Abstract
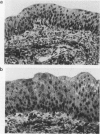
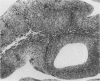
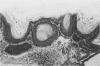
The early and late morphological changes induced in rat bladder urothelium by intravesicular administration of the alkylating agents methyl methanesulphonate (MMS) and ethyl methanesulphonate (EMS) are described. In the short-term, both compounds produced dose-related toxic damage followed by a regenerative hyperplasia of the urothelium. At any given dose-level, the effects of MMS were more severe than those of EMS. Two years after administration of multiple doses of 2.5 mg MMS or 7.5 mg EMS the majority of animals had dose-related simple urothelial hyperplasias with occasional mild dysplasia. However, in three MMS-treated animals the hyperplasias had progressed to well-differentiated transitional-cell carcinomas. No bladder neoplasms were seen in EMS-treated animals. The urothelial response of the rat to MMS and EMS is discussed with reference to the known chemical reactivity of these compounds. It is concluded that EMS is a mitogen for the urothelium and that the few carcinomas which develop following topical exposure of the bladder to MMS do not necessarily reflect any initiating potential in this compound. Rather it is argued that the results are consistent with MMS acting as a promoter in cells which have either been previously initiated or which carry a latent oncogene.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Sasaki M. Chromosome aberrations and sister chromatid exchanges in Chinese hamster cells exposed to various chemicals. J Natl Cancer Inst. 1977 Jun;58(6):1635–1641. doi: 10.1093/jnci/58.6.1635. [DOI] [PubMed] [Google Scholar]
- Chowaniec J., Hicks R. M. Response of the rat to saccharin with particular reference to the urinary bladder. Br J Cancer. 1979 Apr;39(4):355–375. doi: 10.1038/bjc.1979.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei J. V., Lawley P. D. Tissue distribution and mode of DNA methylation in mice by methyl methanesulphonate and N-methyl-N' -nitro-N-nitrosoguanidine: lack of thymic lymphoma induction and low extent of methylation of target tissue DNA at 0-6 of guanine. Chem Biol Interact. 1976 Jun;13(3-4):215–222. doi: 10.1016/0009-2797(76)90075-2. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Swenson D. H., Warren W., Lawley P. D. Alkylation of deoxyribonucleic acid in vivo in various organs of C57BL mice by the carcinogens N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea and ethyl methanesulphonate in relation to induction of thymic lymphoma. Some applications of high-pressure liquid chromatography. Biochem J. 1978 Sep 15;174(3):1031–1044. doi: 10.1042/bj1741031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei J. V. Tumour induction by low molecular weight alkylating agents. Chem Biol Interact. 1971 Apr;3(2):117–121. doi: 10.1016/0009-2797(71)90091-3. [DOI] [PubMed] [Google Scholar]
- Hicks R. M. Multistage carcinogenesis in the urinary bladder. Br Med Bull. 1980 Jan;36(1):39–46. doi: 10.1093/oxfordjournals.bmb.a071611. [DOI] [PubMed] [Google Scholar]
- Hicks R. M., Wakefield J. S. Rapid induction of bladder cancer in rats with N-methyl-N-nitrosourea. I. Histology. Chem Biol Interact. 1972 Jul;5(2):139–152. doi: 10.1016/0009-2797(72)90040-3. [DOI] [PubMed] [Google Scholar]
- Hicks R. M., Wakefield J., Chowaniec J. Evaluation of a new model to detect bladder carcinogens or co-carcinogens; results obtained with saccharin, cyclamate and cyclophosphamide. Chem Biol Interact. 1975 Sep;11(3):225–233. doi: 10.1016/0009-2797(75)90101-5. [DOI] [PubMed] [Google Scholar]
- Hicks R. M., Wright R., Wakefield J. S. The induction of rat bladder cancer by 2-naphthylamine. Br J Cancer. 1982 Oct;46(4):646–661. doi: 10.1038/bjc.1982.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Tumor promoter induces sister chromatid exchanges: relevance to mechanisms of carcinogenesis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6149–6153. doi: 10.1073/pnas.75.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Perry P., Evans H. J. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature. 1975 Nov 13;258(5531):121–125. doi: 10.1038/258121a0. [DOI] [PubMed] [Google Scholar]
- Severs N. J., Barnes S. H., Wright R., Hicks R. M. Induction of bladder cancer in rats by fractionated intravesicular doses of N-methyl-N-nitrosourea. Br J Cancer. 1982 Mar;45(3):337–351. doi: 10.1038/bjc.1982.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield J. S., Hicks R. M. Erythrophagocytosis by the epithelial cells of the bladder. J Cell Sci. 1974 Aug;15(3):555–573. doi: 10.1242/jcs.15.3.555. [DOI] [PubMed] [Google Scholar]
- Wolff S., Rodin B. Saccharin-induced sister chromatid exchanges in Chinese hamster and human cells. Science. 1978 May 5;200(4341):543–545. doi: 10.1126/science.644315. [DOI] [PubMed] [Google Scholar]