Abstract
Fusariosis is an emerging opportunistic mycosis against which currently used antifungals have limited activity. Here, we investigated the in vitro activities of pentamidine (PNT) against 10 clinical isolates of Fusarium species (five Fusarium solani isolates and five non-F. solani isolates) by using the National Committee for Clinical Laboratory Standards microdilution method in three different media (RPMI, RPMI-2, and a yeast nitrogen base medium), disk diffusion testing, and viability dye staining. PNT had significant activities against all 10 Fusarium isolates. Non-F. solani isolates were more susceptible than F. solani isolates (P < 0.05). Additionally, PNT was fungicidal against all non-F. solani isolates, whereas it had fungistatic effects against four of the five F. solani isolates. PNT also exhibited greater activity against conidial than against hyphal development of the fungus. This fungicidal activity against non-F. solani Fusarium isolates was confirmed microscopically after staining of PNT-treated Fusarium oxysporum hyphae with the fluorescent viability dyes 5,(6)-carboxyfluorescein diacetate (CFDA) and bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC). The MICs at which 50% of the isolates were inhibited (2 μg/ml for non-F. solani isolates and 4 μg/ml for F. solani isolates) and the minimum fungicidal concentration at which 50% of the isolates were killed (8 μg/ml for non-F. solani isolates) were much lower than the PNT tissue concentrations previously reported in humans using conventional daily intravenous PNT dosing. Finally, PNT was more active against Fusarium isolates in a hypoxic environment of in vitro growth (P < 0.05). This finding may be clinically significant, because Fusarium, an angiotropic mold, causes tissue infarcts with resultant low tissue perfusion. Our findings suggest that PNT may have a role in the management of Fusarium infections. Future in vivo studies are needed to verify these in vitro findings.
Invasive fusariosis is a severe opportunistic fungal infection that is primarily encountered in patients with leukemia (7, 27, 28). Nevertheless, other profoundly immunocompromised patients, such as allogeneic bone marrow and solid-organ transplant recipients, may experience this infection (7, 27, 28). This devastating mycosis has emerged in recent years as the second most common opportunistic invasive mold infection, behind only invasive aspergillosis, in some tertiary-care cancer centers (7, 27, 28). The most common clinical presentation of fusariosis is pneumonia, which is followed by disseminated disease (27). Among the Fusarium species, Fusarium solani accounts for nearly half of the cases of invasive fusariosis in humans and is the most virulent strain according to tests in animal models (27). Fusarium oxysporum is the second most commonly encountered species, followed by Fusarium moniliforme, Fusarium verticilloides, and Fusarium proliferatum (7, 17, 27, 28).
Several studies have demonstrated the limited activities of antifungal agents against Fusarium spp. both in vitro and in animal models (3, 4, 13, 25). The only somewhat active agents in vitro are amphotericin B (AMB) and the newer broad-spectrum triazoles voriconazole (VRC) and posaconazole (24, 25); nevertheless, their in vivo activity in animal models is mediocre, which is in agreement with the exceedingly high mortality rate associated with this infection in clinical practice (>80%, even with treatment) (7, 27). Irrespective of treatment, the major prognostic determinant in fusariosis cases is neutrophil recovery (D. P. Kontoyiannis, H. Hanna, R. Hachem, M. Boktour, E. Girgawy, M. Mardani, G. P. Bodey, and I. Raad, Abstr. 40th Ann. Meet. Infect. Dis. Soc. Am., abstr. 366, 2002). Thus, the introduction of new, more effective therapeutic approaches is essential to improving the prognosis of fusariosis.
Pentamidine (PNT) is an antimicrobial agent that is active against a broad spectrum of microbes, including fungi (26). Administration of PNT is a well-established approach used for prophylaxis and treatment of pneumonia caused by Pneumocystis carinii, a microorganism that was recently classified taxonomically to be a fungus (21). PNT has also been shown to have in vitro activities against a variety of fungal pathogens, such as Candida albicans, Cryptococcus neoformans, Scedosporium prolificans, and Aspergillus terreus (1, 2, 5, 20). Therefore, in this study, we evaluated the in vitro activities of PNT against 10 clinical isolates of Fusarium spp. in different culture media and various oxygen conditions and against the two developmental programs of these opportunistic mold species.
MATERIALS AND METHODS
Fusarium isolates.
We obtained 10 clinical isolates of Fusarium spp. from the Mycology Laboratory at The University of Texas M. D. Anderson Cancer Center for testing: five isolates of the F. solani complex and five non-F. solani isolates (F. oxysporum, F. proliferatum, and F. pallidoroseum). Molecular identification of all of the isolates tested was conducted using amplification and sequencing of translation elongation factor 1 alpha sequences (23). Candida tropicalis strain ATCC 2697 was used for quality control purposes in all of the experiments.
Susceptibility testing.
