Abstract
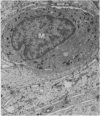
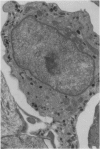
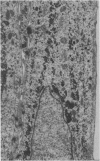
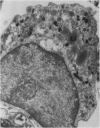
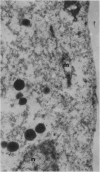
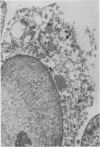
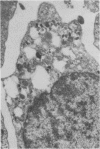
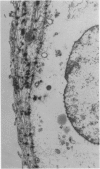
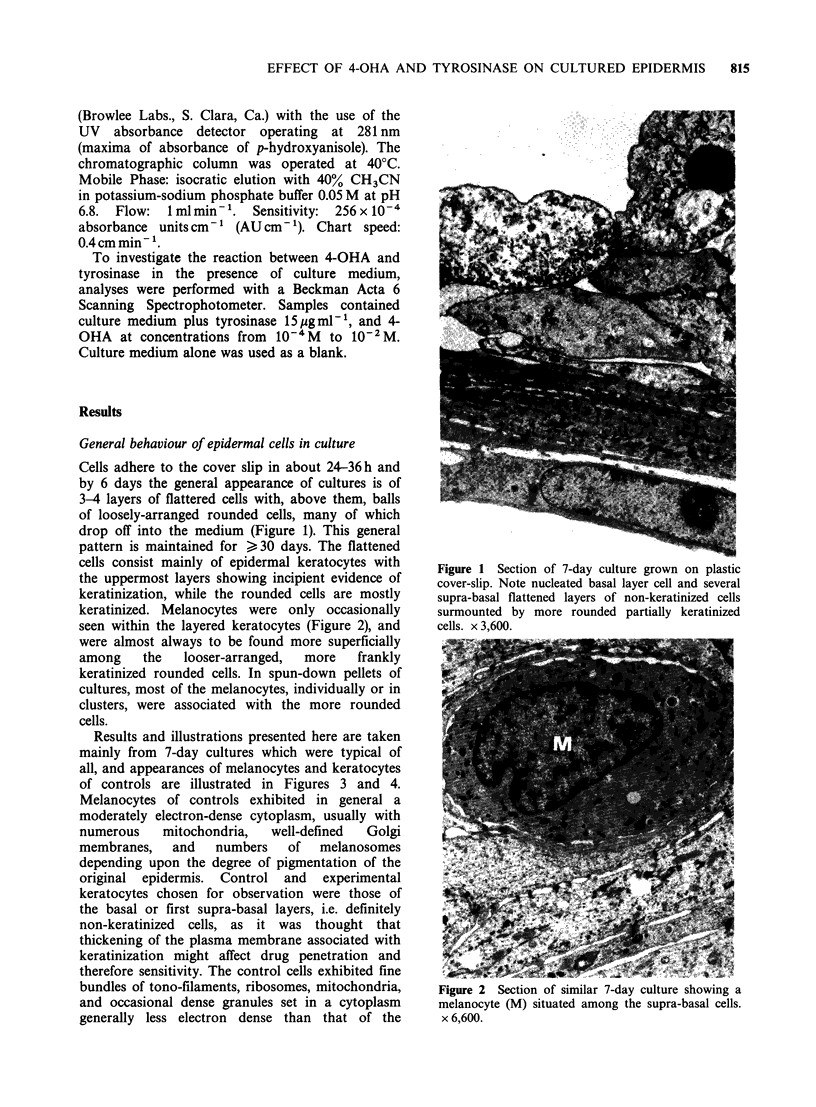
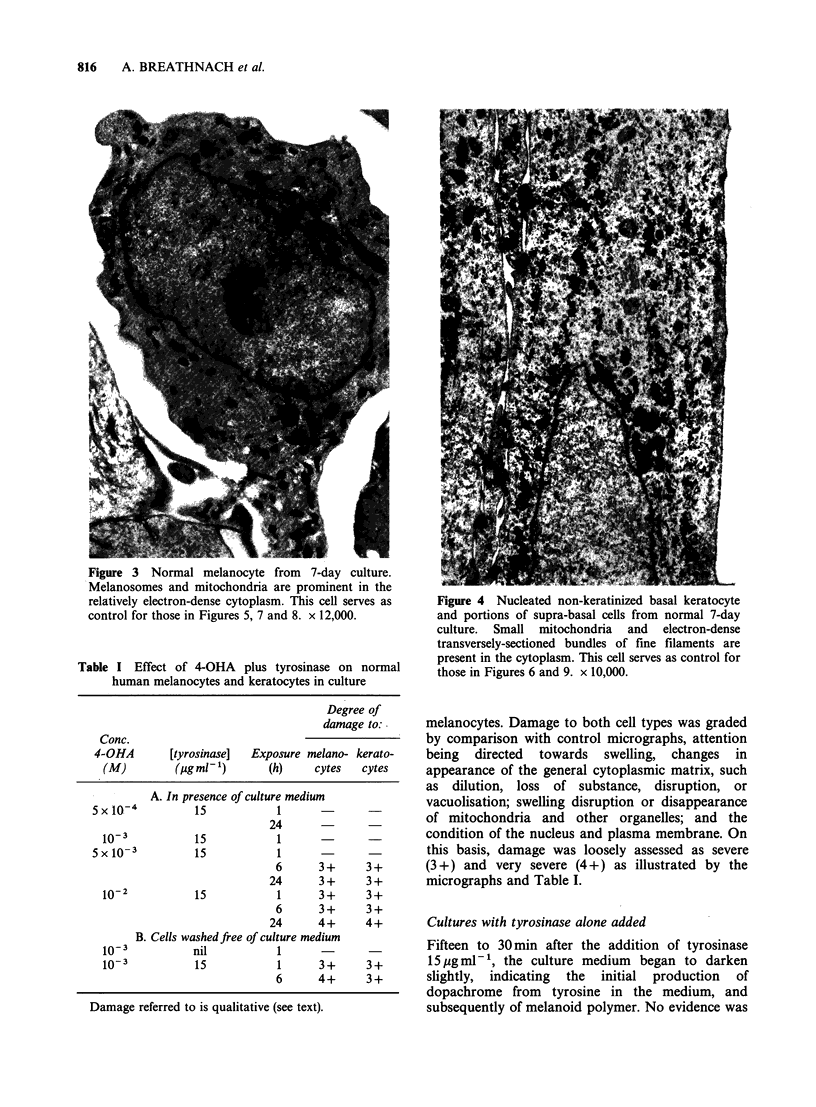
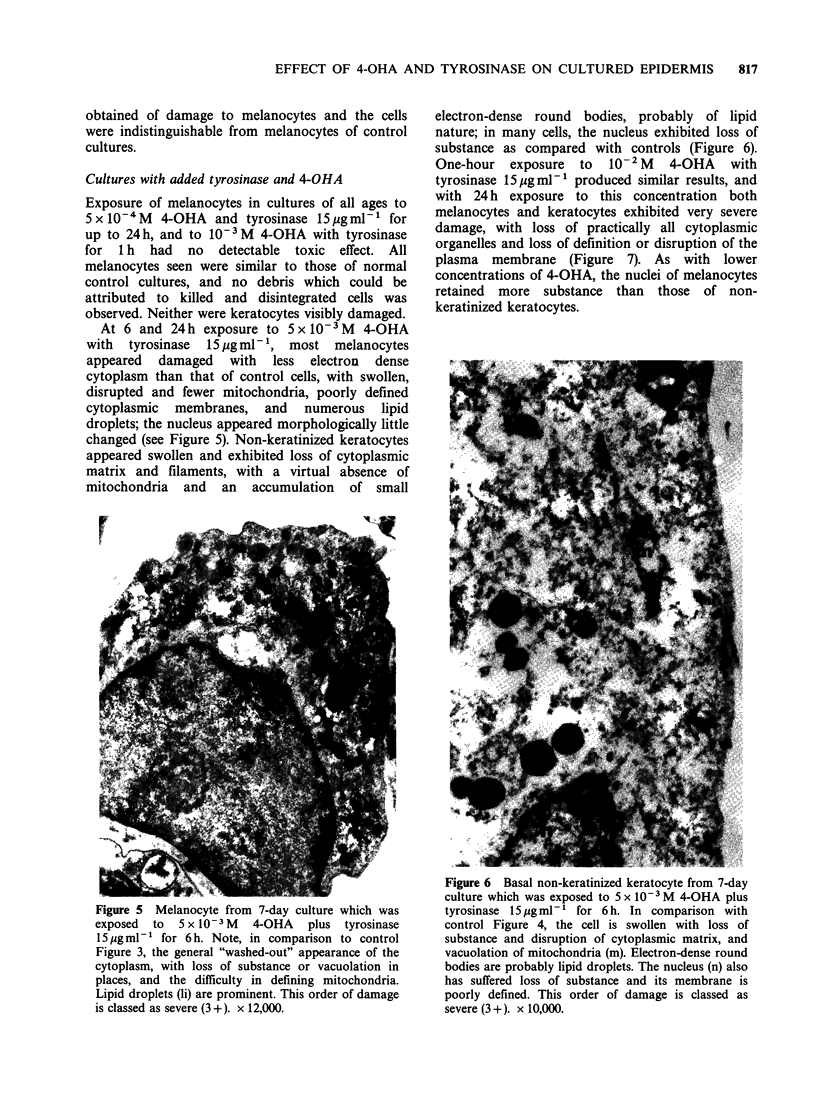
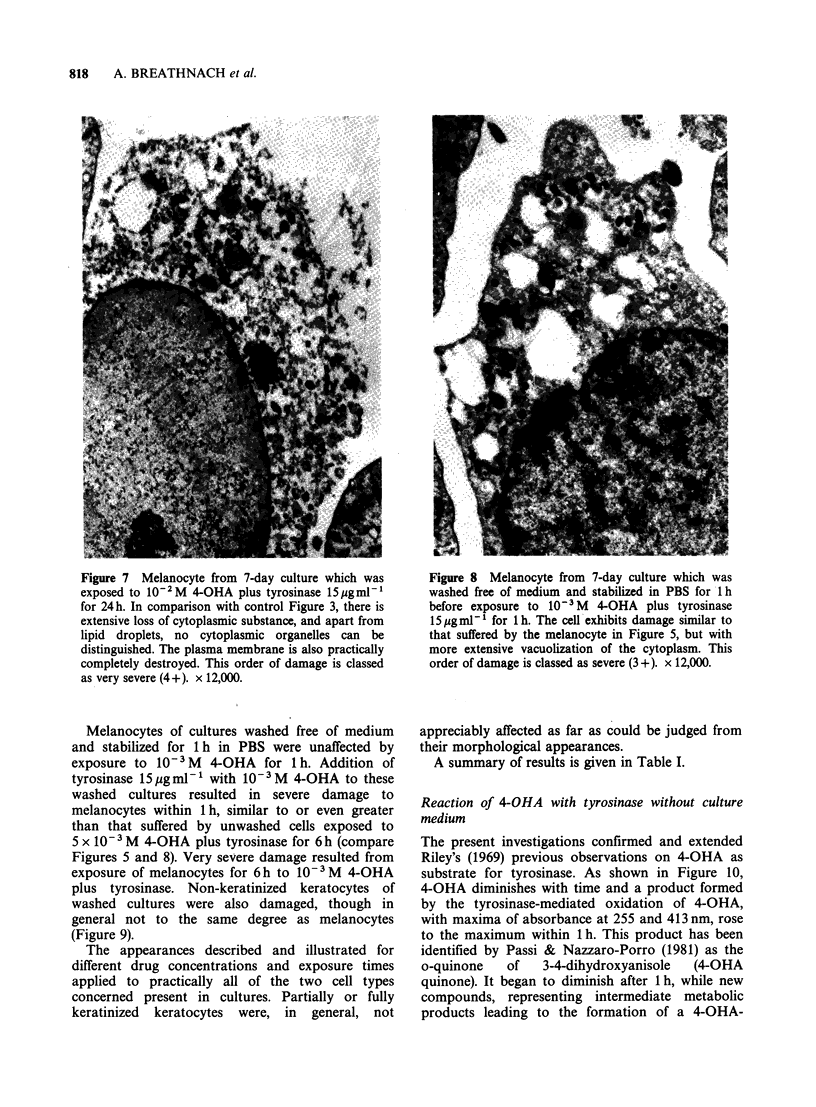
Cultures of melanocytes and keratocytes were exposed to 15 micrograms ml-1 tyrosinase and 4-hydroxyanisole (4-OHA) 5 X 10(-4) M to 5 X 10(-2) M for 1 to 24 h. No damage was suffered by either cell below 5 X 10(-3) M 4-OHA for 6 h, but higher concentrations and longer exposures extensively damaged both cells. Exposure of cells washed free of culture medium to tyrosinase and 4-OHA 1 X 10(-3) M for 1 h resulted also in extensive damage. This indicates that an early-formed toxic product of the reaction between tyrosinase and 4-OHA is inactivated by constituents of the medium. This was confirmed by Liquid Chromatography and Scanning Spectrophotometry which showed that a toxic 4-OHA quinone immediately reacted with nucleophilic substances in the medium resulting in products which, on accumulation, are probably responsible for the later (6 h plus) damage to melanocytes and keratocytes. A possible effect of allegedly specific melanocytotoxic drugs on keratocytes should always be borne in mind with tissue culture experiments.
