Abstract
When the essential and distinctive cell walls of either pathogenic or nonpathogenic fungi break, cytoplasmic membranes rupture and fungi die. This fungicidal activity was discovered previously on nonproliferating Saccharomyces cerevisiae cells treated briefly with the oxidative tool and anticancer drug family of bleomycins. The present studies investigated effects of bleomycin on growing fungal organisms. These included the medically important Aspergillus fumigatus and Cryptococcus neoformans, as well as the emerging human pathogen and fungal model, S. cerevisiae. Bleomycin had its highest potency against A. fumigatus. Scanning electron microscopy and thin-section transmission electron microscopy were used to study morphological growth characteristics. Killing and growth inhibition were also measured. Long, thin, and segmented hyphae were observed when A. fumigatus was grown without bleomycin but were never observed when the mold was grown with the drug. Bleomycin arrested conidial germination, hyphal development, and the progression and completion of cell wall septation. Similarly, the drug inhibited the construction of yeast cell wall septa, preventing cytokinesis and progression in the cell division cycle of S. cerevisiae. Even when cytoplasms of mother and daughter cells separated, septation and cell division did not necessarily occur. Bizarre cell configurations, abnormally thickened cell walls at mother-daughter necks, abnormal polarized growth, large undivided cells, fragmented cells, and empty cell ghosts were also produced. This is the first report of a fungicidal agent that arrests fungal growth and development, septum formation, and cytokinesis and that also preferentially localizes to cell walls and alters isolated cell walls as well as intact cell walls on nongrowing cells.
The unique cell wall that surrounds fungal cells is absolutely essential for their survival. The cell wall of pathogenic fungi has evolved for survival from environmental factors and host defenses (7, 16, 18, 21, 60). The ability of cell walls to evade such factors contributes to the power of pathogenic fungi to cause a wide spectrum of clinical disease (45). Moreover, the unique arrangement of the molecular species forming the structure of the fungal cell wall makes it an excellent target for drug intervention (3, 6, 24, 44, 46, 54). Thus, the elucidation of the mechanisms of action of agents that interact with the molecules forming the cell walls will yield new insights into fungal cell wall structure, formation of cell walls between dividing cells, and cell division. For fungal pathogens, an understanding of the mechanisms of action of cell wall-specific agents should assist in the design of wall-specific antifungal drugs.
Saccharomyces cerevisiae has long been a unicellular fungal model for studies of basic mechanisms. Since this budding yeast is also an emerging opportunistic human pathogen (15, 38, 42) with properties frequently observed in pathogenic fungi (4, 29, 30), the yeast is also developing as a model fungal pathogen (12). The discovery that the fungal cell wall is a target of a family of oxidative agents widely used for anticancer chemotherapy was made in S. cerevisiae (35). The mechanism of action of the low-molecular-weight bleomycins (Mr approximately 1,500 to 1,600 [57, 58]), structurally related metalloglycopeptidic antibiotics (5), involves an initial localization (35) and subsequent injury to cell walls (23, 32, 35), altered anchorage of mannoproteins to the cell wall matrix (2), and the release of specific species of surface mannoproteins from intact as well as isolated cell walls (2).
For the present studies, we investigated the effects of the metalloglycopeptides on fungal cell walls, cell growth, cell wall septation, and cell division. We reasoned that these basic cellular processes could be at risk, since a specific target of the bleomycin drug family is the fungal cell wall (35). The common opportunistic fungal pathogen Aspergillus fumigatus was chosen to study the effects of the drug family on conidial germination, hyphal development, and growth. Aspergillus infections cause a high proportion of opportunistic fungal infections in immunocompromised patients and, worldwide, aspergillosis is the most common mold infection (20). S. cerevisiae was selected to investigate potential blocks in the growth of yeast cells, development of the yeast cell wall, formation of septa, and cytokinesis. The growth inhibitory effects of bleomycin on A. fumigatus, S. cerevisiae, and the opportunistic fungal pathogen Cryptococcus neoformans were also compared. Cryptococcal disease is a prevalent life-threatening infection, particularly in immunocompromised patients such as patients with AIDS or cancer (1, 14, 62). The studies identified targets of the class of compounds represented by the bleomycin drug family.
MATERIALS AND METHODS
Fungal strains and culturing conditions.
A. fumigatus (isolate H11941), C. neoformans (a clinical isolate cultured in the Memorial Hospital Microbiology Laboratory from a patient at The Memorial Sloan-Kettering Center Infectious Disease Service), and S. cerevisiae (ATCC 36375) were inoculated at 2 × 104 CFU per ml in RPMI medium (American Bioorganics, Inc., Niagara Falls, N.Y.) according to our published procedures (39). S. cerevisiae strain CM1069-40 (37) was inoculated at 5 × 103 or 5 × 105 cells per ml into nonsynthetic complete growth medium (YPAD [31]).
The liquid broth microdilution assays we used were previously described (39) and were a modification of the method proposed by the National Committee for Clinical Laboratory Standards (9). The lowest drug concentration that prevented visible growth was considered the MIC. The A. fumigatus, C. neoformans, and S. cerevisiae ATCC 36375 strains are used routinely in our antifungal susceptibility testing to compare responses of different fungal strains. The responses of ATCC 36375 to the lethal effects of bleomycin and in microdilution assays are identical to those of CM1069-40 and many other S. cerevisiae strains we have tested.
