Abstract
The pharmacokinetics of the hypoxic radio-sensitizer nimorazole were studied in 19 individuals after single oral doses of between 0.5-3.5 g. HPLC measurements showed, after a rapid absorption, a linear relationship between peak plasma concentration and given dose. Mean elimination half life was 3.1 h. A tendency to a dose-dependent variation in the apparent volume of distribution, total body clearance and elimination half life suggest non-linear pharmacokinetics of nimorazole. Tumour concentrations measured in 5 patients gave tumour/plasma ratios between 0.8-1.3. No toxicity was observed. The results indicate that nimorazole may have potential as a clinically useful hypoxic radiosensitizer.
Full text
PDF

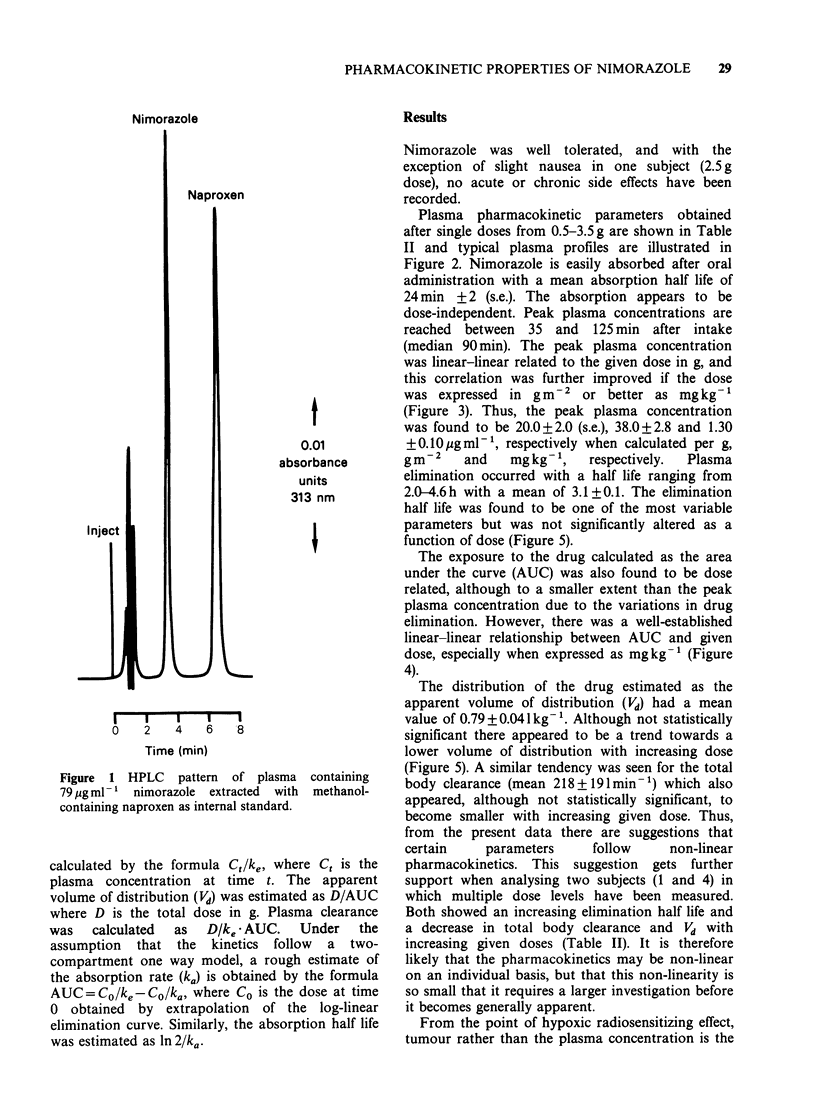
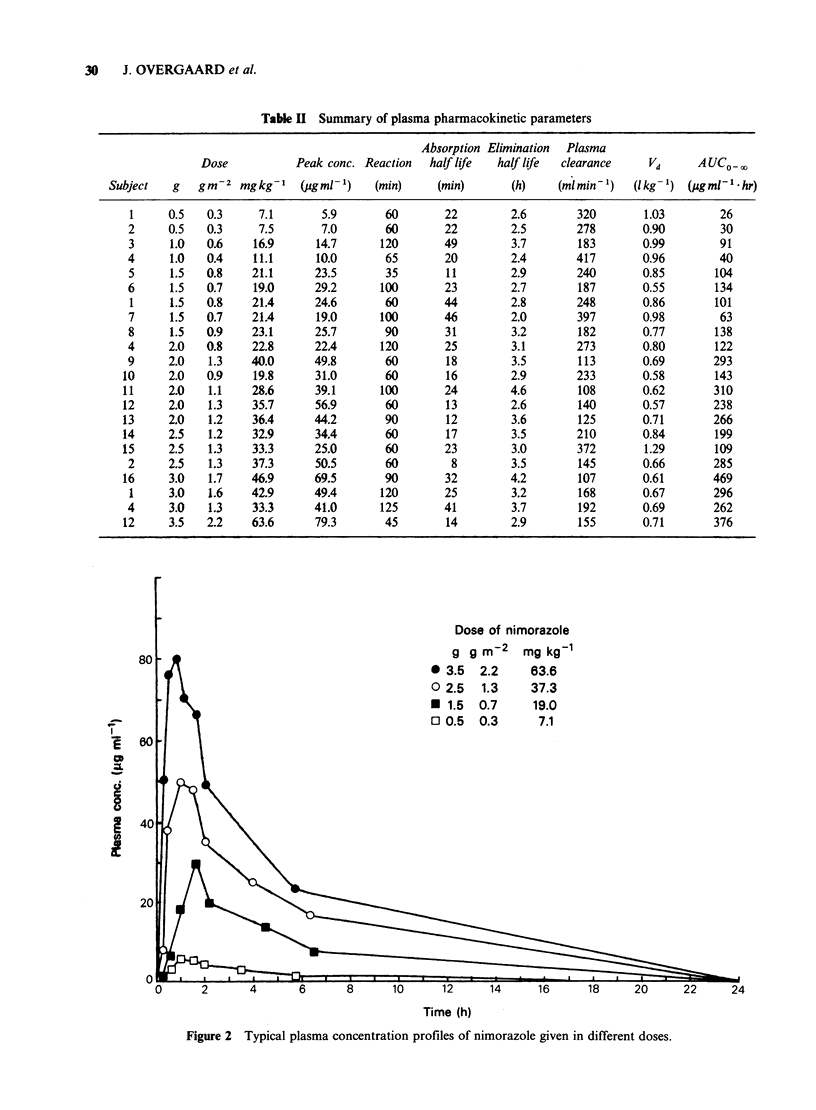
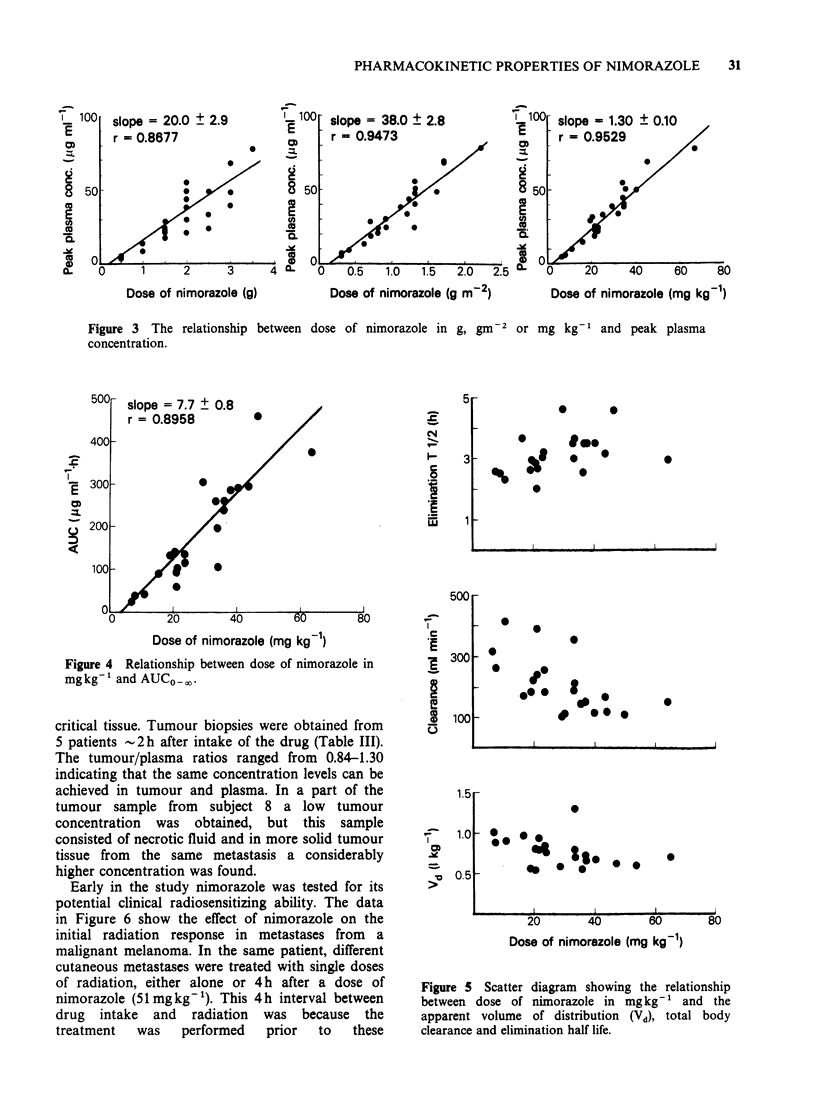
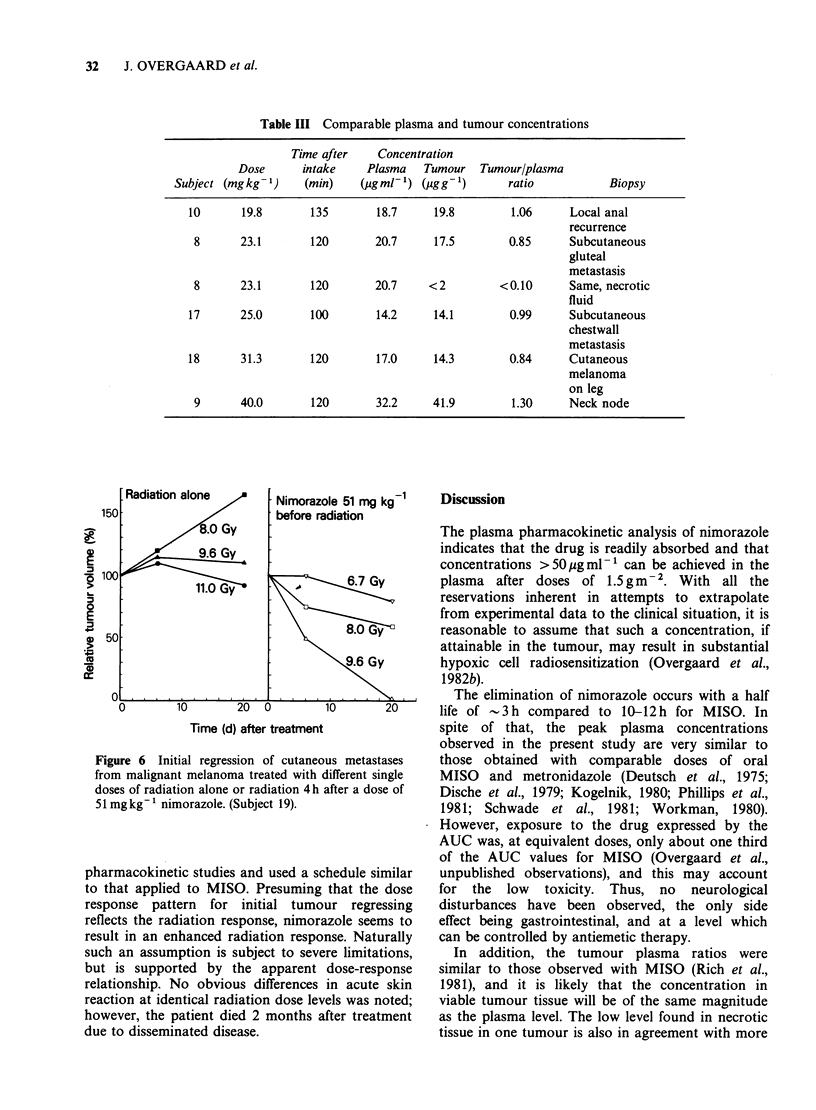


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash D. V., Smith M. R. Causes of apparent low levels of misonidazole in human tumours. Clin Radiol. 1980 Mar;31(2):233–237. doi: 10.1016/s0009-9260(80)80172-3. [DOI] [PubMed] [Google Scholar]
