Abstract
The first line drug against leishmaniasis consists of pentavalent antimony [Sb(V)], but there is general belief that the active form of the metal is the trivalent form [Sb(III)]. In this study, we have quantified the accumulation of Sb(V) and Sb(III) in Leishmania by using inductively coupled plasma mass spectrometry. The accumulation was studied in three Leishmania species at various life stages, sensitive or resistant to antimony. Both Sb(III) and Sb(V) are accumulated in promastigote and amastigote parasites, but through competition experiments with arsenite, we found that the routes of entry of Sb(V) and Sb(III) are likely to differ in Leishmania. The level of accumulation of either Sb(III) or Sb(V), however, was not correlated with the susceptibility of wild-type Leishmania cells to antimony. This suggests that other factors may also be implicated in the mode of action of the drugs. In contrast to metal susceptibility, resistance to Sb(III) correlated well with decreased antimony accumulation. This phenotype was energy dependent and highlights the importance of transport systems in drug resistance of this protozoan parasite.
The protozoan parasite Leishmania is responsible for several pathologies ranging from self-healing cutaneous lesions to visceral infections that can be fatal if untreated (14). No effective vaccines are available against leishmaniasis, and the treatment relies on chemotherapy (1, 22). The first line drug against all forms of Leishmania infection consists of pentavalent antimony [Sb(V)]-containing drugs such as Pentostam and Glucantime. Resistance to this class of drug has been described in several parts of the world (11) but has reached epidemic proportions in the state of Bihar, India (35). Resistance to Sb(V) drugs in Leishmania is one of the World Health Organization's antimicrobial resistance priorities (www.who.int/infectious-disease-report/2000). The recent demonstration of the efficacy of miltefosine is a breakthrough (34), but resistance to this drug, at least in vitro, can easily be achieved (25). It is quite remarkable that even after 50 years of clinical use, the mode of action of antimony is unknown, but there is a general belief that to be active, Sb(V) needs to be reduced to the trivalent form (24). The exact site of reduction is also unknown, although evidence for reduction inside the parasites was recently described (33). An alternative view is that the metal is reduced in the macrophage of the host (31). Reduction could occur either enzymatically, as in yeast (21), or by parasite- or host-derived thiols (30).
The mechanism of resistance to antimony in field strains is unknown, and most of our understanding stems from work based on cells in which resistance was selected in vitro. Leishmania cells have been selected in the past for Sb(V) resistance, and some resistance mechanisms were suggested, including reduced accumulation (7), gene amplification (10, 13), and loss of reduction of the metal (33). Since the active drug is likely to be Sb(III), cells were also selected for Sb(III) resistance (12), and analysis of these mutants led to the proposal of a model for resistance. This model was derived mostly from work carried out while studying resistance mechanisms to arsenite, a metal sharing several characteristics with antimony, but seems to hold true for Sb(III), at least in Leishmania tarentolae promastigotes (12). Once Sb(III) is within the cell, it would be conjugated to trypanothione (24), the parasite-specific spermidine-glutathione conjugate (9). Indeed, trypanothione was found to be increased in arsenite- and antimonite-resistant cells (12, 20). This Sb-trypanothione conjugate could then be sequestered inside a vacuole by the ABC transporter PGPA (16) or extruded from the cell by a thiol-X efflux pump (6), possibly corresponding to one of the five other ABC transporters belonging to the same family as PGPA that were unraveled in the almost completed Leishmania genome (www.genedb.org).
Altered transport of metals appears to be an important determinant for resistance (7), but few studies have dealt with the uptake of antimony in Leishmania. The transport of antimony in Leishmania was first studied by using [125Sb]Pentostam in the promastigote and amastigote stages of Leishmania mexicana and Leishmania donovani (2, 5). The uptake of radioactive arsenite has also been used as a model to investigate transport of metals in Leishmania promastigotes (7, 15). In parallel, a variety of mass spectrometric methods have been developed to measure metal uptake in Leishmania (26, 27, 33), and here we present our analysis and new results on metal transport in three Leishmania species sensitive or resistant to antimony by using inductive coupled plasma mass spectrometry (ICP-MS).
MATERIALS AND METHODS
Reagents.
The additive-free formulations of N-methylglucamine antimoniate (Glucantime, WRAIR lot reference no. BL09186; Rhône Poulenc) and sodium stibogluconate (Pentostam, WRAIR lot reference no. BL06916; Glaxo-Wellcome), were from the Walter Reed Army Institute. Potassium antimonyl tartrate and sodium arsenate were obtained from Aldrich, and sodium m-arsenite, 2,4-dinitrophenol (DNP), and valinomycin were obtained from Sigma.
Cell lines and cultures.