An anticancer formulation, a mixture of 11 bleomycins differing in their terminal amines (approximately 55 to 70% [usually 68 to 69%] bleomycin A2 and approximately 25 to 32% bleomycin B2 [5; W. T. Bradner, Bristol Myers Squibb Laboratories, personal communication]), was used in these studies. Bleomycins were dissolved and diluted in deionized water just prior to use. The final pH was 5. The absorbance of bleomycin, whose extinction coefficient is 1.45 × 104, was monitored at 292 nm. Detailed reaction conditions were described previously (33).
Quantitative measurements of viability
Quantitative measurements were carried out according to published protocols (31, 33, 36). S. cerevisiae cell (strain CM1069-40) was inoculated at 5 × 103 cells/ml (experiment 1) or 5 × 105 cells/ml (experiments 2 and 3) in YPAD medium and incubated with aeration for 18 h at 30°C. At the end of 18 h, the numbers of cells were determined by counting the cells using a light microscope and hemacytometer. This direct microscopic count included both viable and inviable cells. To determine the number of viable cells, appropriate dilutions of the cell populations were plated on YPAD medium and incubated at 30°C for 3 to 7 days. The viability of each cell population was calculated from the ratio of cells that formed colonies to the total number of cells determined by direct microscopic count. The results in Table 1 are the means and standard errors of the three independent experiments.
TABLE 1.
Growth and viability of yeast cells after 18 ha
| Drug (μg/ml) | Expt 1
|
Expt 2
|
Expt 3
|
|||
|---|---|---|---|---|---|---|
| No. of cell doublings | Viable cells (%) | No. of cell doublings | Viable cells (%) | No. of cell doublings | Viable cells (%) | |
| 0 | 13.1 ± 0.6 | 96 | 9.3 ± 0.3 | 95 | 11.3 ± 0.4 | 95 |
| 25 | 0.73 ± 0.08 | 0.72 | 1.3 ± 0.07 | 0.13 | 1.09 ± 0.08 | 0.34 |
Experiments are listed individually to show the numbers of generations that cells grew from the inocula in each experiment and the reproducibility of growth inhibition and killing among the treated cultures. These potential variables would not be factored into a single statistical mean value and its associated standard error.
SEM.
Fungal cells were prepared for scanning electron microscopy (SEM) according to the initial fixation and dehydration steps previously published (35) followed by critical point drying, where the alcohol was exchanged for liquid CO2. The CO2 was removed at the critical temperature and pressure, mounted on an aluminum stub, and coated with a light coat of gold palladium. Specimens were then viewed with an S-530 Hitachi scanning electron microscope.
TEM.
Fungal cells were prepared for transmission electron microscopy (TEM) as previously published (35). KMnO4 was omitted during cell fixation to preserve the integrity of the cell walls and membranes and permit good visualization of these organelles (35). The stage of the electron microscope was tilted at multiple angles to optimize visualization perpendicular to the plane of the electron beam of hyphal branching, bud neck regions, cell walls, and the development of septa. Multiple sections through the specimens were also made to analyze the frequency and consistency of alterations.
RESULTS
A. fumigatus.
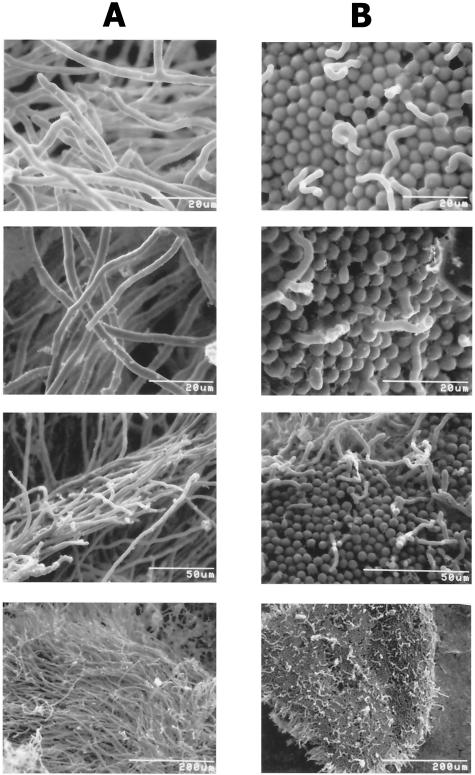
We first set out to determine if conidial germination or hyphal development was in some way affected by the oxidative drug bleomycin. SEM was used to examine the morphologies of A. fumigatus cells after conidia were treated with bleomycin (Fig. 1). Conidia that were inoculated and grown without the drug germinated normally. They developed typically elongated and elaborately branched masses of hyphae forming mycelia (Fig. 1A). In contrast, conidial germination and development of hyphae were severely inhibited in a dose-dependent manner by low concentrations of bleomycin. The profound inhibition of germination and hyphal development in 10 μg of drug/ml is illustrated in Fig. 1B. A. fumigatus cells exhibited virtually no normal hyphal growth compared to their matched untreated controls. Not only was germination rare, but any hyphae that began to develop were aberrant and aborted. The few hyphae that were observed were short, curved, and bulbous at the tips. Conidial germination was completely arrested, and all of the A. fumigatus cells were ungerminated in 100 μg of bleomycin/ml (SEM results not shown).
