Abstract
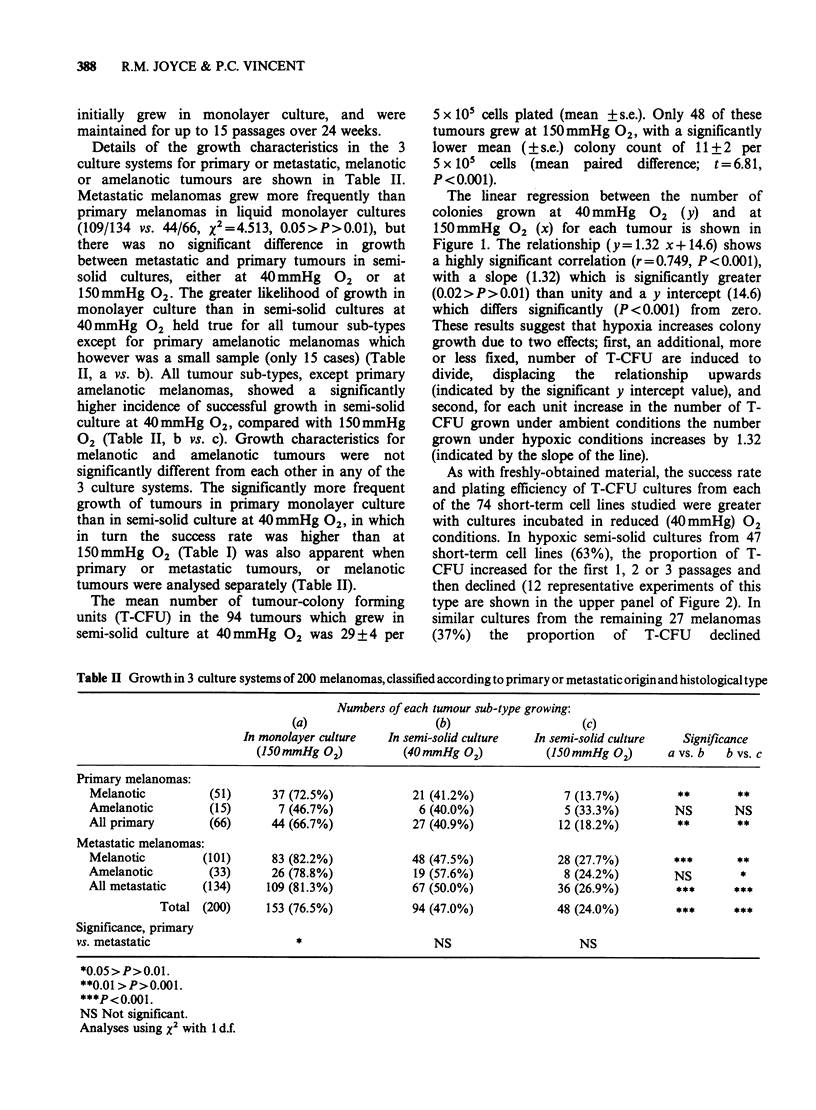
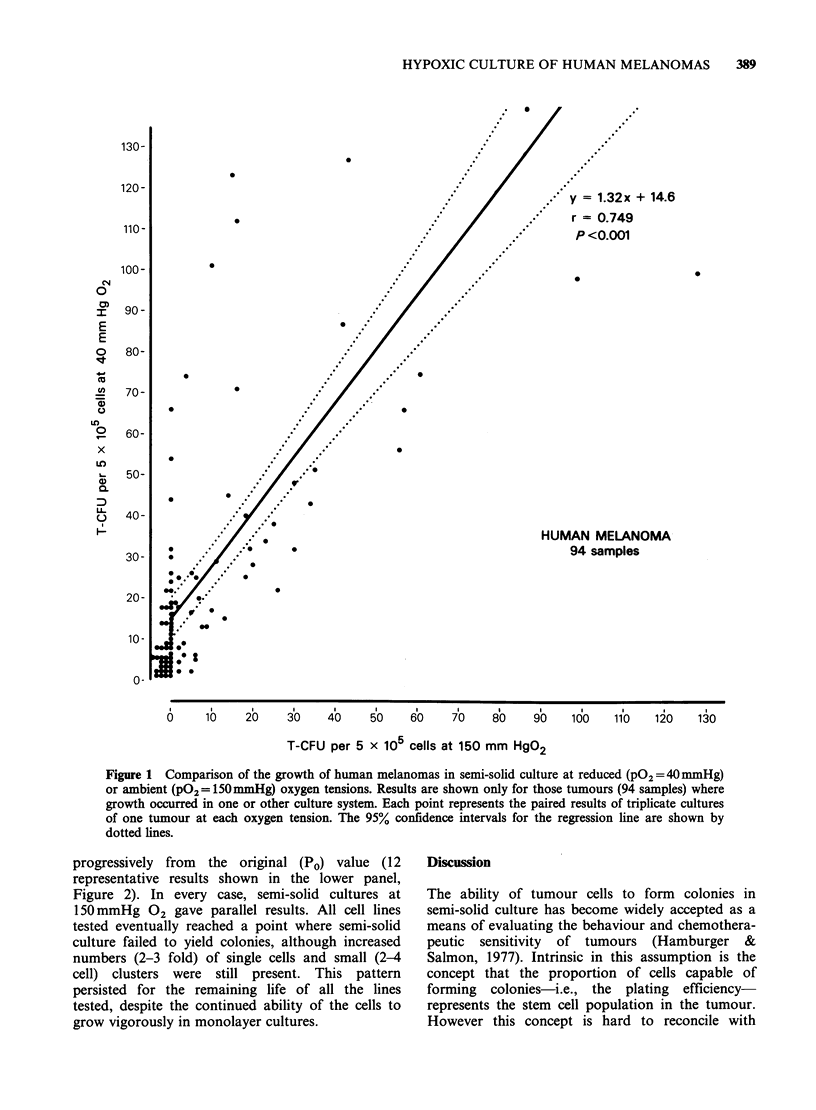
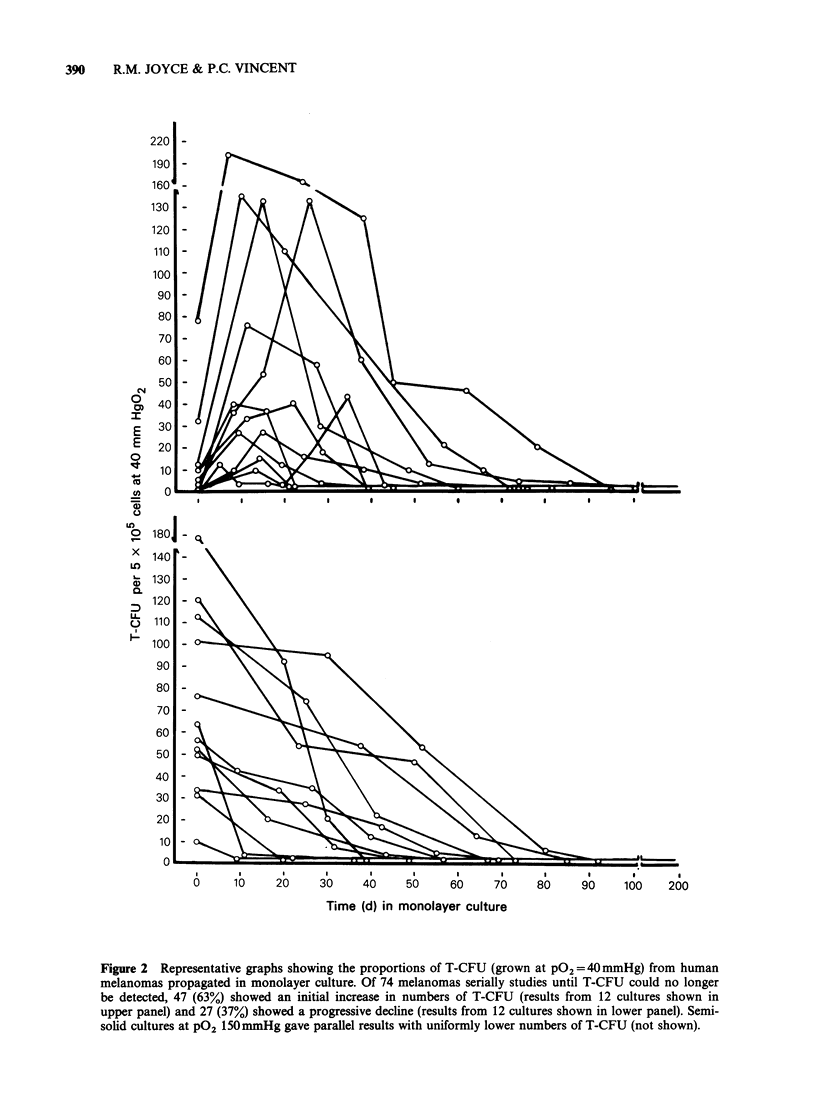
A systematic study was undertaken to compare the growth characteristics of human melanomas in liquid monolayer cultures at ambient oxygen tension, and in semi-solid cultures at ambient or reduced oxygen tension. Physically dispersed single cell suspensions from 200 freshly-excised melanomas (66 primary, 134 secondary) from 169 patients were cultured in monolayers, or plated in semi-solid cultures maintained either in 5% CO2 in room air (20% O2) or in 5% CO2, 5% O2 and 90% N2, to assay tumour colony-forming units (T-CFU). Aliquots were taken at each passage of the monolayer cultures for T-CFU assay in semi-solid culture at ambient and reduced O2 concentrations. Of 200 melanomas tested, 153 (77%) grew in monolayer culture, 94 (47%) in semi-solid culture at 5% O2, and only 48 (24%) in semi-solid culture at 20% O2. The mean number (+/-s.e.) of colonies in the 94 tumours which grew in semi-solid culture at 5% O2 (29 +/- 4 per 5 x 10(5) cells plated) was significantly greater than the mean in the same tumours in semi-solid culture at 20% O2 (11 +/- 2 per 5 x 10(5) cells). Furthermore, hypoxic colonies showed a morphologically different growth pattern. There was a significant correlation (r = 0.749, P less than 0.001) between the number of colonies growing at 5% O2 and the