The L. tarentolae cell lines TarII wild type, TarII As 50.1 (selected for resistance to arsenite), and TarII SbIII 400.1, a parent of TarIISb1.1 (selected for resistance to antimonite), have been described previously (12, 23), as have Leishmania infantum strain MHOM/MA/67/ITMAP-263 (31) and Leishmania viannia panamensis strain MHOM/CO/86/1166 (18). The L. infantum line was grown as axenic amastigotes in the cell-free medium MAA/20 (31), and using a similar protocol, the L. panamensis line could also be grown as axenic amastigotes. We have generated, by step-wise selection starting with a drug concentration corresponding to the 50% effect concentration (EC50) of the strain, Sb(III)-resistant mutants of L. infantum and L. panamensis axenic amastigotes. These mutants, named L. infantum SbIII 2000.1 and L. panamensis 12.3, are each 10-times-more resistant to SbIII than their wild-type parent cells. Cells were grown in SDM-79 at 25°C as promastigotes and in MAA/20 at 37°C (L. infantum) or 33°C (L. panamensis) as axenic amastigotes.
Transport assays.
Log-phase Leishmania cells were washed twice in buffer A [50 mM triethanolamine hydrochloride, 15 mM KCl, 10 mM (NH4)2SO4, and 1 mM MgSO4 (pH 6.9)] and resuspended in buffer A containing 10 mM glucose, at a density of 108 cells/ml. Transport assays were done generally with 100 μM substrate (either sodium arsenite, potassium antimonyl tartrate, or Pentostam). An aliquot was taken at various time points, washed two times with ice-cold HEPES-NaCl buffer, pelleted, dried, and then analyzed by ICP-MS. Each transport experiment was repeated at least twice. Transport experiments using inhibitors (including controls) were performed essentially as described above, except that glucose was omitted in buffer A. Cells were incubated for 15 min on ice with the metabolic inhibitors DNP (5 mM) or valinomycin (1 μM) before the addition of the substrate.
ICP-MS analysis.
All chemicals used for the pretreatment of the samples were of at least analytical reagent grade, unless stated otherwise. Deionized water made from a DDS-11A system (Shuang-Feng Pure Water Equipment Co., Beijing, China) was used throughout the experiments. A standard Sb solution (0.1000 mg/ml) was purchased from the National Standard Center of China (BW3145). Working solutions were prepared daily by dilution. Concentrated HNO3 (0.1 ml) was added to each tube containing 5 × 107 Leishmania cells. The mixture was maintained for 24 h at room temperature to ensure the complete dissolution of the cells. The sample was diluted to 1.5 ml with deionized water and then injected into the ICP-MS (Sciex Elan 5000; Perkin-Elmer) for quantification. The details of the operating parameters are available upon request. Briefly, samples were injected at a flow rate of 1 ml/min, nebulized with argon (flow rate, 0.781 liter/min), and observed at 121 m/z, with 5 replicates for each measurement.
RESULTS
Uptake of metals in Leishmania as determined by ICP-MS.
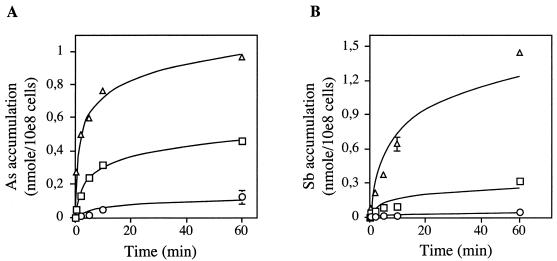
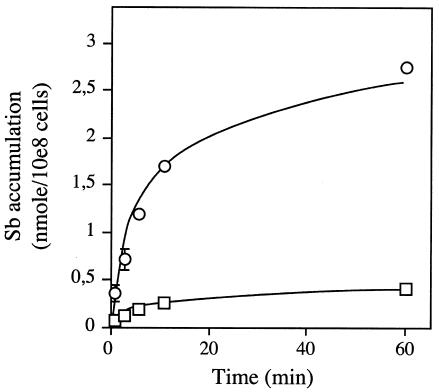
Antimony-containing drugs are the first line of treatment against Leishmaniasis, and we describe here the accumulation of antimony in sensitive and resistant strains of several Leishmania species. Initially, the accumulation of 73As (7), a metal related to antimony and often used as a paradigm to study metal resistance in Leishmania, was reported. The direct measurement of Sb accumulation in Leishmania cells is complicated by the lack of a commercial source of 125Sb. The accumulation of potassium antimony tartrate [Sb(III)] and of sodium arsenite [As(III)], were therefore measured in L. tarentolae using the relatively novel technique of ICP-MS. The accumulations of both metals were found to be concentration dependent (Fig. 1) and consistent with previously reported kinetics with 73As (7). It has been argued, on the basis of cross-resistance data, that As(III) and Sb(III) have similar uptake systems and, possibly, modes of action in Leishmania (7), although this does not appear to be the case for L. donovani (33). In an attempt to further investigate this, we looked at Sb(III) transport in L. tarentolae and found that it was diminished in the presence of As(III) (Fig. 2), demonstrating that in L. tarentolae, as in yeasts and mammals (reviewed in reference 28), As(III) and Sb(III) enter the cell by the same route.