FIG. 1.
Scanning electron micrographs of the fungal pathogen A. fumigatus growing in the absence or presence of bleomycin antibiotics. Conidia from isolate H11941 were inoculated at 2 × 104 conidia per ml according to our published procedures (39) and incubated with aeration for 24 h at 30°C. (A) The four panels (at left) show results of incubation without drug. (B) The four panels (at right) show results of incubation with 10 μg of bleomycin/ml. Magnifications are highest in the micrographs in the first two rows, intermediate in the third row, and lowest in the bottom row.
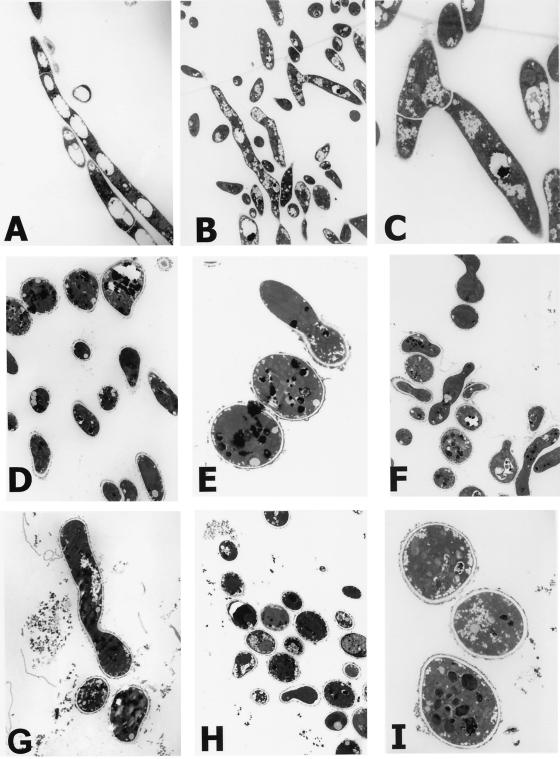
To further investigate the blocks in conidial germination and hyphal development, thin-section TEM was employed (35). Observations of multiple planes and angles of each specimen made from the pellets of untreated populations revealed mixtures of segmented hyphae in all directions. Long, thin hyphae were found distributed throughout sequential slices through the pellets, although it was difficult from the planes that were cut to identify segmentation and branching in all hyphae since hyphae were longitudinal, oblique, and traverse in the thin sections (e.g., Fig. 2A and B). The micrographs in Fig. 2A through C illustrate the normal hyphae and septa formed after cell division when A. fumigatus grew without bleomycin. The septa and typical acute angle branching shown in the field in Fig. 2B are enlarged in Fig. 2C. Cell walls also appeared intact, with no evidence of the loss of contents of hyphae or conidia. Intracellular structures and organelles also appeared normal.
FIG. 2.
Transmission electron micrographs of A. fumigatus cells grown with or without drug. Conidia were inoculated and grown as described in the legend for Fig. 1. Specimens were not treated with potassium permanganate (KMnO4). (A to C) 0 μg/ml; (D to G) 10 μg/ml; (H and I) 100 μg/ml. Magnifications for the panels were as follows: A, ×2.3K; B, ×1.7K; C, ×6.1K; D, ×2.8K; E, ×8.5K; F, ×2.2K; G, ×3.9K; H, ×1.7K; I, ×8.5K.
In contrast, A. fumigatus structures were rounded, and long, thin, or segmented hyphae were never observed in pellets of treated populations. The TEM of cultures grown in the presence of 10 μg of bleomycin/ml revealed the ultrastructure of nongerminated conidia and aborted hyphae (Fig. 2D through G) and that some conidia became quite irregularly shaped. The rare hyphae observed in thin sections (Fig. 2F and G) had started to grow and somewhat resembled pseudohyphae except that they were highly irregular and aborted. They did not continue to grow, linearize, or branch normally, and septa were not created. The outer layers of the walls were highly irregular and focally thickened, a feature that was a hallmark of yeast forms exposed to bleomycin in 30-min treatments (35). In 100 μg of drug/ml, none of the conidia germinated (e.g., Fig. 2H and I), and many lysed cells were observed by light microscopy of the cultures and by TEM (data not shown). The TEM micrographs presented in Fig. 1 and 2 together with the findings from the direct observations in tilted and multiple planes of sections from untreated and treated populations confirm that the distinctions between the untreated and treated populations were due to the bleomycin treatments.