- Deutsch G., Foster J. L., McFadzean J. A., Parnell M. Human studies with "high dose" metronidazole: a non-toxic radiosensitizer of hypoxic cells. Br J Cancer. 1975 Jan;31(1):75–80. doi: 10.1038/bjc.1975.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disch S., Saunders M. I., Anderson P., Urtasun R. C., Karcher K. H., Kogelnik H. D., Bleehen N., Phillips T. L., Wasserman T. H. The neurotoxicity of misonidazole: pooling of data from five centres. Br J Radiol. 1978 Dec;51(612):1023–1024. doi: 10.1259/0007-1285-51-612-1023. [DOI] [PubMed] [Google Scholar]
- Dische S., Saunders M. I., Flockhart I. R., Lee M. E., Anderson P. Misonidazole-a drug for trial in radiotherapy and oncology. Int J Radiat Oncol Biol Phys. 1979 Jun;5(6):851–860. doi: 10.1016/0360-3016(79)90070-1. [DOI] [PubMed] [Google Scholar]
- Giraldi P. N., Tosolini G. P., Dradi E., Nannini G., Longo R., Meinardi G., Monti G., de Carneri I. Studies on antiprotozoans. 3. Isolation, identification and quantitative determination in humans of the metabolites of a new trichomonacidal agent. Biochem Pharmacol. 1971 Feb;20(2):339–349. doi: 10.1016/0006-2952(71)90068-2. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Wagner F. C., Lawrence R. Glioblastoma multiforme: treatment by large dose fraction irradiation and metronidazole. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):351–355. doi: 10.1016/0360-3016(82)90638-1. [DOI] [PubMed] [Google Scholar]