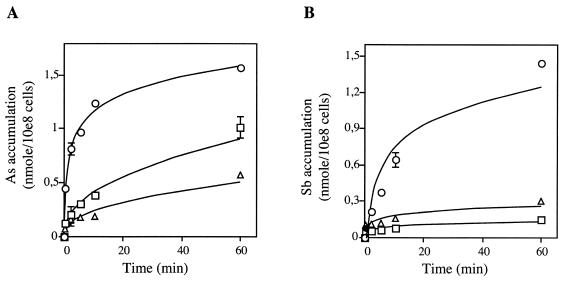
FIG. 1.
Accumulation of metal in Leishmania as measured by ICP-MS. Dose-dependent accumulation of As(III) (A) and Sb(III) (B) in wild-type L. tarentolae. Parasites were incubated with 1 μM (○), 10 μM (□), or 100 μM (▵) substrate as described in Materials and Methods. Data are shown as the means ± standard errors (symbols are frequently wider than error bars).
FIG. 2.
Effect of As(III) competition on accumulation of Sb(III) in L. tarentolae cells. The accumulation of Sb with 5 μM Sb(III) as the substrate (○) was lost when 100 μM As(III) was added (□). Data are shown as the means ± standard errors (symbols are frequently wider than error bars).
Antimony uptake throughout the Leishmania life cycle.
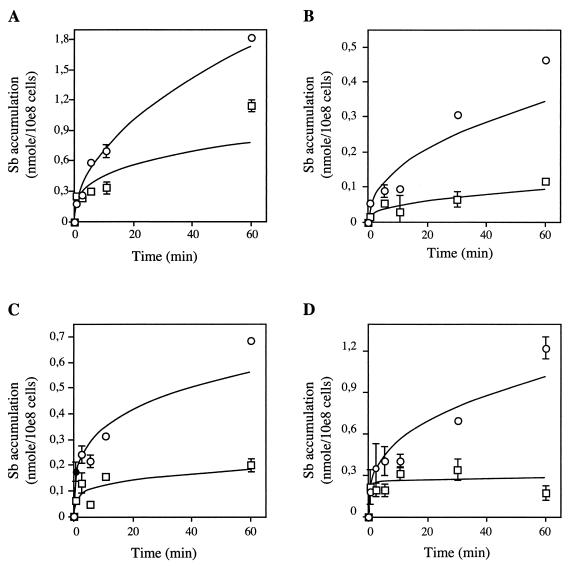
Our work with L. tarentolae promastigotes has validated the use of ICP-MS for the examination of Sb(III) transport in Leishmania cells. We were interested in determining whether other species of Leishmania, as promastigotes or amastigotes, were capable of accumulating Sb(III). We have developed L. infantum and L. panamensis strains that can be grown both as promastigotes and axenic amastigotes. The axenic amastigote stage parasites of both species were found to be more sensitive to Sb(III) and As(III) than the promastigote parasites (Table 1). Also, the pathogenic species of Leishmania were intrinsically more resistant than L. tarentolae to both As(III) and Sb(III) (Table 1). However, and as described at length previously (5, 19, 26, 31, 32), neither promastigotes nor axenic amastigotes were sensitive to a pharmacological concentration of the preservative-free Sb(V) Pentostam (Table 1). We show here that despite various susceptibilities to Sb(III) (Table 1), the drug accumulates well in the promastigote stage of all species. The ability to accumulate the drug does not seem to correlate with drug susceptibility, since while four times more Sb(III) accumulates in L. infantum promastigotes than in amastigotes, the latter are 15-fold more sensitive (Fig. 3 and Table 1). We also show that Sb(III) is accumulated in axenic amastigotes of both L. infantum and L. panamensis (Fig. 3). There is no clear trend as to whether one life stage accumulates more Sb(III), since in L. infantum, a greater accumulation of Sb(III) was noted in promastigotes but in L. panamensis, more Sb(III) was measured in axenic amastigotes (Fig. 3).
TABLE 1.