A. fumigatus, C. neoformans, and S. cerevisiae: comparisons of growth inhibition.
Like A. fumigatus, C. neoformans, and S. cerevisiae were grown in the presence of a wide range of bleomycin concentrations and compared to control populations grown without the drug (50). Low drug concentrations inhibited the growth of all three fungal organisms. After 24 h, A. fumigatus and S. cerevisiae were equally sensitive (MIC = 3.2 μg/ml) and C. neoformans was slightly less sensitive (MIC = 6.4 μg/ml) to the growth inhibitory effects of the drug. In fact, A. fumigatus was the most sensitive of all the opportunistic fungal pathogens we tested, and it was more sensitive than most of the S. cerevisiae strains we studied (data not shown).
Loss of viability of cells cultured with bleomycin.
Quantitative measurements of the killing and numbers of cell generations (doublings) were made among S. cerevisiae populations grown in the presence and absence of bleomycin. As illustrated in Table 1, cell populations on the average never completed more than one doubling in the presence of the drug, consistent with the blocks before cytokinesis recorded in the SEM images. Approximately 33% of the cells appeared arrested with a daughter cell nearly the same size as the mother cell while attempting to divide. However, more than 99% of the cells in each population died (Table 1), and killing was even higher when cells were incubated longer than 18 h or incubated with higher concentrations of bleomycin (data not shown). Under the same growth conditions, cells grew 9 to 13 generations without the drug and were viable (Table 1). Less than 1% of these cells had a bud of substantial size.
Budding yeast: SEM and TEM.
S. cerevisiae was studied in more detail by using SEM and TEM. In the absence of drug, the sizes and shapes of the yeast cells were normal, as illustrated in the SEM micrographs in Fig. 3A and B. Typical of cells in the stationary phase of growth, cells were also predominantly singlet and contained multiple bud scars, indicating they had divided multiple times. In contrast to the normal cell division in these healthy cell populations, high frequencies of the cells grown with bleomycin had not divided (Fig. 3C through F). Cell shapes were highly irregular, and cell sizes varied a great deal. In addition, bizarre cell configurations were frequent, cells were mostly budded, and very few bud scars were seen. These observations indicated that cell cycle progression was blocked in these cells and the cells were unable to complete division.
FIG. 3.
Representative scanning electron micrographs illustrating aberrant cell division and inhibition of cytokinesis in the model yeast, S. cerevisiae. Similar to the conidia of A. fumigatus, the yeast cells (strain CM1069-40) were inoculated at 5 × 103 cells/ml into YPAD growth medium and incubated with aeration in replicate cultures without drug (A and B) or with 25 μg of bleomycin/ml (C to F) for 18 h at 30°C.
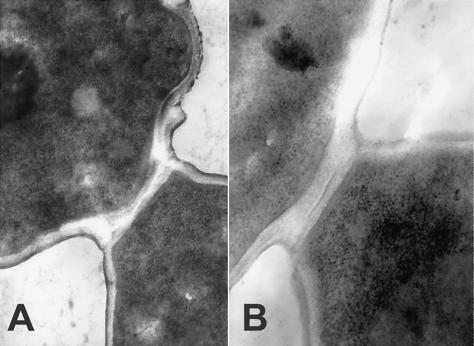
Thin sections of these cells were studied to examine cell septation and determine how cell walls were affected, why the cells died, and where in the cell cycle cells were arrested. Constructing a septum between mother and daughter cells is part of normal cell growth and division. Figure 4 illustrates the progression of normal septum formation in untreated cells that follows bud emergence and nuclear division. As buds enlarge and become equal in size to parental cells, the nucleus and cytoplasm distribute equally between mother and daughter, and normal constriction progresses between the two cells prior to full septum formation and cell division.
FIG. 4.
Transmission electron micrographs illustrating normal septum formation in S. cerevisiae (strain CM1069-40) during cell division. Yeast were inoculated and grown without drug as described in the legend for Fig. 3. After the progression from a mother cell with a small bud to a mother cell with a bud of the same size, a constriction forms at the mother-bud neck prior to septum formation and cell division. Magnifications were as follows: A, ×24.75K; B, ×36K.