Full text
PDF

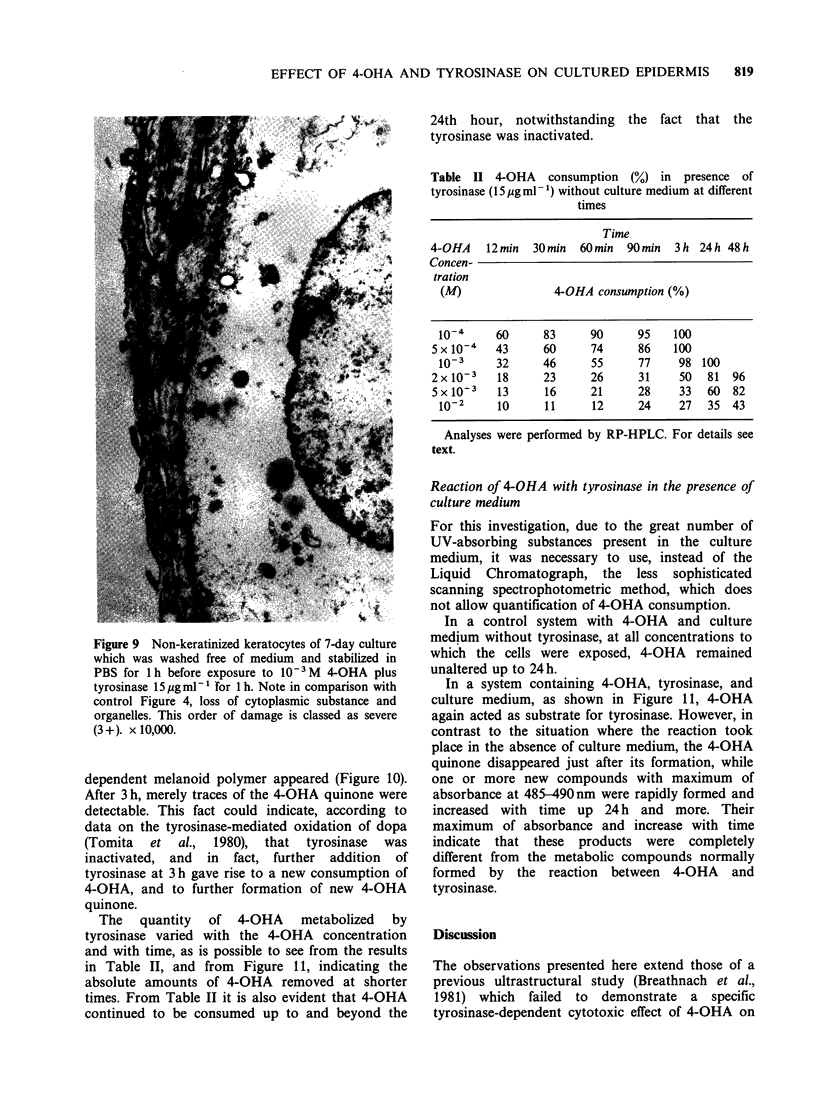
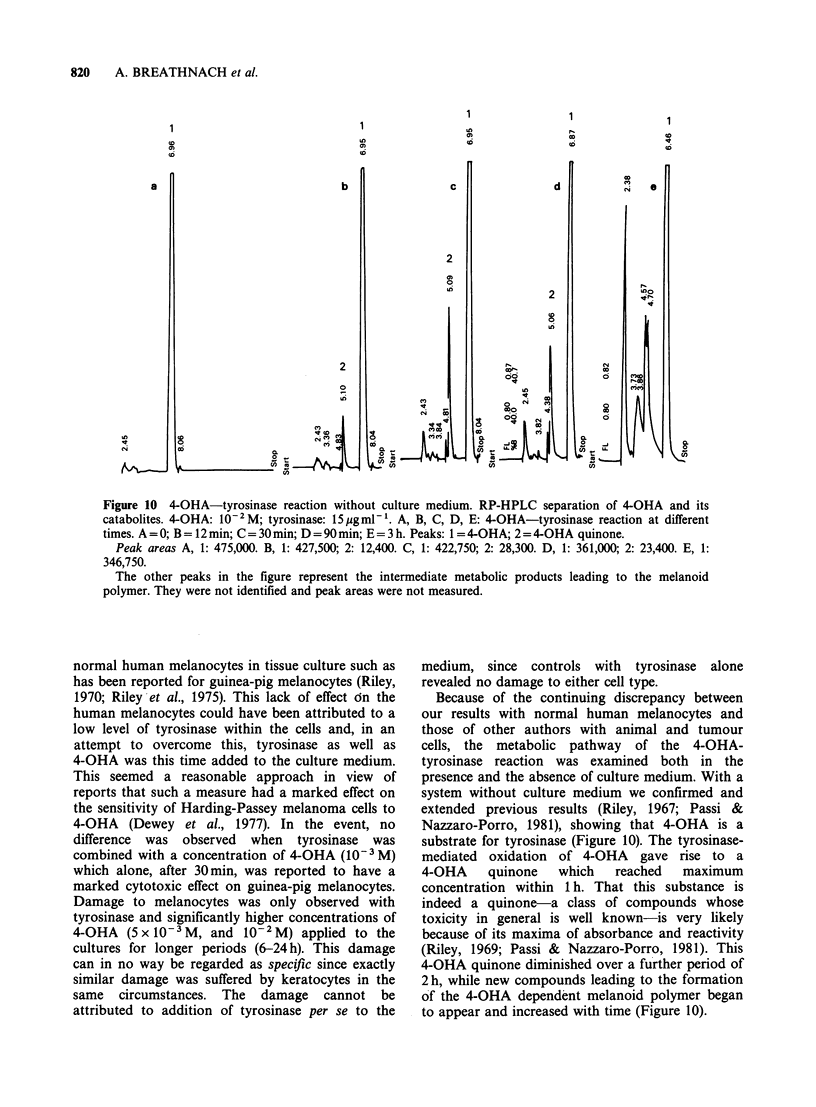
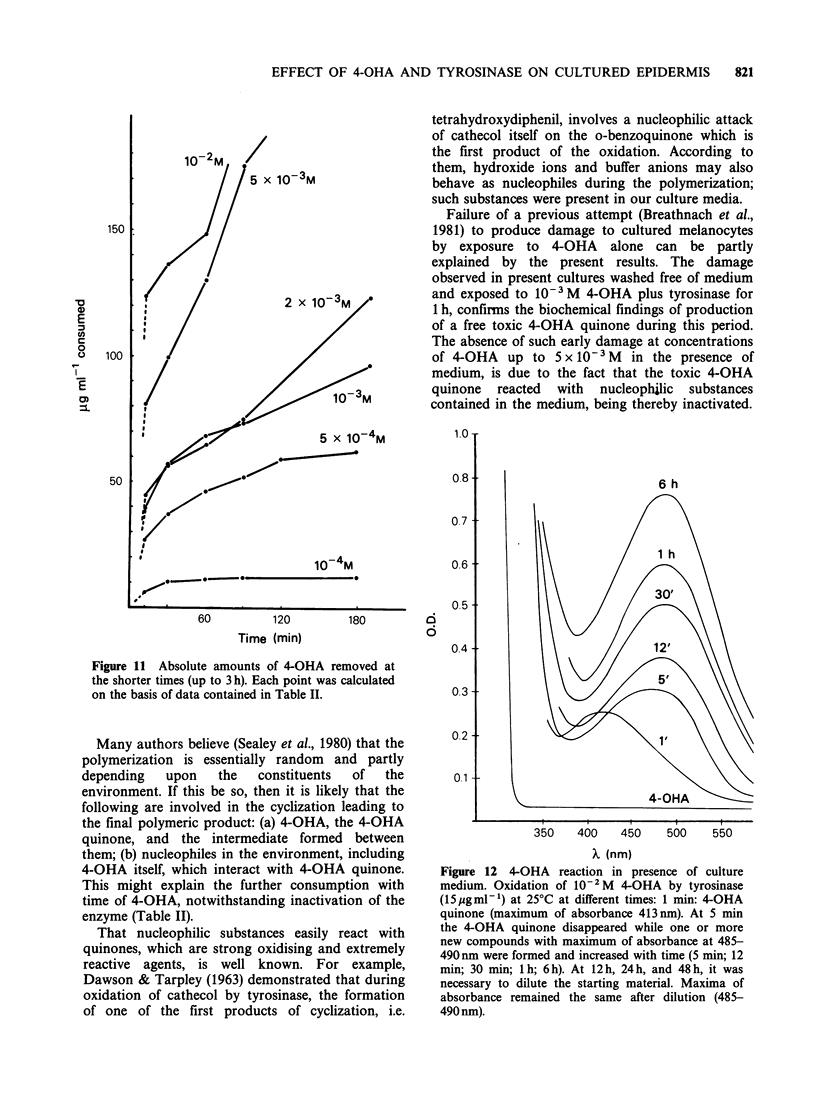

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breathnach A. S., Diala E. B., Gallagher S. M., Porro M. N., Passi S. Ultrastructural observations on the effect of 4-hydroxyanisole on normal human melanocytes in tissue culture. J Invest Dermatol. 1981 Sep;77(3):292–296. doi: 10.1111/1523-1747.ep12482465. [DOI] [PubMed] [Google Scholar]
- DAWSON C. R., TARPLEY W. B. On the pathway of the catecholtyrosinase reaction. Ann N Y Acad Sci. 1963 Feb 15;100:937–950. [PubMed] [Google Scholar]