- Karim A. B. Prolonged metronidazole administration with protracted radiotherapy: a pilot study on response of advanced tumours. Br J Cancer Suppl. 1978 Jun;3:299–301. [PMC free article] [PubMed] [Google Scholar]
- Kogelnik H. D. Clinical experience with misonidazole: high dose fractions versus daily low doses. Cancer Clin Trials. 1980 Summer;3(2):179–186. [PubMed] [Google Scholar]
- Orozco García O., Pérez Nava J., Gonzálex Díaz R. Valoración clínica del nimorazol en absceso hepático amibiano. Prensa Med Mex. 1975 Nov-Dec;40(11-12):378–382. [PubMed] [Google Scholar]
- Overgaard J., Overgaard M., Nielsen O. S., Pedersen A. K., Timothy A. R. A comparative investigation of nimorazole and misonidazole as hypoxic radiosensitizers in a C3H mammary carcinoma in vivo. Br J Cancer. 1982 Dec;46(6):904–911. doi: 10.1038/bjc.1982.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T. L., Wasserman T. H., Johnson R. J., Levin V. A., VanRaalte G. Final report on the United States Phase I Clinical Trial of the hypoxic cell radiosensitizer, misonidazole (Ro-07-0582; NSC #261037). Cancer. 1981 Oct 15;48(8):1697–1704. doi: 10.1002/1097-0142(19811015)48:8<1697::aid-cncr2820480802>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Rich T. A., Dische S., Saunders M. I., Stratford M. R., Minchinton A. A serial study of the concentration of misonidazole in human tumors correlated with histologic structure. Int J Radiat Oncol Biol Phys. 1981 Feb;7(2):197–203. doi: 10.1016/0360-3016(81)90437-5. [DOI] [PubMed] [Google Scholar]
