Abstract
In vitro linezolid resistance was selected at a higher frequency in Enterococcus faecalis JH2-2 than in recombination-deficient E. faecalis UV202. Resistance in JH2-2 was related to accumulated G2576T mutations in 23S rRNA genes, with the least resistance conferred by mutations in two of four copies. UV202 resistance was associated with a G2505A mutation present in a single copy in mutants with different MICs.
The oxazolidinone antimicrobial agent linezolid inhibits bacterial protein synthesis by binding to the ribosomal peptidyltransferase center, domain V of 23S rRNA, and blocking translation initiation (6, 14). Resistance to linezolid is conferred by point mutations within this region, which is responsible for binding fMet-tRNA (2, 6). A variety of mutations have been observed, but in vitro investigations and clinical studies implicate a G2576U (Escherichia coli numbering scheme) mutation in rRNA in linezolid resistance for Enterococcus faecalis, Enterococcus faecium, and Staphylococcus aureus (7, 11, 13). Further, a recent study showed a direct correlation between the level of resistance and the percentage of 23S genes with the G2576T mutation (7). E. faecalis has four copies of the 23S rRNA gene, while E. faecium has six copies. The frequency with which strains having the mutation in multiple gene copies are found clinically suggests that homologous recombination between mutated and wild-type 23S rRNA genes may promote the development of high-level resistance under consistent antimicrobial selective pressure. This phenomenon, generally referred to as gene conversion, has been implicated in increasing levels of resistance to aminoglycosides in Mycobacterium. smegmatis (12) and to evernimicin in Streptococcus pneumoniae (1). To test this hypothesis, we compared the ability to select linezolid-resistant mutants in vitro from E. faecalis JH2-2 and its recombination-deficient relative UV202.
Strain JH2-2 is a fusidic acid- and rifampin-resistant mutant of E. faecalis JH2 (5). E. faecalis UV202 is a recombination-deficient mutant of JH2-2 (15). MICs were determined by the agar dilution method in brain heart infusion (BHI) broth (9). In vitro selection of resistant mutants was performed for both strains by serial plate selection described for generating resistance to quinupristin-dalfopristin (8) and linezolid (13). When resistant mutants of UV202 could not be selected using this method, a second technique was developed in which initial cultures were grown overnight on agar containing linezolid (1 μg/ml, the linezolid MIC for strain UV202). Individual colonies that grew at this concentration were grown overnight in BHI broth containing linezolid (1 μg/ml) and standardized to a McFarland no. 1 reference density at 3 × 107 CFU/ml. One hundred microliters of the suspension, or 1 × 106 to 3 × 106 CFU, was streaked onto a BHI agar plate containing linezolid (1.5 μg/ml) and incubated, first for 24 h at 37°C and then for 72 h at room temperature. The resulting colonies from this plate were transferred to a gradient plate of linezolid (0.5 to 4.0 μg/ml) and incubated for 48 h at 37°C. The colonies growing closest to the highest diffused concentration were incubated overnight in 3 ml of BHI broth without antibiotic. These overnight-grown cultures were again standardized to a McFarland no. 1 reference, and 100-μl culture samples were streaked onto plates containing 2, 4, 8, and 16 μg of linezolid per ml. Colonies grew successfully on the three lower concentrations, but all colonies were inhibited by linezolid at 16 μg/ml. Two strains were chosen for further analysis. One strain was derived from the original selection on gradient plates and had a linezolid MIC of 2 μg/ml. The other strain resulted from a further selection performed on this UV202 mutant, and its linezolid MIC was 16 μg/ml.
The G2576T mutation was detected by using a MaeI digestion assay performed by the method of Marshall et al. (7). Details of the assay using EcoRV to digest amplification products from UV202 were identical, except for the specific restriction enzyme employed.
Cloned PCR products were sequenced as previously described (3) using an A.L.F. automated sequencer (Amersham Pharmacia, Piscataway, N.J.). Enterococcal DNA was extracted, digested with restriction enzyme EcoRV (Promega, Madison, Wis.), transferred, and hybridized with digoxigenin-labeled probes as previously described (3).