We evaluated the in vitro activities of PNT and a variety of antifungal agents against the 10 Fusarium isolates by performing a broth microdilution method according to NCCLS guidelines (M38-P) (22). In addition to PNT, the drugs used were AMB, 5-flucytosine (5FC), fluconazole (FLC), itraconazole (ITZ), VRC, and caspofungin (CAS). FLC and VRC were obtained from Pfizer Inc. (New York, N.Y.), while ITZ was obtained from Janssen Pharmaceutica (Titusville, N.J.). All of the antifungal agents were obtained in assay powder form. Drug dilutions were prepared in 100% dimethyl sulfoxide (for AMB and ITZ) or distilled water (for all other agents) followed by further dilutions (1:50) in the NCCLS standard RPMI 1640 medium to yield twice the final strength required for testing. The RPMI 1640 medium was prepared according to the manufacturer's (Sigma Chemical Co., St. Louis, Mo.) instructions. RPMI medium with l-glutamine but without bicarbonate was buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid. To test the in vitro activity of PNT, the NCCLS method was performed using two other media in addition to RPMI medium: RPMI medium plus 2% glucose (RPMI-2) and a yeast nitrogen base (YNB) medium (Difco, Detroit, Mich.). The final concentrations of the tested drugs ranged from 0.03 to 16.00 μg/ml for AMB, ITZ, and VRC; 0.125 to 64.00 μg/ml for 5FC and FLC; 0.06 to 32.00 μg/ml for CAS; and 0.125 to 128.00 μg/ml for PNT. The tests were performed in 96-well flat-bottom microtitration plates (Corning Inc., Corning, N.Y.) that were kept frozen at −70°C until the day of the experiment.
Stock inoculum suspensions were prepared from 7-day-old cultures grown on potato dextrose agar slants as described in the NCCLS M38-P document and adjusted spectrophotometrically to optical densities (ODs) ranging from 0.09 to 0.30 (60 to 82% transmittance). On the day of the experiment, each microdilution well containing 100 μl of the twice-diluted drug concentrations was inoculated with 100 μl of the twice-diluted conidial suspensions, producing a final volume in each well of 200 μl. The concentrations of final inocula ranged from 0.4 × 104 to 5.0 × 104 CFU/ml.
Following agitation, microtitration plates were incubated at 35°C for 48 h, and the MICs of the antifungal agents were estimated. The MIC was defined as the lowest drug concentration at which there was complete absence (for AMB) or prominent reduction of growth (corresponding to approximately 50% growth reduction for the azoles, 5FC, and CAS). Because the MIC endpoints of PNT have not yet been established in fungi, we evaluated both the MIC-2 and MIC-0 endpoints. The former was defined as the lowest drug concentration that caused visually prominent inhibition of growth (approximately >50%), whereas the latter was defined as the lowest drug concentration that caused complete visual growth inhibition (>95%). All of the isolates were tested in triplicate on three different days. When discordant MICs were found, the higher values were reported.
The minimum fungicidal concentration (MFC) of each agent was determined using a recently proposed methodology (11). Briefly, 20 μl of the suspensions from each well that showed complete inhibition of growth was streaked on YNB plates with 2% glucose and 2% agar prepared according to the manufacturer's instructions. The YNB plates were then incubated at 35°C for 48 to 72 h. The MFC was defined as the lowest drug concentration at which fewer than three colonies were observed, which corresponds to a killing activity of approximately 99.0 to 99.5%.
XTT colorimetric assay.
NCCLS microtitration plates were prepared as described above and incubated for 48 h at 35°C. The 2,3-bis{2-methoxy-4-n-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide} (XTT) solution was prepared as described elsewhere (19). Incubation was continued at 35°C for 2 h in the dark to allow for conversion of XTT to its formazan derivatives. After shaking, the ODs at 492 and 690 nm were measured by using a microplate spectrophotometer (Powerwave X; Bio-Tek Instruments, Winooski, Vt.). The color was then assessed spectrophotometrically based on the relative OD at 492 and 690 nm, the latter of which is a reference wavelength subtracted from 492 nm.
Staining of Fusarium isolates with CFDA and DiBAC.
Staining with the fluorescent dyes CFDA [5,(6)-carboxyfluorescein diacetate] and DiBAC [bis-(1,3-dibutylbarbituric acid) trimethine oxonol] was performed as described previously by Bowman et al. (8). Briefly, conidia from two representative Fusarium isolates—one each from the F. solani and the F. oxysporum complexes—were suspended in RPMI medium to obtain twice the desired final concentration of 0.4 × 104 to 5.0 × 104 conidia/ml. Aliquots of 100 μl each were inoculated in NCCLS microtitration plates and incubated at 35°C for 48 h to allow for conversion of the conidia to hyphae. At 48 h, 100-μl aliquots of the hyphae were mixed with 100-μl aliquots of PNT at various concentrations corresponding to the MIC-0 and eight times the MIC-0 of PNT for the tested F. solani isolate (32 and 256 μg/ml, respectively) and to the MFC and four times the MFC of PNT for the tested F. oxysporum isolate (8 and 32 μg/ml, respectively). After incubation at 35°C for 10 h, hyphae exposed to different PNT concentrations were mixed with the CFDA or DiBAC solution (8). As controls, Fusarium hyphae of the same isolates were also incubated for 10 h with AMB (4 μg/ml; equal to the MFC for the tested isolates) and 1.5% H2O2. Photomicrographs of the hyphae were taken using a triple-band fluorescent microscope (BX-51; Olympus, Melville, N.Y.) as described previously (8).
XTT-based time-kill assay.