Susceptibility to metals in Leishmania
| Strain | Parasite stage | EC50(μM)a of:
|
||
|---|---|---|---|---|
| As(III) | Sb(III) | Sb(V) | ||
| L. tarentolae | ||||
| Wild-type | 0.4 | 0.1 | 170 | |
| As 50.1 | 50 | >100 | >1,300 | |
| Sb 400.1 | 240 | 400 | >1,300 | |
| L. infantum | ||||
| Wild-type | Promastigote | 37 | 225 | >4,000 |
| Axenic amastigote | 9 | 60 | >4,000 | |
| Intracellular amastigote | NDb | 50 | 100 | |
| Sb 2000.1 | Axenic amastigote | 60 | >4,000 | ND |
| L. panamensis | ||||
| Wild-type | Promastigote | 3.5 | 7.5 | ND |
| Axenic amastigote | 0.25 | 1.5 | ND | |
| Sb 12.3 | Axenic amastigote | 2.5 | 25 | ND |
EC50s are averages of at least three independent determinations.
Not done.
FIG. 3.
Reduced accumulation of antimony in Sb(III)-resistant pathogenic Leishmania strains in both promastigote and amastigote forms. Time-dependent uptake of Sb [100 μM Sb(III)] was determined in L. infantum promastigote (A) and amastigote (B) forms and in L. panamensis promastigote (C) and amastigote (D) forms. For L. infantum: ○, wild type; □, Sb2000.1. For L. panamensis: ○, wild type; □, Sb12.3. Data are shown as the means ± standard errors (symbols are frequently wider than error bars).
Pentavalent and trivalent antimony are not taken up by the same route.
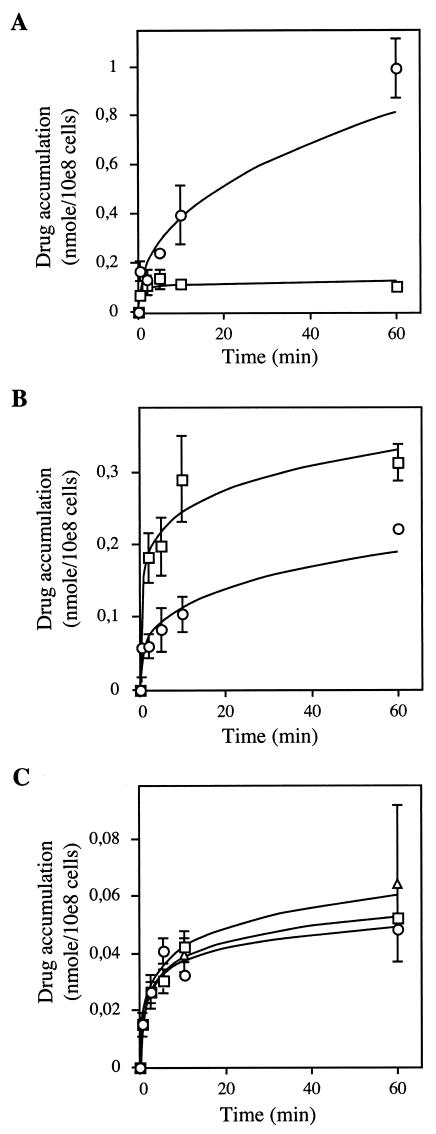
While Sb(V) is the drug used for treating Leishmaniasis, it is generally agreed that the active oxidation level of antimony is Sb(III). If reduction occurs primarily in the host, Leishmania should then encounter Sb(III). However, at this time, we cannot exclude the possibility that reduction occurs primarily in the parasite, in which case it would transport mostly Sb(V). We thus tested whether pentostam was taken up by Leishmania cells. We showed accumulation of pentostam in wild-type promastigotes of L. infantum, although levels were lower than for Sb(III) (Fig. 4A). The accumulation of Pentostam was greater in L. infantum axenic amastigotes than in promastigotes, and in these amastigotes, the accumulation of Pentostam was higher than that of Sb(III) (Fig. 4B). While Sb(V) enters both promastigotes and axenic amastigotes, the drug has no activity against these parasites at relevant pharmacological concentrations, displaying an EC50 of >4 mM (>3,000 μg/ml) (31) (Table 1). The same drug, however, is highly active against the intracellular form of the parasite with an EC50 of 100 μM (70 μg/ml) (31) (Table 1). We have shown that the uptake of Sb(III) is competitively inhibited by As(III) (Fig. 2). We then tested whether As(III) could compete with uptake of pentostam. As(III) was used instead of Sb(III), since both appear to be transported by the same system (Fig. 2), and As and Sb can easily be discriminated by ICP-MS. It was observed that As(III) could not compete with the uptake of Sb(V) (Fig. 4C), suggesting that As(III) and, more importantly, Sb(III) enter the cells by a different route than Sb(V). Using a similar strategy, we found that arsenate [As(V)] was also not inhibiting Sb(V) accumulation (Fig. 4C).
FIG. 4.