number at 20% O2; hypoxia appeared to act both by recruiting additional T-CFU and by increasing the proliferative activity of those already present. Short-term monolayer cultured cell lines showing evidence of persistent tumour cell characteristics were successfully established from 74 tumours, and the proportions of T-CFU assayed at each passage. In 63% of cultures the proportion of T-CFU increased initially and then declined, while in the remainder it declined progressively throughout. Although monolayer cultures were successfully maintained for up to 15 passages, T-CFU became undetectable by the eighth passage and remained so thereafter.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano S., Riglar C. Colony growth in agar by human melanoma cells. Cancer Res. 1981 Mar;41(3):1199–1204. [PubMed] [Google Scholar]
- BROSEMER R. W., RUTTER W. J. The effect of oxygen tension on the growth and metabolism of a mammalian cell. Exp Cell Res. 1961 Oct;25:101–113. doi: 10.1016/0014-4827(61)90311-1. [DOI] [PubMed] [Google Scholar]
- Bateman A. E., Peckham M. J., Steel G. G. Assays of drug sensitivity for cells from human tumours: in vitro and in vivo tests on a xenografted tumour. Br J Cancer. 1979 Jul;40(1):81–88. doi: 10.1038/bjc.1979.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Selby P. J., Smith I. E., Mills J., Peckham M. J. Growth of human tumour cell colonies from biopsies using two soft-agar techniques. Br J Cancer. 