Special 96-well flat-bottom microtitration plates were prepared by inoculating PNT into all of their rows (final concentration range, 0.125 to 128.00 μg/ml). We prepared conidial suspensions from three F. solani and three F. oxysporum isolates in RPMI-2 medium as described above (final concentration, 0.4 × 104 to 5.0 × 104/ml). At the beginning of each incubation, 100-μl aliquots of the conidial suspensions were added to each well of the plates, which were then incubated at 35°C. Subsequently, at regular time points following the addition of the conidial suspensions (in particular, at 4, 12, 24, 36, and 48 h), 50-μl aliquots of XTT solution that were prepared as described previously (19) were added to each well of single rows corresponding to each time point. The ODs at 492 and 690 nm were then measured in each individual row in which the XTT solution was added following a 2-h incubation in the dark. The color was assessed as described above. Using this method, we assessed the time-dependent conversion of Fusarium conidia to hyphae and the effects of PNT on the growth of Fusarium isolates. An analogous experiment was performed using YNB medium instead of RPMI-2. In a similar manner, we evaluated the inhibitory effect of PNT when introduced to already formed hyphae of two F. solani and two F. oxysporum isolates.
Activity of PNT against Fusarium isolates in a hypoxic chamber of in vitro growth.
Microtitration plates were prepared as described above and placed in chambers along with BBL gas generator envelopes (CampyPak Plus and GasPak Plus for microaerophilic and anaerobic conditions, respectively; Becton Dickinson and Co., Sparks, Md.). We then estimated the MIC-2s, MIC-0s, and MFCs of PNT for the 10 Fusarium isolates under both microaerophilic and anaerobic conditions. We also determined the MICs and MFCs of AMB, 5FC, FLC, ITZ, VRC, and CAS against Fusarium under these hypoxic conditions.
Disk diffusion susceptibility testing.
We prepared a stock solution of PNT (60 mg/ml in distilled water) and stored it at −70°C until use. Disk diffusion testing was performed on RPMI agar plates previously prepared using standardized methods. Two hundred microliters of a standardized suspension of Fusarium conidia (106 conidia/ml) of each isolate was plated. After the plates were allowed to dry, a sterile 1/4-inch-thick paper disk (Schleicher & Schuell, Keene, N.H.) was placed on the agar surface and inoculated with 8.3 μl of PNT, producing a final PNT concentration in each plate of 20 μg/ml. Next, plates were placed in chambers containing envelopes that produced microaerophilic or anaerobic conditions as described above or were incubated in room air for 48 h at 35°C. The radius of the zone of inhibition was measured using a micrometer after 48 h. AMB was used as a control. Fifty microliters of a stock solution of AMB (5 mg/ml in dimethyl sulfoxide) was inoculated onto the paper disk, resulting in a final AMB concentration in each plate of 10 μg/ml. Three independent experiments were performed at different time points.
Statistical analysis.
The MIC-2s and MIC-0s of PNT for the 10 Fusarium isolates in RPMI medium were compared with those in YNB medium. Additionally, the MIC-2s, MIC-0s, and MFCs of PNT and the MICs and MFCs of VRC for F. solani isolates were compared with those for non-F. solani Fusarium isolates. The Mann-Whitney two-tailed t test was used to assess significant differences in the corresponding MIC-2s, MIC-0s, and MFCs. In addition, the MIC-2s and MIC-0s (as well as the radius of the zone of growth inhibition) of PNT for the 10 Fusarium isolates under aerobic conditions were compared with those under microaerophilic and anaerobic conditions. Analysis of variance was used to assess differences in the corresponding MICs, and Kruskal-Wallis one-way analysis of variance with Dunn's test was used with the GraphPad Prism 3 software program to assess statistically significant differences (GraphPad Software, Inc., San Diego, Calif.).
The 50% (EC50) and 90% (EC90) effective concentrations of PNT were determined by fitting XTT time-kill data to a sigmoidal dose-response model by using the following four-parameter logistic equation:
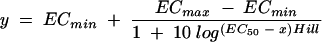 |
where ECmin represents the lowest PNT concentration producing inhibitory activity, ECmax represents the maximal PNT concentration that achieves maximal inhibitory activity, and Hill is the variable slope factor, or Hill coefficient of the dose-response curve.
RESULTS
Susceptibility of Fusarium spp. to antifungal agents and PNT.
We found that AMB, VRC, and PNT were the only active agents against the Fusarium isolates, whereas 5FC, FLC, ITZ, and CAS had no activity. The MICs and MFCs of AMB, 5FC, FLC, ITZ, VRC, and CAS are listed in Table 1. Specifically, AMB had comparable fungicidal activities against the solani and non-F. solani Fusarium isolates. Also, VRC was fungicidal against the non-F. solani Fusarium isolates, whereas it was fungistatic against the F. solani isolates. In comparison, ITZ had very limited fungistatic activity against the non-solani Fusarium isolates. The MIC-2s, MIC-0s, and MFCs of PNT against Fusarium isolates in RPMI and YNB media are shown in Table 2. PNT showed activity against all 10 of the Fusarium isolates tested. However, the MICs of PNT against non-F. solani Fusarium isolates were lower than those against F. solani isolates (P < 0.05). In addition, based on the MFC/MIC ratios, PNT was fungicidal against all five non-F. solani Fusarium isolates and one F. solani isolate and was fungistatic against the remaining four F. solani isolates. The PNT MICs were identical in the RPMI and RPMI-2 media (data for RPMI-2 medium not shown), whereas they were significantly lower in the YNB medium (P < 0.001). In contrast, the MFCs of PNT in the RPMI and YNB media did not differ significantly.
TABLE 1.