Sb accumulation in L. infantum. Accumulation of Sb(III) (○) and Sb(V) (Pentostam) (□) in L. infantum in its promastigote (A) and amastigote (B) forms. The substrate was 100 μM Sb(III) or Sb(V) (Pentostam). In panel C, the accumulation of Sb with 5 μM Sb(V) (Pentostam) as the substrate (○) was unaffected in the presence of 100 μM As(III) (□) or As(V) (▵) in L. infantum amastigotes. Data are shown as the means ± standard errors.
Reduced accumulation of Sb(III) in Leishmania-resistant mutants.
Our ability to measure the uptake of specific metals in Leishmania cells by using ICP-MS allowed us to look at the transport of these metals in a number of Leishmania species and stages selected for metal resistance. We first analyzed L. tarentolae As 50.1, a mutant for which a reduced accumulation of 73As was described (7). We confirmed that this mutant has a reduced accumulation of As compared to wild-type cells (Fig. 5A), and for the first time, we have been able to show directly a decreased accumulation of Sb (Fig. 5B), which is consistent with the cross-resistance pattern of this mutant (Table 1). We also describe here the transport properties of TarII SbIII 400.1, an L. tarentolae Sb(III)-resistant mutant selected for Sb(III) resistance and cross-resistant to As(III) (Table 1). As with the As(III)-resistant mutant, we found a reduced accumulation of both arsenite and Sb(III) in this mutant (Fig. 5). Two new axenic amastigote Sb(III)-resistant mutants were generated. Similarly, as seen for L. tarentolae, the L. infantum and L. panamensis amastigote mutants selected for Sb(III) resistance were cross-resistant to As(III) (Table 1). The resistant amastigote mutants displayed a markedly decreased accumulation of Sb(III) (Fig. 3B and D). Interestingly, when resistant amastigotes were switched to promastigotes, the transport defect selected in the amastigote stage of the parasites (Fig. 3B and D) was retained in the promastigote stage of both species (Fig. 3A and C), suggesting that the transport defect is not stage specific.
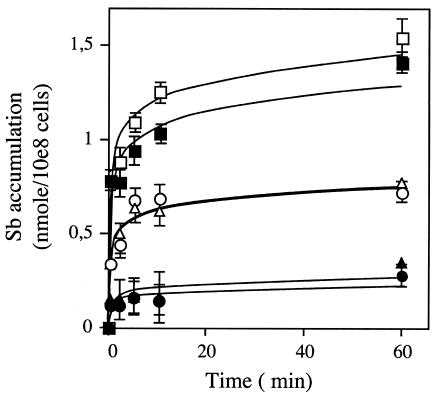
FIG. 5.
Accumulation of As(III) (A) and Sb(III) (B) in L. tarentolae metal-resistant mutants. Time-dependent uptake of metal was measured as described in Materials and Methods with 100 μM substrate. ○, wild-type L. tarentolae; □, Sb400.1; ▵, As 50.1. Data are shown as the means ± standard errors (symbols are frequently wider than error bars).
Energetics of Sb(III) transport in Leishmania.
We further characterized the Leishmania transport properties of Sb(III) by using metabolic inhibitors, including DNP, an ATPase inhibitor, and valinomycin, an ionophore that disrupts the membrane potential. The wild-type L. tarentolae cells and the Sb(III)-resistant mutant TarII SbII 400.1 were incubated with these inhibitors. Neither the wild-type nor the mutant strain with a decreased accumulation phenotype showed altered transport properties upon incubation with valinomycin (Fig. 6). Treatment of wild-type cells with DNP increases by twofold the accumulation of Sb(III) in a susceptible strain, and upon DNP treatment, the Sb(III) accumulation in the mutant becomes indistinguishable from the accumulation of similarly treated wild-type cells (Fig. 6). These results are suggestive of an active efflux system in both wild-type and mutant cells. Indeed, a metal efflux system has been inferred in Leishmania from work with 73As (6, 7). We were indeed capable of showing active efflux of Sb(III) in wild-type cells, and we also detected efflux in TarII SbIII 400.1, but this efflux system did not appear higher in the mutant than in the wild-type cells (results not shown).
FIG. 6.
Effect of energy uncouplers on accumulation of Sb(III) in L. tarentolae cells. Time-dependent uptake of Sb was determined as described in Materials and Methods with 100 μM substrate in the presence of DNP or valinomycin. For wild-type L. tarentolae: ○, control; □, 5 mM DNP; ▵, 1 μM valinomycin. For Sb400.1: •, control; ▪, 5 mM DNP; ▴, 1 μM valinomycin. Data are shown as the means ± standard errors (symbols are frequently wider than error bars).