- Dewey D. L., Butcher F. W., Galpine A. R. Hydroxyanisole-induced regression of the Harding-Passey melanoma in mice. J Pathol. 1977 Jul;122(3):117–127. doi: 10.1002/path.1711220302. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZPATRICK T. B., BREATHNACH A. S. DAS EPIDERMALE MELANIN-EINHEIT-SYSTEM. Dermatol Wochenschr. 1963 May 18;147:481–489. [PubMed] [Google Scholar]
- Morgan B. D., O'Neill T., Dewey D. L., Galpine A. R., Riley P. A. Treatment of malignant melanoma by intravascular 4-hydroxyanisole. Clin Oncol. 1981 Sep;7(3):227–234. [PubMed] [Google Scholar]
- Passi S., Nazzaro-Porro M. Molecular basis of substrate and inhibitory specificity of tyrosinase: phenolic compounds. Br J Dermatol. 1981 Jun;104(6):659–665. doi: 10.1111/j.1365-2133.1981.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Riley P. A. Hydroxyanisole depigmentation: in-vitro studies. J Pathol. 1969 Feb;97(2):193–206. doi: 10.1002/path.1710970203. [DOI] [PubMed] [Google Scholar]
- Riley P. A. Mechanism of pigment-cell toxicity produced by hydroxyanisole. J Pathol. 1970 Jun;101(2):163–169. doi: 10.1002/path.1711010211. [DOI] [PubMed] [Google Scholar]
- Riley P. A., Sawyer B., Wolf M. A. The melanocytotoxic action of 4-hydroxyanisole. J Invest Dermatol. 1975 Feb;64(2):86–89. doi: 10.1111/1523-1747.ep12510304. [DOI] [PubMed] [Google Scholar]