Susceptibilities of 10 clinical isolates of Fusarium spp. to antifungal agents in RPMI medium (NCCLS microdilution method M-38P)
| Agent |
F. solani isolates (n = 5)
|
Non-F. solani isolates (n = 5)
|
||||||
|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MFC50 | MFC90 | MIC50 | MIC90 | MFC50 | MFC90 | |
| AMB | 2a | 4 | 2 | 4 | 2 | 4 | 2 | 4 |
| 5FC | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| FLC | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| ITC | >16 | >16 | >16 | >16 | 8 | 8 | >16 | >16 |
| VRC | 4b | 8 | >16c | >16 | 1b | 1 | 8c | 8 |
| CAS | 32 | >32 | >32 | >32 | 32 | >32 | >32 | >32 |
Units are micrograms per milliliter throughout.
P = 0.09 (not significant) for VRC MICs against F. solani isolates versus those for non-F. solani Fusarium isolates.
P < 0.05 for VRC MFCs against F. solani isolates versus those for non-solani Fusarium isolates.
TABLE 2.
MIC-2s, MIC-0s, and MFCs of PNT against 10 clinical isolates of Fusarium spp. in RPMI and YNB media (NCCLS microdilution method M-38P)
| Fusarium sp. (no. of isolates) | RPMI medium
|
YNB medium
|
||||
|---|---|---|---|---|---|---|
| MIC50 (range, GMa)
|
MFC50 (range, GM) | MIC50 (range, GM)
|
MFC50 (range, GM) | |||
| MIC-2b | MIC-0c | MIC-2b | MIC-0c | |||
| F. solani (5) | 4g (2-4, 3.6)d | 32 (8-32, 24)e | >128 (8->128, 206.4)e | 1 (1-2, 1.4) | 8 (4-16, 10.4) | >128 (8->128, 206.4) |
| Non-F. solani (5) | 2 (1-2, 1.8)d | 8 (4-8, 6.4)e | 8 (4-16, 8.8)f | 0.5 (0.5-1.0, 0.6) | 1 (1-2, 1.2) | 8 (4-8, 6.4) |
GM, geometric mean.
P < 0.001 for MIC-2s of PNT against Fusarium spp. in RPMI medium versus those in YNB medium.
P < 0.05 for MIC-0s of PNT against Fusarium spp. in RPMI medium versus those in YNB medium.
P < 0.05 for MIC-2s of PNT against F. solani isolates versus those for non-F. solani Fusarium isolates.
P < 0.05 for MIC-0s of PNT against F. solani isolates versus those for non-F. solani Fusarium isolates.
P < 0.001 for MFCs of PNT against F. solani isolates versus those for non-F. solani Fusarium isolates.
Units are micrograms per milliliter throughout.
In agreement with the NCCLS results, use of the disk diffusion method showed that the radii of the zones of growth inhibition of F. solani isolates caused by PNT were smaller than those of non-F. solani Fusarium isolates. The mean radii for the F. solani and non-F. solani isolates were 5.867 mm (standard deviation [SD], ±2.694 mm) and 14.660 mm (SD, ±1.65 mm), respectively (P < 0.05).
Comparison of the visual and spectrophotometric readings of the NCCLS method for Fusarium spp.
The MIC-2 and MIC-0 endpoints of PNT as measured spectrophotometrically using the XTT colorimetric assay were comparable to the respective MICs estimated visually using the NCCLS method. More specifically, by using the MIC-2 endpoint, in 8 of the 10 Fusarium isolates the visual and spectrophotometric MIC-2s were identical, while in 2 isolates the values differed for one drug dilution. By using the MIC-0 endpoint, 9 of the 10 isolates had identical visual and spectrophotometric MIC-0s, whereas in 1 isolate those values differed for one drug dilution. Furthermore, in 9 of the 10 isolates tested, the MICs of AMB were identical when assessed both visually and spectrophotometrically.
XTT-based pharmacodynamic analysis of PNT activity.
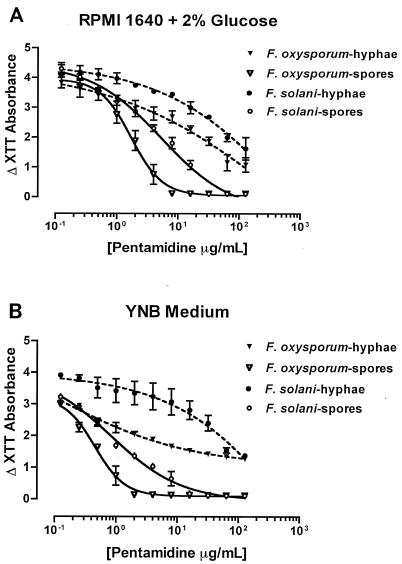
We generated dose-response curves of PNT and calculated the effective PNT concentrations that caused 50% (EC50) and 90% (EC90) reductions in XTT absorbance compared with drug-free XTT absorbance at 48 h after the addition of PNT. The EC50s and EC90s of PNT against the two developmental programs of growth of F. solani and F. oxysporum in two culture media are summarized in Table 3. Again, PNT showed preferential activity against the conidial development program of Fusarium spp. compared with the hyphal development program (Fig. 1). Also, in agreement with the MIC and MFC results, the EC50s and EC90s of PNT were lower against F. oxysporum than against F. solani and were lower in YNB medium than in RPMI-2 medium.
TABLE 3.