Growth curves were performed on wild-type parents and resistant mutants. Freshly grown cultures (grown overnight) were diluted 1:100 in BHI broth. Thirty microliters of the diluted culture was then mixed with 3 ml of BHI broth with no antibiotic. This culture was grown at 37°C without shaking. The optical density at 600 nm (OD600) was recorded hourly for the first 3 h and then every 30 min for the next 4 h. Twenty microliters was removed when the OD600 reached 1.0 in order to perform colony counts. Measurements were stopped at 8 h.
The initial linezolid MICs for both JH2-2 and UV202 were 1 μg/ml. Serial passage on doubling concentrations of linezolid led to stable high-level resistance in E. faecalis JH2-2. MICs of 128 μg/ml were achieved with sequential passage onto plates with higher concentrations of linezolid, with intermediate isolates maintained with MICs equal to 4, 8, 16, 32, and 64 μg/ml. Despite several attempts, we were unable to isolate any linezolid-resistant mutants from E. faecalis UV202 by this method. Linezolid-resistant mutants of UV202 were obtained by the gradient plate method. The colonies that were recovered grew well on agar plates containing 2, 4, and 8 μg of linezolid per ml. Colonies from the plate containing 8 μg of linezolid per ml failed to grow at higher concentrations, even when the inoculum was increased to a McFarland no. 2 standard.
Digestion of amplification products from resistant E. faecalis JH2-2 mutants with MaeI showed a correlation between the percentage of 23S rRNA genes possessing the G2576T mutation and the linezolid MIC for the isolate (Table 1). The least resistant mutant that we recovered was inhibited by 4 μg of linezolid per ml, two doubling dilutions above the MIC of the wild-type strain. Even at this low MIC, two of four 23S rRNA genes possessed the G2576T mutation. Isolates screened with MICs of 16, 32, and 64 μg/ml had mutations in three of four gene loci, whereas the isolate with an MIC of 128 μg/ml had the mutation present at all 23S rRNA loci. We were unable to identify a JH2-2 mutant with the G2576T mutation at only one locus. Digestion of cloned, PCR-amplified fragments from UV202 and two mutants (linezolid MICs of 2 and 16 μg/ml) showed, in both instances, absence of the MaeI restriction site, indicating that the G2576T mutation did not occur and was not the basis of resistance seen in these strains.
TABLE 1.
Correlation of linezolid MICs to the number of G2576T mutated 23S rRNA genes in E. faecalis JH2-2 mutants
| Linezolid MIC (μg/ml) | No. of amplification products digested with MaeI/no. of colonies screened (%) | No. of G2576T mutated genes/total no. of 23S rRNA genes (%) |
|---|---|---|
| 4 | 5/10 (50) | 2/4 (50) |
| 8 | 4/12 (33) | 2/4 (50) |
| 16 | 8/12 (67) | 3/4 (75) |
| 32 | 9/11 (81) | 3/4 (75) |
| 64 | 10/12 (83) | 3/4 (75) |
| 128 | 17/17 (100) | 4/4 (100) |
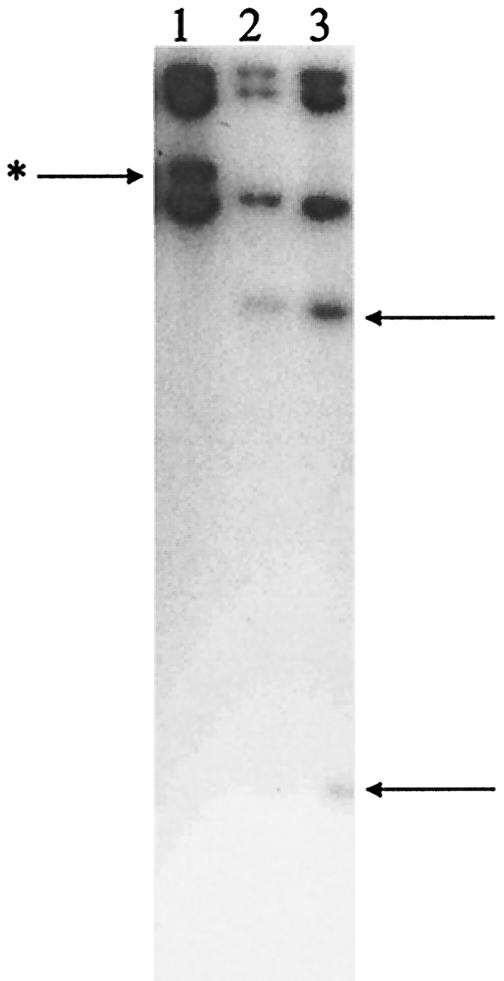
Sequence analysis of PCR amplification products from E. faecalis UV202 and its mutants confirmed the wild-type nucleotide at position 2576 but revealed a G2505A mutation in both UV202 mutants (MICs of 2 and 16 μg/ml). This G2505A mutation creates an EcoRV restriction site in the 23S rRNA gene. Using the rapid restriction enzyme digest assay, the presence of the G2505A mutation in linezolid-resistant UV202 mutants was confirmed. It was also shown to be absent from susceptible wild-type strains and from resistant mutants of E. faecalis JH2-2. Digestion of amplification products from UV202 and its two resistant mutants suggested that the mutation was present in one of four loci in both isolates (data not shown). To confirm this determination, Southern hybridization was performed on EcoRV digests of UV202 and its mutants, using a labeled amplification product of 23S rRNA (that spanned the EcoRV site) as a probe (Fig. 1). This Southern hybridization confirmed the mutation at only one locus in mutants with linezolid MICs of 2 and 16 μg/ml.