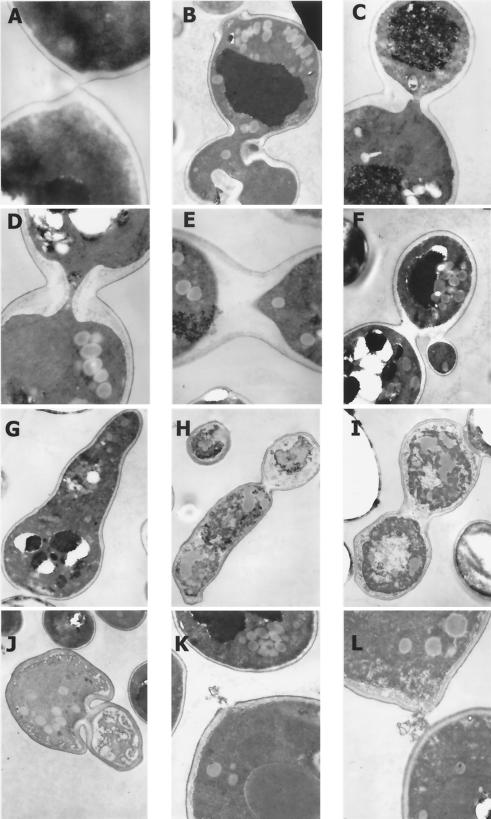
The normal progression of septation (Figs. 4) and cytokinesis (Fig. 5A) as well as the intact cell perimeters (Fig. 5A) observed among cells growing without bleomycin contrast with the abnormal cell division observed at high frequencies among cells that tried to divide in the presence of the drug. Representative and common configurations are illustrated in Fig. 5B through J. Many cells were enlarged, elongated, empty ghosts, or fragmented, consistent with the extremely low viability and absence of cell divisions revealed in the quantitative analyses (Table 1). Typically during growth, the mother cell and daughter cell did not pinch off completely, cell walls were abnormally thick and irregularly shaped, and polarized growth was abnormal. In Fig. 5B, interruptions and thickening of the cell wall around both mother and bud, focal thickening of the cell wall at the bud neck, and a shredded and broken wall on the bud scar of the mother cell all accompanied the distorted shape of the mother cell and its large bud. The cell wall in Fig. 5C was focally thickened in different regions, most severely at the constriction between mother and bud. Moreover, the cytoplasm was distributed asymmetrically between mother and bud at the constrictions in Fig. 5B and C. In Fig. 5D, the wall is grossly thickened at the constriction between mother and bud, in contrast to normal division (Fig. 4 and 5A). In Fig. 5E and F, the cytoplasms of the mother and daughter cells completely divided but without cell division. A bud is shown emerging abnormally from the daughter cell in Fig. 5F.
FIG. 5.
Representative transmission electron micrographs illustrating normal cytokinesis and examples of antifungal effects of bleomycin on S. cerevisiae (strain CM1069-40). Cells were inoculated and grown as described for Fig. 3 and 4. KMnO4 was not added during cell fixation. (A) 0 μg/ml; (B to L) 25 μg/ml. Magnifications were as follows: A, ×33K; B, ×6.4K; C, ×8.2K; D and E, ×10.24K; F, ×8.2K; G, ×5.6K; H, ×4.3K; I and J, ×8.2K; K and L, ×10K.
A cell representative of those that grew abnormally large and elongated is shown in Fig. 5G, and the thin-section micrograph showed that neither its cytoplasm nor cell wall divided. Focal thickening is observed along the cell wall in several places, and a part of the cell wall is missing. In addition, cell division was not completed between this giant cell and an adjacent cell (Fig. 5G, lower left). Another peculiar yet common form is illustrated in Fig. 5H, where an irregularly shaped cell elongated without dividing. The cell wall also developed abnormally, as it focally thickened at the constriction as well as in other places. In the singlet ovoid cell in the upper left of Fig. 5H, most of the wall is actually missing, indicating severe damage. In Fig. 5I, the cytoplasms divided in the triplet cell, but an abnormal septum formed at the upper construction and the wall thickened without septation at the bottom constriction. Cytokinesis failed in both divisions.
Cell wall development and thickening are also grossly abnormal throughout the bizarre yeast form in Fig. 5J, where the cytoplasm but not the cell wall divided. Among some of the cells that remained somewhat intact, parts of the cell wall, membrane, and cytoplasm were lost (Fig. 5K and L).
DISCUSSION
This is the first report of a chemical or drug that blocks fungal septum formation and cytokinesis and that also preferentially localizes to cell walls (35) and alters intact cell walls on nongrowing cells (2, 23, 32, 35, 40) and isolated cell walls (2). In this regard, the bleomycin drug family represents a previously unknown class of cidal antifungal agents. The results presented in this report indicate that the blocks in the growth and development of A. fumigatus and S. cerevisiae cells stopped progression of the cell division cycle, preventing cytokinesis. From the collective data from quantitative measurements of cell growth and division, the scanning and transmission microscopy studies, and the MICs, the model that emanates is that cells finish a division if formation of the cell wall septum progresses sufficiently to permit cytokinesis, but cells are unable to undergo another division. Practically all of the S. cerevisiae cells died after 18 h (Table 1).
Bleomycin causes the destruction of cell wall components by an oxidative mechanism (2, 23, 32, 35, 40). Because bleomycin destroys both intact and isolated cell walls, we believe that the drug damages newly formed cell wall polymers as well as preexisting polymers. In these respects, the bleomycin action is different from the action of RO-09-3143, which was shown by Sudoh and coworkers to be an inhibitor highly specific to one of the three fungal chitin synthases in Candida albicans, CaChs1p (55). Although our belief that bleomycin affects cell wall polymers that are already formed is supported by our group's bleomycin studies thus far (2, 23, 32, 34, 35, 40; present report), the findings do not rule out the possibility that the drug also affects the synthesis of one or more cell wall components.
