 11;290(15):813–819. doi: 10.1056/NEJM197404112901501. [DOI] [PubMed] [Google Scholar]
- Janossy G., Goldstone A. H., Capellaro D., Greaves M. F., Kulenkampff J., Pippard M., Welsh K. Differentiation linked expression of p28,33 (Ia-like) structures on human leukaemic cells. Br J Haematol. 1977 Nov;37(3):391–402. doi: 10.1111/j.1365-2141.1977.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Kung P. C., Berger C. L., Goldstein G., LoGerfo P., Edelson R. L. Cutaneous T cell lymphoma: characterization by monoclonal antibodies. Blood. 1981 Feb;57(2):261–266. [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R., Dilley J., Fox R. I., Warnke R. A human thymus-leukemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6552–6556. doi: 10.1073/pnas.76.12.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukes R. J., Taylor C. R., Parker J. W., Lincoln T. L., Pattengale P. K., Tindle B. H. A morphologic and immunologic surface marker study of 299 cases of non-Hodgkin lymphomas and related leukemias. Am J Pathol. 1978 Feb;90(2):461–486. [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Rust N. A., Pilch J. R., Sochynsky R., Morton J., Mason D. Y., Ruan C., Tobelem G., Caen J. Monoclonal antibody to human platelet glycoprotein I. I. Immunological studies. Br J Haematol. 1981 Dec;49(4):501–509. doi: 10.1111/j.1365-2141.1981.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Maloney D. G., Warnke R., Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982 Mar 4;306(9):517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- Minowada J., Janossy G., Greaves M. F., Tsubota T., Srivastava B. I., Morikawa S., Tatsumi E. Expression of an antigen associated with acute lymphoblastic leukemia in human leukemia-lymphoma cell lines. J Natl Cancer Inst. 1978 Jun;60(6):1269–1277. doi: 10.1093/jnci/60.6.1269. [DOI] [PubMed] [Google Scholar]
- Murphy S. B. Childhood non-Hodgkin's lymphoma. N Engl J Med. 1978 Dec 28;299(26):1446–1448. doi: 10.1056/NEJM197812282992606. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Birbeck M. S., Wallis V. J., Forrester J. A., Davies A. J. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980 Mar 27;284(5754):364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Habeshaw J. A., Kennedy R., Sloane J., Wiltshaw E., Davies A. J. Binding of peanut lectin to germinal-centre cells: a marker for B-cell subsets of follicular lymphoma? Br J Cancer. 1981 Jul;44(1):68–74. doi: 10.1038/bjc.1981.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston I., Majda J. A., Baird S. M., Meserve B. L., Griffiths J. C. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J Immunol. 1980 Aug;125(2):725–731. [PubMed] [Google Scholar]
- Stathopoulos G., Papamichail M., Holborow E. J., Davies A. J. Description of the cells from the lymph nodes of patients with non-Hodgkin's lymphoma according to some B- and T-cell characteristics. Br J Exp Pathol. 1977 Feb;58(1):95–100. [PMC free article] [PubMed] [Google Scholar]
- Stein H. Immunchemische und immunzytologische Befunde bei Non-HODGKIN-Lymphomen. Hamatol Bluttransfus. 1976;18:167–183. [PubMed] [Google Scholar]
- Strauchen J. A., Young R. C., DeVita V. T., Jr, Anderson T., Fantone J. C., Berard C. W. Clinical relevance of the histopathological subclassification of diffuse "histiocytic" lymphoma. N Engl J Med. 1978 Dec 21;299(25):1382–1387. doi: 10.1056/NEJM197812212992503. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]