DISCUSSION
A number of studies have been conducted to examine the uptake of antimony in Leishmania cells. Earlier studies have used radioactive 125Sb and 73As, a metal related to antimony, to look at the transport properties of Leishmania cells (5, 7). However, the availability of the metals in a radioactive form is limiting. Thus, electrothermal atomic absorption spectroscopy (27) and, more recently, ICP-MS techniques (33) have been used to monitor the uptake of antimony in Leishmania cells. We describe here our results with ICP-MS to further characterize the uptake of antimony and to monitor the decreased accumulation of the metal in resistant Leishmania cells. We first confirmed that both trivalent and pentavalent antimony in the form of potassium antimonyl tartrate and Pentostam are taken up by both promastigotes and amastigotes of various Leishmania species (Fig. 3 and 4). Significantly, Sb(V) accumulation is higher in axenic amastigotes of L. infantum than in their promastigote stage (Fig. 5), and this was also observed in Leishmania amazonensis (5) and in L. donovani (33). Despite the fact that Sb(V) accumulates in both stages of the parasite, at pharmacological concentrations this drug has no antileishmanial activity (19, 26, 31). This was reconfirmed with axenic amastigotes of L. infantum and L. panamensis (Table 1 and unpublished observations). These observations are consistent with several other studies, but it is salient to point out that in some studies, axenic amastigotes were found to be as susceptible as intracellular ones (3, 4, 33). This discrepancy will need to be resolved and will require standardization in assessing resistance, as several factors such as medium, pH, and thiol concentration are likely to influence metal susceptibility (4, 30). Since Sb(III) is highly active against Leishmania and, in our hands, Sb(V) is active only when used against intracellular parasites, it is logical to hypothesize that reduction of Sb(V) to Sb(III) is necessary for activity and that this activity is present primarily in the macrophages. There is one report, however, that shows that the parasite itself in its amastigote stage can reduce Sb(V) to Sb(III) (33), which suggests that under some conditions, reduction may indeed occur primarily in the parasite. The pH of the medium and even the concentration of thiols such as cysteine (30) could influence the reduction of the metal. Consequently, further work is required to determine where most of the reducing activity takes place. Wherever the site of reduction is, it is clear that more Sb(V) enters the axenic amastigote stage than Sb(III) in L. infantum (Fig. 4).
The differential accumulation of Sb(III) and Sb(V) in both stages of Leishmania suggests that Sb(V) and Sb(III) do not enter by the same route. This was further substantiated by competition experiments with the related metal As(III). Indeed, the accumulation of Sb(III) is competitively inhibited efficiently with As(III) (Fig. 2), whereas the accumulation of Sb(V) is not (Fig. 4C). In E. coli, As(III) and Sb(III) enter cells through GlpF, the glycerol facilitator (29). GlpF is a member of the aquaglyceroporin family, which are channels that transport neutral organic solutes. Yeast (36) and mammalian (17) aquaglyceporins were also shown to catalyze the uptake of As(III) and Sb(III). It remains to be seen how trivalent metals enter Leishmania cells. The fact that Sb(III) uptake is not decreased in cells treated with DNP (Fig. 6) is also consistent with entry through an energy-independent channel like protein. In both E. coli and yeast, arsenate [As(V)] uptake is catalyzed by a number of phosphate transporters (reviewed in reference 28). It is not known yet whether Sb(V) enters by the same transporters as As(V) in different types of cells. However, neither As(V) nor phosphate could compete with the uptake of Pentostam in Leishmania (Fig. 4C and result not shown). Pentostam consists of Sb(V) complexed to gluconate, and it is possible that Sb(V) enters via a protein recognizing a sugar-like structure shared with gluconate. Gluconate was shown to competitively inhibit the uptake of Sb(V) by 35% in axenic amastigotes (result not shown), suggesting that this could be one possible route of entry.
More Sb(III) enters L. infantum promastigotes than axenic amastigotes (Fig. 3A and B), but nonetheless, Sb(III) is 15 times more active against the axenic amastigote stage (Table 1). A similar 15-fold increase in susceptibility was observed for the axenic L. panamensis parasites (Table 1), although in this case, slightly more Sb(III) enters the amastigote parasite. This increased Sb(III) susceptibility in the amastigote stage was seen in other species (8, 26), suggesting that targets are expressed differently in amastigotes than in promastigotes or that detoxification of Sb(III) is more efficient in the promastigote stage. Selecting for Sb(III) resistance in the amastigote stage led to resistant parasites (Table 1), and in these parasites, we report for the first time a marked decrease in the accumulation of the drug (Fig. 3 and 4). This decreased accumulation could be due to decreased uptake (e.g., through a point mutation in the putative channel) or increased efflux of the metal. By de-energizing the resistant cells with DNP, we increased Sb(III) accumulation in resistant cells, suggesting that there is an energy-dependent system either preventing the entry or increasing the efflux of the metal. Interestingly, we could also increase slightly Sb(III) accumulation in wild-type cells upon DNP treatment (Fig. 6), suggesting a basal activity of this energy-dependent system in sensitive isolates. Using 73As, we were able to observe an active efflux system whose activity was increased in drug-resistant mutants (7). While here we were able to measure an efflux of Sb(III) by ICP-MS, we could not see a difference between sensitive and resistant cells (results not shown). This could suggest that the resistance mechanisms between Sb(III) and As(III) are not similar, which we think is unlikely, or that efflux is very rapid in the resistant cells and technically not accurately measurable during the first 30 s. Further work will be required to understand the molecular basis of the decreased accumulation of Sb(III) in resistant cells.