It would be useful to determine efficacies against aspergillosis and cryptococcosis in model systems. In many clinical situations, A. fumigatus is enormously difficult to control by drugs and is lethal (8, 20). The sequence of infectious events is that the asexual spores, the conidia, germinate and produce multicellular hyphae which elongate and branch into networks of hyphae (7, 45, 51). Various Aspergillus species are airborne throughout the world all of the time, and the inhalation of conidia of A. fumigatus can cause fulminating infections in immunocompromised organisms as well as acute toxic reactions (8, 20). C. neoformans is now a major opportunistic pathogen worldwide, and its infections are deadly (14, 15, 41, 52). With the widespread epidemic of AIDS, occurrences of cryptococcosis of the central nervous system have increased sharply (10, 26, 61), and C. neoformans has become a leading cause of morbidity and mortality in these patients (19, 43, 56). Cryptococcal meningitis is the most common life-threatening mycosis in individuals with human immunodeficiency virus type 1 infection, and additional central nervous system disorders caused by Cryptococcus include toxoplasma encephalitis, primary central nervous system lymphoma, cytomegalovirus encephalitis, and progressive multifocal leukoencephalopathy (26). In organ transplant recipients, aspergillosis is the second and cryptococcosis is the third most common invasive fungal infection (17, 59).
We further propose that it would be beneficial to determine the minimal part of the bleomycin molecule responsible for producing the cell wall injury and developmental blocks. A new class of fungicidal drugs would be of significant medical value because of the shortage of effective antifungals (20) and because of developing fungal resistance to current antifungal therapies (25, 28, 47-49). The drugs are particularly needed for use in immunocompromised AIDS and cancer patients. The potential for lung fibrosis that sometimes develops in genetically susceptible cancer patients after extensive therapeutic modalities with bleomycin or bleomycin and radiation (22, 27, 53) may well be abated with taurine (11, 13), a naturally occurring amino acid with antioxidant properties. Bleomycin or a substructure may also be useful as a tool to study fungal cell wall architecture, septa, the cell division cycle, and cytokinesis.
Acknowledgments
We are grateful for the helpful advice and assistance provided by Ronald Uson, Fitzroy Edwards, Joseph Samet, Ajay Pramanik, Edward Bottone, Peter Lipke, Darline Davermann, and Ronald Edwards. We also thank Bristol Laboratories of Bristol-Myers Squibb Company, Pharmaceutical Research and Development Division (William T. Bradner, Syracuse, N.Y., and Linda Sanders and Daniel T. Elliott, Evansville, Ind.) for providing Blenoxane.
This work was supported by The National Science Foundation, National Institutes of Health (including the RCMI AIDS Infrastructure and Minority Biomedical Research Programs), Aaron Diamond Foundation, City University of New York Medical School, and The Sophie Davis School of Biomedical Education, Mt. Sinai School of Medicine, and Memorial Sloan-Kettering Cancer Center.
REFERENCES
- 1.Armstrong, D. 1989. Problems in management of opportunistic fungal diseases. Rev. Infect. Dis. 11(Suppl. 7):S1591-S1599. [DOI] [PubMed] [Google Scholar]
- 2.Beaudouin, R., S. T. Lim, J. A. Steide, M. Powell, J. McKoy, A. J. Pramanik, E. Johnson, C. W. Moore, and P. N. Lipke. 1993. Bleomycin affects cell wall anchorage of mannoproteins in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 37:1264-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabib, E., D. H. Roh, M. Schmidt, L. B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679-19682. [DOI] [PubMed] [Google Scholar]
- 4.Clemons, K. V., J. H. McCusker, K. Davis, and D. A. Stevens. 1994. Comparative pathogenesis of clinical and nonclinical isolates of Saccharomyces cerevisiae. J. Infect. Dis. 169:859-867. [DOI] [PubMed] [Google Scholar]
- 5.Crooke, S. T., and W. T. Bradner. 1976. Bleomycin, a review. J. Med. 7:333-428. [PubMed] [Google Scholar]
- 6.de Nobel, H., E. H. van Den, and F. M. Klis. 2000. Cell wall maintenance in fungi. Trends Microbiol. 8:344-345. [DOI] [PubMed] [Google Scholar]
- 7.Diamond, R. D. 1988. Fungal surfaces: effects of interactions with phagocytic cells. Rev. Infect. Dis. 10(Suppl. 2):S428-S431. [DOI] [PubMed] [Google Scholar]
- 8.Dupont, B., M. Richardson, P. E. Verweij, and J. F. Meis. 2000. Invasive aspergillosis. Med. Mycol. 38(Suppl. 1):215-224. [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A., K. Dawson, M. Pfaller, E. Anaissie, B. Breslin, D. Dixon, A. Fothergill, V. Paetznick, J. Peter, and M. Rinaldi. 1995. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob. Agents Chemother. 39:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez, C. O., N. C. Fernandez, A. Ariosa, and N. J. Fernandez. 2003. Characterization of a group of patients with cryptococcosis of the central nervous system. Rev. Neurol. 36:316-321. [PubMed] [Google Scholar]