XTT-based pharmacodynamic analysis of PNT activities against two Fusarium spp. and two developmental programs in two culture mediaa
| Medium | Species | Developmental program | PNT conc (μg/ml)
|
|
|---|---|---|---|---|
| EC50 | EC90 | |||
| RPMI-2 | F. solani | Conidial | 3.9 | 29.7 |
| Hyphal | 53.1 | >128 | ||
| F. oxysporum | Conidial | 1.6 | 5.8 | |
| Hyphal | 30.3 | >128 | ||
| YNB | F. solani | Conidial | 1.4 | 8.6 |
| Hyphal | 50.5 | >128 | ||
| F. oxysporum | Conidial | 0.5 | 1.57 | |
| Hyphal | 17.8 | >128 | ||
The pharmacodynamic analysis of PNT against conidia was based on three isolates each of F. solani and F. oxysporum, whereas that of PNT against hyphae was based on two isolates each of F. solani and F. oxysporum.
FIG. 1.
XTT-based pharmacodynamic analysis of PNT activities against isolates of F. solani and F. oxysporum, the two developmental programs of growth of the fungi (conidia and hyphae), and two culture media (RPMI-2 and YNB). Graphs show reductions in XTT absorbance caused by PNT against F. solani and F. oxysporum conidia (three isolates each) and hyphae (two isolates each) in RPMI-2 medium (A) and YNB medium (B). Error bars show standard deviations, dashed lines represent fitted hyphal curves, and solid lines represent fitted conidial curves. The EC50s and EC90s were calculated using the four-parameter logistic equation. When starting with conidial inocula, PNT abrogated the conversion of conidia to hyphae in a dose-dependent fashion. Specifically, the germination of conidia to hyphal elements was completely inhibited at a concentration equal to the MIC-0 for the tested isolates. At a concentration approaching the MIC-2 for the tested isolates, PNT caused a nearly 50% reduction in XTT absorbance compared with the drug-free measured absorbance. When starting with hyphal inocula, PNT attenuated the increase in XTT absorbance compared with the drug-free absorbance in a dose-dependent fashion but it did not result in complete inhibition of hyphal growth in any of the isolates tested.
CFDA and DiBAC staining.
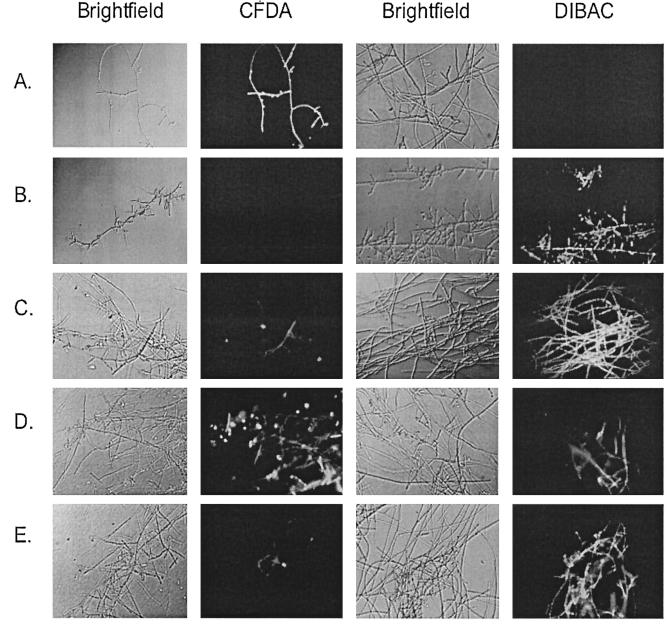
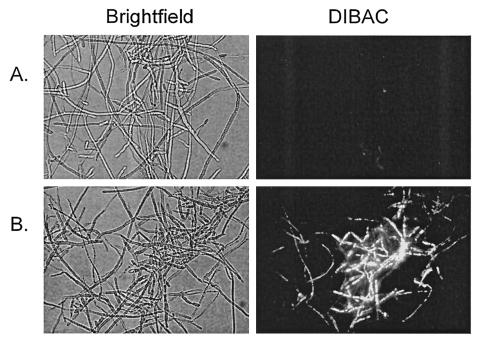
After staining with CFDA and DiBAC, we found that PNT killed apical and subapical compartments of viable F. oxysporum hyphae at a concentration equal to the MFC against the F. oxysporum isolate used. This fungicidal activity was more pronounced when hyphae were exposed to higher PNT concentrations. Figure 2D and E show the CFDA and DiBAC staining in a F. oxysporum isolate after challenge with PNT (at a concentration equal to the MFC and four times the MFC, respectively). Interestingly, AMB did not kill all of the hyphal elements at a concentration equal to the MFC (Fig. 2C). In the F. solani isolate tested, PNT had no fungicidal effects when added at the MIC-0; however, at a much higher concentration (equal to eight times the MIC-0 against that isolate), compartments of dead hyphae were seen (Fig. 3).
FIG. 2.
CFDA and DiBAC staining of F. oxysporum hyphae (isolate no. 2) exposed to H2O2, AMB, and PNT. Shown are untreated hyphae (A), H2O2-treated hyphae (1.5%) (B), AMB-treated hyphae (MFC, 4 μg/ml) (C), PNT-treated hyphae (MFC, 8 μg/ml) (D), and PNT-treated hyphae (four times the MFC, 32 μg/ml) (E).
FIG. 3.
DiBAC staining of F. solani hyphae (isolate no. 2) exposed to PNT. Shown are PNT-treated hyphae (MIC-0, 32 μg/ml) (A) and PNT-treated hyphae (eight times the MIC-0, 256 μg/ml) (B).
Activity of PNT under hypoxic conditions.