FIG. 1.
Southern hybridization of EcoRV-digested genomic DNA from E. faecalis with a 23S rRNA probe. The position of the 23S rRNA gene copy that acquires the mutation is indicated by the arrow with the asterisk to the left of the gel. The positions of the two bands resulting from EcoRV digestion of the 23S rRNA gene that acquires the G2505A mutation in the two resistant UV202 mutants are indicated by the arrows to the right of the gel. Lane 1, E. faecalis UV202; lane 2, linezolid-resistant UV202 mutant (MIC of 2 μg/ml); lane 3, linezolid-resistant UV202 mutant (MIC of 16 μg/ml).
The data presented in this paper support the association between a mutation at position 2576 of the E. faecalis 23S rRNA gene and resistance to linezolid. They also support the fact that the level of resistance expressed correlates with the number of 23S genes possessing the G2576T mutation. It is of interest that even the lowest level of resistance detectable in E. faecalis JH2-2 was conferred by mutation of two of four 23S loci, suggesting that mutation of only one of four loci does not confer a level of resistance that can be detected by our technique. In this context, our failure to obtain G2576T mutations conferring resistance in E. faecalis UV202 is consistent with the hypothesis that greater percentages of 23S loci possessing the mutation arise through gene conversion (homologous recombination between resistant and susceptible loci) within the individual organism. The recombination-deficient strain may be able to mutate a single site at the same rate as a proficient strain (we have no evidence either for or against this possibility), but it cannot then perform the subsequent recombination necessary to yield a high enough percentage of mutant loci to confer detectable levels of resistance.
The G2505A mutation observed in the resistant E. faecalis UV202 mutants has been associated with linezolid resistance in a previous in vitro study (13). No other mutations were noted within the amplified region of the 23S rRNA gene, but we did not go further to amplify the entire gene to look for additional mutations. The different levels of resistance associated with the presence of this mutation in our study suggests the accumulation of additional 23S mutations that we are not aware of or suggests that there may be other mechanisms that serve to amplify the level of resistance associated with specific mutations.
The predominance of the G2576T mutation in clinical isolates is not explained by our work. In an effort to examine the relative fitness of the two mutations, we plotted growth curves of the mutants compared to their wild-type parents. An inverse association was noted between the number of 23S rRNA genes with the G2576T mutation and growth rate in E. faecalis JH2-2 and its mutants (data not shown). Moreover, even among strains that had three of four genes mutated, growth rates were slightly slower in strains exhibiting higher MICs (data not shown). In contrast, increasing levels of resistance in E. faecalis UV202 mutants was associated with more rapid growth compared to wild-type UV202 (data not shown).