- 11.Giri, S. N., R. Blaisdell, R. B. Rucker, Q. Wang, and D. M. Hyde. 1994. Amelioration of bleomycin-induced lung fibrosis in hamsters by dietary supplementation with taurine and niacin: biochemical mechanisms. Environ. Health Perspect. 102(Suppl. 10):137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein, A. L., and J. H. McCusker. 2001. Development of Saccharomyces cerevisiae as a model pathogen. A system for the genetic identification of gene products required for survival in the mammalian host environment. Genetics 159:499-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon, R. E., R. F. Heller, and R. F. Heller. 1992. Taurine protection of lungs in hamster models of oxidant injury: a morphologic time study of paraquat and bleomycin treatment. Adv. Exp. Med. Biol. 315:319-328. [DOI] [PubMed] [Google Scholar]
- 14.Gottfredsson, M., and J. R. Perfect. 2000. Fungal meningitis. Semin. Neurol. 20:307-322. [DOI] [PubMed] [Google Scholar]
- 15.Hazen, K. C. 1995. New and emerging yeast pathogens. Clin. Microbiol. Rev. 8:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan, L. H., B. S. Klein, and S. M. Levitz. 1996. Virulence factors of medically important fungi. Clin. Microbiol. Rev. 9:469-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzheimer, R. G., and H. Dralle. 2002. Management of mycoses in surgical patients—review of the literature. Eur. J. Med. Res. 7:200-226. [PubMed] [Google Scholar]
- 18.Kurokawa, C. S., M. F. Sugizaki, and M. T. Peracoli. 1998. Virulence factors in fungi of systemic mycoses. Rev. Inst. Med. Trop. Sao Paulo 40:125-135. [DOI] [PubMed] [Google Scholar]
- 19.Kwon-Chung, K. J., T. C. Sorrell, F. Dromer, E. Fung, and S. M. Levitz. 2000. Cryptococcosis: clinical and biological aspects. Med. Mycol. 38(Suppl. 1):205-213. [PubMed] [Google Scholar]
- 20.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latge, J. P., J. P. Debeaupuis, J. Sarfati, M. Diaquin, and S. Paris. 1993. Cell wall antigens in Aspergillus fumigatus. Arch. Med. Res. 24:269-274. [PubMed] [Google Scholar]
- 22.Lazo, J. S. 1999. Bleomycin. Cancer Chemother. Biol. Response Modif. 18:39-45. [PubMed] [Google Scholar]
- 23.Lim, S. T., C. K. Jue, C. W. Moore, and P. N. Lipke. 1995. Oxidative cell wall damage mediated by bleomycin-Fe(II) in Saccharomyces cerevisiae. J. Bacteriol. 177:3534-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipke, P. N., and R. Ovalle. 1998. Cell wall architecture in yeast: new structure and new challenges. J. Bacteriol. 180:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeffler, J., and D. A. Stevens. 2003. Antifungal drug resistance. Clin. Infect. Dis. 36:S31-S41. [DOI] [PubMed] [Google Scholar]
- 26.Mamidi, A., J. A. DeSimone, and R. J. Pomerantz. 2002. Central nervous system infections in individuals with HIV-1 infection. J. Neurovirol. 8:158-167. [DOI] [PubMed] [Google Scholar]
- 27.Marshall, R. P., R. J. McAnulty, and G. J. Laurent. 1997. The pathogenesis of pulmonary fibrosis: is there a fibrosis gene? Int. J. Biochem. Cell Biol. 29:107-120. [DOI] [PubMed] [Google Scholar]
- 28.Masia, C. M., and R. F. Gutierrez. 2002. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2:550-563. [DOI] [PubMed] [Google Scholar]
- 29.McCusker, J. H., K. V. Clemons, D. A. Stevens, and R. W. Davis. 1994. Genetic characterization of pathogenic Saccharomyces cerevisiae isolates. Genetics 136:1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCusker, J. H., K. V. Clemons, D. A. Stevens, and R. W. Davis. 1994. Saccharomyces cerevisiae virulence phenotype as determined with CD-1 mice is associated with the ability to grow at 42 degrees C and form pseudohyphae. Infect. Immun. 62:5447-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, C. W. 1982. Ligase-deficient yeast cells exhibit defective DNA rejoining and enhanced gamma ray sensitivity. J. Bacteriol. 150:1227-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, C. W. 1982. Modulation of bleomycin cytotoxicity. Antimicrob. Agents Chemother. 21:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore, C. W. 1990. Degradation of DNA and structure-activity relationship between bleomycins A2 and B2 in the absence of DNA repair. Biochemistry 29:1342-1347. [DOI] [PubMed] [Google Scholar]