In summary, we have used a novel technique to quantify intracellular antimony uptake in Leishmania. We have provided evidence that Sb(V), the drug used for treating patients, and Sb(III), likely the active form of the metal, accumulate in these parasites by different routes. Leishmania selected for antimony resistance in the amastigote stage also shows a decrease in the accumulation of the drug. Thus, resistance to antimony in Leishmania might be mediated by transporters, and further work is required to test how this decreased accumulation is achieved.
Acknowledgments
We thank our colleagues from the Ouellette lab for useful comments on the manuscript.
This work was supported by the CIHR group and operating grants to M.O., through a Wellcome Trust-Burroughs Wellcome Fund new initiative in infectious diseases program grant (to M.O. and N.G.S), and through an FRSQ collaborative travel grant between M.O. and J.W. C.B. was the recipient of a CIHR studentship. M.O. is a Burroughs Wellcome Fund Scholar in Molecular Parasitology and holds a Canada Research Chair in Antimicrobial Resistance.
C.B. and J.W. contributed equally to this work.
REFERENCES
- 1.Berman, J. D. 1997. Human Leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. [DOI] [PubMed] [Google Scholar]
- 2.Berman, J. D., J. V. Gallalee, and B. D. Hansen. 1987. Leishmania mexicana: uptake of sodium stibogluconate (Pentostam) and pentamidine by parasite and macrophages. Exp. Parasitol. 64:127-131. [DOI] [PubMed] [Google Scholar]
- 3.Callahan, H. L., A. C. Portal, R. Devereaux, and M. Grogl. 1997. An axenic amastigote system for drug screening. Antimicrob. Agents Chemother. 41:818-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrio, J., M. de Colmenares, C. Riera, M. Gallego, M. Arboix, and M. Portus. 2000. Leishmania infantum: stage-specific activity of pentavalent antimony related with the assay conditions. Exp. Parasitol. 95:209-214. [DOI] [PubMed] [Google Scholar]
- 5.Croft, S. L., K. D. Neame, and C. A. Homewood. 1981. Accumulation of [125Sb]sodium stibogluconate by Leishmania mexicana amazonensis and Leishmania donovani in vitro. Comp. Biochem. Physiol. C 68:95-98. [DOI] [PubMed] [Google Scholar]
- 6.Dey, S., M. Ouellette, J. Lightbody, B. Papadopoulou, and B. P. Rosen. 1996. An ATP-dependent As(III)-glutathione transport system in membrane vesicles of Leishmania tarentolae. Proc. Natl. Acad. Sci. USA 93:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey, S., B. Papadopoulou, A. Haimeur, G. Roy, K. Grondin, D. Dou, B. P. Rosen, and M. Ouellette. 1994. High level arsenite resistance in Leishmania tarentolae is mediated by an active extrusion system. Mol. Biochem. Parasitol. 67:49-57. [DOI] [PubMed] [Google Scholar]
- 8.Ephros, M., A. Bitnun, P. Shaked, E. Waldman, and D. Zilberstein. 1999. Stage-specific activity of pentavalent antimony against Leishmania donovani axenic amastigotes. Antimicrob. Agents Chemother. 43:278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairlamb, A. H., and A. Cerami. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695-729. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira-Pinto, K. C., A. L. Miranda-Vilela, C. Anacleto, A. P. Fernandes, M. C. Abdo, M. L. Petrillo-Peixoto, and E. S. Moreira. 1996. Leishmania (V.) guyanensis: isolation and characterization of glucantime-resistant cell lines. Can. J. Microbiol. 42:944-949. [DOI] [PubMed] [Google Scholar]
- 11.Guerin, P. J., P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral Leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 12.Haimeur, A., C. Brochu, P. Genest, B. Papadopoulou, and M. Ouellette. 2000. Amplification of the ABC transporter gene PGPA and increased trypanothione levels in potassium antimonyl tartrate (SbIII) resistant Leishmania tarentolae. Mol. Biochem. Parasitol. 108:131-135. [DOI] [PubMed] [Google Scholar]
- 13.Haimeur, A., and M. Ouellette. 1998. Gene amplification in Leishmania tarentolae selected for resistance to sodium stibogluconate. Antimicrob. Agents Chemother. 42:1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 15.Légaré, D., B. Papadopoulou, G. Roy, R. Mukhopadhyay, A. Haimeur, S. Dey, K. Grondin, C. Brochu, B. P. Rosen, and M. Ouellette. 1997. Efflux systems and increased trypanothione levels in arsenite-resistant Leishmania. Exp. Parasitol. 87:275-282. [DOI] [PubMed] [Google Scholar]