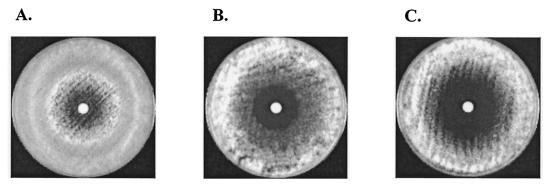
The MIC-2s and MIC-0s of PNT were lower under microaerophilic and anaerobic conditions than under normal oxygen concentrations (Table 4). The MFCs of PNT against all of the Fusarium isolates did not differ from those estimated under normal oxygen conditions. In contrast, the low-oxygen environment did not affect the MICs and MFCs of AMB, FLC, 5FC, ITZ, VRC, and CAS. By using the disk diffusion method, we found that the radius of the zone of growth inhibition caused by PNT was larger under microaerophilic and anaerobic conditions than under normal oxygen levels (P < 0.05) (Table 5) (Fig. 4). In contrast, the zone of growth inhibition caused by AMB was not affected by the different oxygen conditions, as the geometric means of the radii of the zones in a normal oxygen environment and under microaerophilic and anaerobic conditions were 1.83, 1.73, and 1.92 mm, respectively (P value was not significant).
TABLE 4.
MIC-2s and MIC-0s of PNT against 10 clinical isolates of Fusarium spp. under different oxygen conditions in RPMI medium (NCCLS microdilution method M-38P)
| Fusarium sp. (no. of isolates) | MIC50e (range, GMa)
|
|||||
|---|---|---|---|---|---|---|
| Aerobic conditions
|
Microaerophilic conditions
|
Anaerobic conditions
|
||||
| MIC-2b,c | MIC-0d | MIC-2b | MIC-0 | MIC-2c | MIC-0d | |
| F. solani (5) | 4 (2-4, 3.6) | 32 (8-32, 24) | 2 (1-2, 1.8) | 16 (8-16, 12.8) | 1 (1-2, 1.2) | 8 (4-16, 8.8) |
| Non-F. solani (5) | 2 (1-2, 1.8) | 8 (4-8, 6.4) | 0.5 (0.5-1, 0.6) | 2 (1-2, 1.6) | 0.250 (0.125-0.500, 0.220) | 1.0 (0.5-1.0, 0.9) |
GM, geometric mean.
P < 0.05 for MIC-2s of PNT against Fusarium spp. under aerobic conditions versus those under microaerophilic conditions.
P < 0.001 for MIC-2s of PNT against Fusarium spp. under aerobic conditions versus those under anaerobic conditions.
P < 0.05 for MIC-0s of PNT against Fusarium spp. under aerobic conditions versus those under anaerobic conditions.
Units are micrograms per milliliter throughout.
TABLE 5.
Disk diffusion susceptibility testing of PNT activities against Fusarium spp. under different oxygen conditions
| Fusarium sp. (no. of isolates) | GMa ± SD of the radii (mm) of the zones of inhibition caused by PNTb
|
||
|---|---|---|---|
| Aerobic conditions | Microaerophilic conditions | Anaerobic conditions | |
| All Fusarium spp. (10) | 10.267 ± 3.829c | 17.283 ± 3.695 | 20.783 ± 5.168c |
| F. solani (5) | 5.867 ± 2.694d,e | 13.2 ± 3.532 | 16.6 ± 3.227e |
| Non-F. solani (5) | 14.66 ± 1.65d,f | 21.367 ± 2.162 | 24.967 ± 2.434f |
GM, geometric mean.
Each disk contained 8.3 μl of PNT (from a stock solution of 60 mg/ml).
P < 0.05 for the radii of the zones of growth inhibition caused by PNT against all Fusarium spp. tested under aerobic conditions versus those under anaerobic conditions.
P < 0.05 for the radii of the zones of growth inhibition caused by PNT against F. solani isolates versus non-F. solani Fusarium isolates in room air.
P < 0.05 for the radii of the zones of growth inhibition caused by PNT against F. solani isolates under aerobic conditions versus those under anaerobic conditions.
P < 0.05 for the radii of the zones of growth inhibition caused by PNT against non-F. solani Fusarium isolates under aerobic conditions versus those under anaerobic conditions.
FIG. 4.
Effects of PNT against an F. solani isolate (isolate no. 2) under different oxygen conditions, i.e., natural oxygen conditions (A), microaerophilic conditions (B), and anaerobic conditions (C), as seen in disk diffusion susceptibility testing (each disk contained 8.3 μl of PNT from a 60-mg/ml stock solution).
DISCUSSION
We investigated the in vitro activities of PNT against 10 clinical isolates of Fusarium spp. and against the different developmental programs of the fungus by using independent susceptibility methods, different media, and various environmental conditions. The susceptibilities of Fusarium isolates to the other antifungals tested were in agreement with those reported in previous studies (4, 13, 25). Specifically, AMB and VRC were the only other active agents. However, the MIC50s and MFC50s of AMB were higher than the previously reported tissue concentration of AMB in the lungs (0.5 μg/ml) when conventional intravenous AMB doses (1.0 to 1.5 mg/kg of body weight/day) were used (15). Furthermore, the activity of VRC was variable and depended on the Fusarium species tested. Although VRC was fungicidal against the non-F. solani Fusarium isolates, it had moderate fungistatic effects against the F. solani isolates. As previously reported, CAS, FLC, and ITZ had no activity against the Fusarium isolates that were tested (4, 13, 25).