- 34.Moore, C. W. 1999. Bleomycin, p. 292-297. In T. Creighton (ed.), Encyclopedia of molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 35.Moore, C. W., R. Del Valle, J. McKoy, A. Pramanik, and R. E. Gordon. 1992. Lesions and preferential initial localization of [S-methyl-3H]bleomycin A2 on Saccharomyces cerevisiae cell walls and membranes. Antimicrob. Agents Chemother. 36:2497-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore, C. W., J. McKoy, M. Dardalhon, D. Davermann, M. Martinez, and D. Averbeck. 2000. DNA damage-inducible and RAD52-independent repair of DNA double-strand breaks in Saccharomyces cerevisiae. Genetics 154:1085-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore, C. W., and A. Schmick. 1979. Recombinogenicity and mutagenicity of saccharin in Saccharomyces cerevisiae. Mutat. Res. 67:215-219. [DOI] [PubMed] [Google Scholar]
- 38.Murphy, A. R., and K. A. Kavanagh. 2001. Adherence of clinical isolates of Saccharomyces cerevisiae to buccal epithelial cells. Med. Mycol. 39:123-127. [DOI] [PubMed] [Google Scholar]
- 39.Murphy, M., E. M. Bernard, T. Ishimaru, and D. Armstrong. 1997. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:696-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ovalle, R., S. T. Lim, B. Holder, C. K. Jue, C. W. Moore, and P. N. Lipke. 1998. A spheroplast rate assay for determination of cell wall integrity in yeast. Yeast 14:1159-1166. [DOI] [PubMed] [Google Scholar]
- 41.Perfect, J. R., and A. Casadevall. 2002. Cryptococcosis. Infect. Dis. Clin. North Am. 16:837-874. [DOI] [PubMed] [Google Scholar]
- 42.Ponton, J., R. Ruchel, K. V. Clemons, D. C. Coleman, R. Grillot, J. Guarro, D. Aldebert, P. Ambroise-Thomas, J. Cano, A. J. Carrillo-Munoz, J. Gene, C. Pinel, D. A. Stevens, and D. J. Sullivan. 2000. Emerging pathogens. Med. Mycol. 38(Suppl. 1):225-236. [DOI] [PubMed] [Google Scholar]
- 43.Powderly, W. G. 2000. Cryptococcal meningitis in HIV-infected patients. Curr. Infect. Dis. Rep. 2:352-357. [DOI] [PubMed] [Google Scholar]
- 44.Reiss, E., V. M. Hearn, D. Poulain, and M. G. Shepherd. 1992. Structure and function of the fungal cell wall. J. Med. Vet. Mycol. 30(Suppl. 1):143-156. [DOI] [PubMed] [Google Scholar]
- 45.Rippon, J. W. 1988. Medical mycology. W. B. Saunders Company, Philadelphia, Pa.
- 46.Ruiz-Herrera, J. 1992. Fungal cell wall: structure, synthesis and assembly. CRC Press, Boca Raton, Fla.
- 47.Saint Georgiev, V. 2000. Membrane transporters and antifungal drug resistance. Curr. Drug Targets 1:261-284. [DOI] [PubMed] [Google Scholar]
- 48.Sanglard, D. 2002. Clinical relevance of mechanisms of antifungal drug resistance in yeasts. Enferm. Infecc. Microbiol. Clin. 20:462-469. [DOI] [PubMed] [Google Scholar]
- 49.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt, H. J., E. M. Bernard, J. Andrade, F. Edwards, B. Schmitt, and D. Armstrong. 1988. MIC and fungicidal activity of terbinafine against clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 32:780-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitt, H. J., F. Edwards, J. Andrade, Y. Niki, and D. Armstrong. 1992. Comparison of azoles against aspergilli in vitro and in an experimental model of pulmonary aspergillosis. Chemotherapy 38:118-126. [DOI] [PubMed] [Google Scholar]
- 52.Sepkowitz, K. A. 2002. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin. Infect. Dis. 34:1098-1107. [DOI] [PubMed] [Google Scholar]
- 53.Sleijfer, S. 2001. Bleomycin-induced pneumonitis. Chest 120:617-624. [DOI] [PubMed] [Google Scholar]
- 54.Smits, G. J., J. C. Kapteyn, E. H. van Den, and F. M. Klis. 1999. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 2:348-352. [DOI] [PubMed] [Google Scholar]
- 55.Sudoh, M., T. Yamazaki, K. Masubuchi, M. Taniguchi, N. Shimma, M. Arisawa, and H. Yamada-Okabe. 2000. Identification of a novel inhibitor specific to the fungal chitin synthase. Inhibition of chitin synthase 1 arrests the cell growth, but inhibition of chitin synthase 1 and 2 is lethal in the pathogenic fungus Candida albicans. J. Biol. Chem. 275:32901-32905. [DOI] [PubMed] [Google Scholar]
- 56.Thomas, I., and R. A. Schwartz. 2001. Cutaneous manifestations of systemic cryptococcosis in immunosupressed patients. J. Med. 32:259-266. [PubMed] [Google Scholar]
- 57.Umezawa, H. 1976. Structure and action of bleomycin. Prog. Biochem. Pharmacol. 11:18-27. [PubMed] [Google Scholar]
- 58.Umezawa, H., K. Maeda, T. Takeuchi, and Y. Okami. 1966. New antibiotics, bleomycin A and B. J. Antibiot. (Tokyo) 19:200-209. [PubMed] [Google Scholar]
- 59.Vilchez, R. A., J. Fung, and S. Kusne. 2002. Cryptococcosis in organ transplant recipients: an overview. Am. J. Transplant. 2:575-580. [DOI] [PubMed] [Google Scholar]
- 60.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38(Suppl. 1):335-347. [PubMed] [Google Scholar]
- 61.Wheat, L. J., M. Goldman, and G. Sarosi. 2002. State-of-the-art review of pulmonary fungal infections. Semin. Respir. Infect. 17:158-181. [DOI] [PubMed] [Google Scholar]
- 62.White, M. H., and D. Armstrong. 1994. Cryptococcosis. Infect. Dis. Clin. North Am. 8:383-398. [PubMed] [Google Scholar]