The number of 23S rRNA genes present in enterococci was initially considered a reason for optimism that linezolid would not easily yield resistant mutants. Early clinical experience was at odds with this hope, as several investigators reported the emergence of linezolid-resistant isolates during therapy (4, 10). Our data suggest that the reason for this relative ease lies in the host recombination machinery. The proficiency of this machinery in clinical enterococcal isolates indicates that mutation of a single 23S locus is the critical and perhaps rate-limiting step in the emergence of linezolid resistance and that the subsequent development of high levels of resistance may be rapid.
Acknowledgments
This work was supported in part by the Medical Research Service of the Department of Veterans Affairs and by a grant from the Steris Corporation.
REFERENCES
- 1.Adrian, P. V., C. Mendrick, D. Loebenberg, P. McNicholas, K. J. Shaw, K. P. Klugman, R. S. Hare, and T. A. Black. 2000. Evernimicin (SCH27899) inhibits a novel ribosome target site: analysis of 23S ribosomal DNA mutants. Antimicrob. Agents Chemother. 44:3101-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki, H., L. Ke, S. M. Poppe, T. J. Poel, E. A. Weaver, R. C. Gadwood, R. C. Thomas, D. L. Shinabarger, and M. C. Ganoza. 2002. Oxazolidinone antibiotics target the P site on Escherichia coli ribosomes. Antimicrob. Agents Chemother. 46:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carias, L. L., S. D. Rudin, C. J. Donskey, and L. B. Rice. 1998. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J. Bacteriol. 180:4426-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 5.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloss, P., L. Xiong, D. L. Shinaberger, and A. S. Mankin. 1999. Resistance mutations in 23S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center. J. Mol. Biol. 294:93-101. [DOI] [PubMed] [Google Scholar]
- 7.Marshall, S. H., C. J. Donskey, R. Hutton-Thomas, R. A. Salata, and L. B. Rice. 2002. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 46:3334-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millichap, J., T. A. Ristow, G. A. Noskin, and L. R. Peterson. 1996. Selection of Enterococcus faecium strains with stable and unstable resistance to the streptogramin RP 59500 using stepwise in vitro exposure. Diagn. Microbiol. Infect. Dis. 25:15-20. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Pai, M. P., K. A. Rodvold, P. C. Schreckenberger, R. D. Gonzales, J. M. Petrolatti, and J. P. Quinn. 2002. Risk factors associated with the development of infection with linezolid- and vancomycin-resistant Enterococcus faecium. Clin. Infect. Dis. 35:1269-1272. [DOI] [PubMed] [Google Scholar]
- 11.Pillai, S. K., G. Sakoulas, C. Wennersten, G. M. Eliopoulos, R. C. Moellering, Jr., M. J. Ferraro, and H. S. Gold. 2002. Linezolid resistance in Staphylococcus aureus: characterization and stability of resistant phenotype. J. Infect. Dis. 186:1603-1607. [DOI] [PubMed] [Google Scholar]
- 12.Prammananan, T., P. Sander, B. Springer, and E. C. Bottger. 1999. RecA-mediated gene conversion and aminoglycoside resistance in strains heterozygous for rRNA. Antimicrob. Agents Chemother. 43:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prystowsky, J., F. Siddiqui, J. Chosay, D. L. Shinabarger, J. Millichap, L. R. Peterson, and G. A. Noskin. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 45:2154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong, L., P. Kloss, S. Douthwaite, N. M. Andersen, S. Swaney, D. L. Shinabarger, and A. S. Mankin. 2000. Oxazolidinone resistance mutations in 23S rRNA of Escherichia coli reveal the central region of domain V as the primary site of drug action. J. Bacteriol. 182:5325-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yagi, Y., and D. Clewell. 1980. Recombination-deficient mutants of Streptococcus faecalis. J. Bacteriol. 143:966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]