Here, we demonstrate that PNT has notable in vitro activity against Fusarium spp. PNT had higher activity and was fungicidal against the non-F. solani Fusarium isolates, while it had fungistatic effects against the majority of the F. solani isolates. Several studies have shown that F. solani is, in general, more resistant to antifungals than are non-F. solani Fusarium spp. (4, 13, 25, 27). In our study, this higher resistance of F. solani isolates versus non-F. solani isolates against PNT was also observed with the newer triazoles ITZ and VRC.
In addition, the activity of PNT was not medium dependent, as it was seen in all culture media tested. Nevertheless, PNT had significantly enhanced activities against all of the isolates in YNB medium. A similar discrepancy in the efficacies of several drugs (including PNT) in the rich YNB medium was described previously (2). It has been postulated that the YNB medium facilitates fungal growth, with resultant higher levels of metabolic activity and better drug penetration into the intracellular site of action (18). In fact, Meletiadis et al. (18) showed poorer growth of Aspergillus and Zygomycetes isolates in RPMI and RPMI-2 media than in YNB medium. Nonetheless, we were not able to account for the enhanced activity of PNT against Fusarium spp. in YNB medium based on the aforementioned hypothesis. We found that Fusarium growth was poorer in YNB medium (data not shown), an observation previously described for S. prolificans (18).
The fungicidal activities of PNT against the non-F. solani Fusarium isolates were further confirmed microscopically via staining of a PNT-treated F. oxysporum isolate with the fluorescent dyes CFDA and DiBAC, which have been successfully used to evaluate the fungicidal effects of AMB and CAS against C. albicans and Aspergillus fumigatus (8, 16). In the present study, we found microscopically that administration of PNT appeared to result in the death of parts of viable F. oxysporum hyphae at a PNT concentration equal to the MFC of the isolate used. Interestingly, PNT had similar effects against F. solani, although at much higher concentrations.
The limited number of pharmacokinetic studies using tissue samples obtained from PNT-treated humans upon autopsy have shown high concentrations of PNT in tissues when the drug is administered at a conventional therapeutic dose (4 mg/kg/day intravenously) (6). Blood PNT concentrations have been reported to range from 0.5 to 3.2 μg/ml (6). However, much higher levels are typically seen in tissues, with concentrations of up to 56 μg/g in the lungs, 123 μg/g in the kidneys, 300 μg/g in the liver, and 368 μg/g in the spleen (6). When compared with these reported tissue concentrations of PNT, the MIC50s, MIC90s, and MFC50s of PNT in our study are much lower.
By using XTT colorimetric assay, we demonstrated preferentially increased activity of PNT against the conidial versus the hyphal developmental state of the fungus. This observation is consistent with the results of several studies showing that the hyphal forms of filamentous fungi are inherently more resistant to antifungal agents than are the conidial forms (12, 14). Guarro et al. (12) showed that the MICs and MFCs of AMB, 5FC, FLC, miconazole, ketoconazole, and ITZ against Cladosporium, Paecilomyces, Scopulariopsis, and Cladophialophora species were significantly (2- to 512-fold) higher when these antifungals were tested against already formed hyphae than when the antifungals were tested against the conidial forms of these molds. Similar results have been observed for Aspergillus species (14).
We found that the EC50s and EC90s of PNT against the conidial forms of Fusarium spp. were significantly lower than the PNT concentrations that can be achieved in tissues (6). The respective PNT values against the hyphal forms of the Fusarium spp. were appreciably higher, yet the EC50s were again lower than the achievable PNT concentrations. Conidial colonization is the first step in the pathogenesis of invasive fusariosis. In immunocompetent hosts, conidia are inhaled into the lungs in large numbers and are efficiently phagocytosed there by the resident macrophages (9). However, in immunocompromised patients with defects in macrophage function, some conidia escape phagocytosis, germinate to hyphae, and establish an invasive infection in the setting of prolonged neutropenia (9). Thus, our findings imply that PNT could be more promising for prophylaxis against Fusarium infections.
Even though PNT has been used clinically for more than 2 decades, the mechanisms of its action have not been definitively established. Several mechanisms have been proposed, such as inhibition of DNA, RNA, phospholipid, and protein synthesis (26). Interestingly, we found that PNT was more effective against Fusarium spp. under low-oxygen conditions, as shown using two independent methods: the NCCLS microdilution method and disk diffusion susceptibility testing. Even though this difference may reflect less vigorous growth of the fungus in an environment of low-oxygen tension, these data may indicate that PNT actually has preferentially greater efficacy against Fusarium spp. under such oxygen conditions, as the hypoxic environment did not alter the activities of the other antifungals tested. Whether this observation reflects the conversion of PNT to an active prodrug in the setting of low-oxygen tension in a mechanism reminiscent of the activity of metronidazole against bacteria (10) remains to be determined. However, these results have promise for further in vivo testing of PNT against fusariosis. Like Aspergillus, Fusarium is an angiotropic mold that invades vessels, resulting in tissue infarcts, low tissue perfusion, and suboptimal efficacy of antifungals (P. J. Patterson, E. M. Johnson, S. Ainscough, H. G. Prentice, M. Potter, and C. C. Kibbler, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1328, 2000). Given the establishment of a semianaerobic environment as a result of invasive fusariosis, this in vitro enhanced activity of PNT under hypoxic conditions may be of clinical importance.