- Schwade J. G., Strong J. M., Gangji D. I.V. misonidazole (NSC 261037). Report of initial clinical experience. Cancer Clin Trials. 1981;4(1):33–39. [PubMed] [Google Scholar]
- Urtasun R. C., Chapman J. D., Band P., Rabin H. R., Fryer C. G., Sturmwind J. Phase 1 study of high-dose metronidazole: a specific in vivo and in vitro radiosensitizer of hypoxic cells. Radiology. 1975 Oct;117(1):129–133. doi: 10.1148/117.1.129. [DOI] [PubMed] [Google Scholar]
- Urtasun R., Feldstein M. L., Partington J., Tanasichuk H., Miller J. D., Russell D. B., Agboola O., Mielke B. Radiation and nitroimidazoles in supratentorial high grade gliomas: a second clinical trial. Br J Cancer. 1982 Jul;46(1):101–108. doi: 10.1038/bjc.1982.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P. Analysis of the basic 5-nitroimidazole nimorazole in blood by reversed-phase high-performance liquid chromatography, and its application to pharmacokinetic studies in individual mice. J Chromatogr. 1979 Aug 21;163(4):396–402. doi: 10.1016/s0378-4347(00)81643-6. [DOI] [PubMed] [Google Scholar]
- Workman P. Pharmacokinetics of hypoxic cell radiosensitizers: a review. Cancer Clin Trials. 1980 Fall;3(3):237–251. [PubMed] [Google Scholar]