- 16.Légaré, D., D. Richard, R. Mukhopadhyay, Y. D. Stierhof, B. P. Rosen, A. Haimeur, B. Papadopoulou, and M. Ouellette. 2001. The Leishmania ABC protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 276:26301-26307. [DOI] [PubMed] [Google Scholar]
- 17.Liu, Z., J. Shen, J. M. Carbrey, R. Mukhopadhyay, P. Agre, and B. P. Rosen. 2002. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA 99:6053-6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucumi, A., S. Robledo, V. Gama, and N. G. Saravia. 1998. Sensitivity of Leishmania viannia panamensis to pentavalent antimony is correlated with the formation of cleavable DNA-protein complexes. Antimicrob. Agents Chemother. 42:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mottram, J. C., and G. H. Coombs. 1985. Leishmania mexicana: enzyme activities of amastigotes and promastigotes and their inhibition by antimonials and arsenicals. Exp. Parasitol. 59:151-160. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay, R., S. Dey, N. Xu, D. Gage, J. Lightbody, M. Ouellette, and B. P. Rosen. 1996. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc. Natl. Acad. Sci. USA 93:10383-10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay, R., J. Shi, and B. P. Rosen. 2000. Purification and characterization of Acr2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 275:21149-21157. [DOI] [PubMed] [Google Scholar]
- 22.Murray, H. W. 2001. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 45:2185-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouellette, M., E. Hettema, D. Wust, F. Fase-Fowler, and P. Borst. 1991. Direct and inverted DNA repeats associated with P-glycoprotein gene amplification in drug resistant Leishmania. EMBO J. 10:1009-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouellette, M., and S. Ward. 2002. Drug resistance in parasites, p. 395-430. In J. Marr, T. Nielsen, and R. Komuniecki (ed.), Molecular medical parasitology. Academic Press, New York, N.Y.
- 25.Perez-Victoria, J. M., F. J. Perez-Victoria, A. Parodi-Talice, I. A. Jimenez, A. G. Ravelo, S. Castanys, and F. Gamarro. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45:2468-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts, W. L., J. D. Berman, and P. M. Rainey. 1995. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob. Agents Chemother. 39:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, W. L., and P. M. Rainey. 1993. Antimony quantification in Leishmania by electrothermal atomic absorption spectroscopy. Anal. Biochem. 211:1-6. [DOI] [PubMed] [Google Scholar]
- 28.Rosen, B. P. 2002. Biochemistry of arsenic detoxification. FEBS Lett. 529:86-92. [DOI] [PubMed] [Google Scholar]
- 29.Sanders, O. I., C. Rensing, M. Kuroda, B. Mitra, and B. P. Rosen. 1997. Antimonite is accumulated by the glycerol facilitator GlpF in Escherichia coli. J. Bacteriol. 179:3365-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos Ferreira, C., P. S. Martins, C. Demicheli, C. Brochu, M. Ouellette, and F. Frezard. 2003. Thiol-induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals 16:441-446. [DOI] [PubMed] [Google Scholar]
- 31.Sereno, D., M. Cavaleyra, K. Zemzoumi, S. Maquaire, A. Ouaissi, and J. L. Lemesre. 1998. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob. Agents Chemother. 42:3097-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sereno, D., G. Roy, J. L. Lemesre, B. Papadopoulou, and M. Ouellette. 2001. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob. Agents Chemother. 45:1168-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaked-Mishan, P., N. Ulrich, M. Ephros, and D. Zilberstein. 2001. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J. Biol. Chem. 276:3971-3976. [DOI] [PubMed] [Google Scholar]
- 34.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 35.Sundar, S., D. K. More, M. K. Singh, V. P. Singh, S. Sharma, A. Makharia, P. C. Kumar, and H. W. Murray. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104-1107. [DOI] [PubMed] [Google Scholar]
- 36.Wysocki, R., C. C. Chery, D. Wawrzycka, M. Van Hulle, R. Cornelis, J. M. Thevelein, and M. J. Tamas. 2001. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 40:1391-1401. [DOI] [PubMed] [Google Scholar]