In conclusion, this report is the first description of the in vitro activities of PNT against a variety of pathogenic Fusarium spp. Our findings suggest that the role of PNT in the treatment of Fusarium infections should be explored further. More studies using appropriate animal models and possibly clinical studies will be needed to elucidate the potential of this drug for prophylaxis or treatment of Fusarium infections in immunocompromised patients. Such studies are currently under way in our laboratory.
Acknowledgments
We thank Kerry O'Donnell (U.S. Department of Agriculture) for his help in the molecular identification of the Fusarium isolates tested in this study.
REFERENCES
- 1.Afeltra, J., E. Dannaoui, J. F. Meis, J. L. Rodriguez-Tudela, and P. E. Verweij. 2002. In vitro synergistic interaction between amphotericin B and pentamidine against Scedosporium prolificans. Antimicrob. Agents Chemother. 46:3323-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afeltra, J., J. F. Meis, R. G. Vitale, J. W. Mouton, P. E. Verweij, and the Eurofung Network. 2002. In vitro activities of pentamidine, pyrimethamine, trimethoprim, and sulfonamides against Aspergillus species. Antimicrob. Agents Chemother. 46:2029-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anaissie, E. J., R. Hachem, C. Legrand, P. Legenne, P. Nelson, and G. P. Bodey. 1992. Lack of activity of amphotericin B in systemic murine fusarial infection. J. Infect. Dis. 165:1155-1157. [PubMed] [Google Scholar]
- 4.Arikan, S., M. Lozano-Chiu, V. Paetznick, S. Nangia, and J. H. Rex. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchiesi, F., M. Del Poeta, V. Morbiducci, F. Ancarani, and G. Scalise. 1994. Effect of pentamidine on the growth of Cryptococcus neoformans. J. Antimicrob. Chemother. 33:1229-1232. [DOI] [PubMed] [Google Scholar]
- 6.Bernard, E. M., H. J. Donnelly, M. P. Maher, and D. Armstrong. 1985. Use of a new bioassay to study pentamidine pharmacokinetics. J. Infect. Dis. 152:750-754. [DOI] [PubMed] [Google Scholar]
- 7.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 8.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons, K. V., V. L. Calich, E. Burger, S. G. Filler, M. Grazziutti, J. Murphy, E. Roilides, A. Campa, M. R. Dias, J. E. Edwards, Jr., Y. Fu, G. Fernandes-Bordignon, A. Ibrahim, H. Katsifa, C. G. Lamaignere, L. H. Meloni-Bruneri, J. Rex, C. A. Savary, and C. Xidieh. 2000. Pathogenesis I: interactions of host cells and fungi. Med. Mycol. 38:99-111. [PubMed] [Google Scholar]
- 10.Edwards, D. I. 1979. Mechanism of antimicrobial action of metronidazole. J. Antimicrob. Chemother. 5:499-502. [DOI] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarro, J., C. Llop, C. Aguilar, and I. Pujol. 1997. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of filamentous fungi. Antimicrob. Agents Chemother. 41:2760-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarro, J., I. Pujol, and E. Mayayo. 1999. In vitro and in vivo experimental activities of antifungal agents against Fusarium solani. Antimicrob. Agents Chemother. 43:1256-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig, H., and M. Kremer. 1979. A propos des discordances observees dans les resultants des CMI faites sur spores ou filaments d'Aspergillus. Bull. Soc. Fr. Mycol. Med. 8:237-242. [Google Scholar]
- 15.Koizumi, T., K. Kubo, T. Kaneki, M. Hanaoka, T. Hayano, T. Miyahara, K. Okada, K. Fujimoto, H. Yamamoto, T. Kobayashi, and M. Sekiguchi. 1998. Pharmacokinetic evaluation of amphotericin B in lung tissue: lung lymph distribution after intravenous injection and airspace distribution after aerosolization and inhalation of amphotericin B. Antimicrob. Agents Chemother. 42:1597-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao, R. S., R. P. Rennie, and J. A. Talbot. 1999. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob. Agents Chemother. 43:1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayayo, E., I. Pujol, and J. Guarro. 1999. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J. Med. Microbiol. 48:363-366. [DOI] [PubMed] [Google Scholar]
- 18.Meletiadis, J., J. F. Meis, J. W. Mouton, and P. E. Verweij. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 39:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, P. E. Verweij, and the Eurofung Network. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miletti, K. E., and M. J. Leibowitz. 2000. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob. Agents Chemother. 44:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris-Jones, S. D., and P. J. Easterbrook. 1997. Current issues in the treatment and prophylaxis of Pneumocystis carinii pneumonia in HIV infection. J. Antimicrob. Chemother. 40:315-318. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.O'Donnell, K., H. C. Kistler, E. Cigelnik, and R. C. Ploetz. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 95:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro activities of investigational triazoles against Fusarium species: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob. Agents Chemother. 46:3298-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pujol, I., J. Guarro, J. Gene, and J. Sala. 1997. In vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrob. Chemother. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 26.Sands, M., M. A. Kron, and R. B. Brown. 1985. Pentamidine: a review. Rev. Infect. Dis. 7:625-634. [DOI] [PubMed] [Google Scholar]
- 27.Torres, H. A., and D. P. Kontoyiannis. 2003. Hyalohyphomycoses, p. 252-270. In W. E. Dismukes, P. G. Pappas, and J. D. Sobel (ed.), Oxford textbook of clinical mycology. Oxford University Press, New York, N.Y.
- 28.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]