1978 Jul;38(1):77–81. doi: 10.1038/bjc.1978.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney L., Berman E. R. Oxygen toxicity: membrane damage by free radicals. Invest Ophthalmol. 1976 Oct;15(10):789–792. [PubMed] [Google Scholar]
- Gan E. V., Haberman H. F., Menon I. A. Electron transfer properties of melanin. Arch Biochem Biophys. 1976 Apr;173(2):666–672. doi: 10.1016/0003-9861(76)90304-0. [DOI] [PubMed] [Google Scholar]
- Graham D. G., Tiffany S. M., Vogel F. S. The toxicity of melanin precursors. J Invest Dermatol. 1978 Feb;70(2):113–116. doi: 10.1111/1523-1747.ep12541249. [DOI] [PubMed] [Google Scholar]
- Gupta V., Krishan A. Effect of oxygen concentration on the growth and drug sensitivity of human melanoma cells in soft-agar clonogenic assay. Cancer Res. 1982 Mar;42(3):1005–1007. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E., Kim M. B., Trent J. M., Soehnlen B. J., Alberts D. S., Schmidt H. J. Direct cloning of human ovarian carcinoma cells in agar. Cancer Res. 1978 Oct;38(10):3438–3444. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VANDENBRENK H. A. EFFECT OF ELECTRODE DIMENSIONS ON TISSUE PO-2 MEASUREMENT IN VIVO. Nature. 1964 Mar 21;201:1227–1228. doi: 10.1038/2011227a0. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VANDENBRENK H. A. OXYGEN TENSION IN HUMAN MALIGNANT DISEASE UNDER HYPERBARIC CONDITIONS. Br J Cancer. 1965 Mar;19:139–150. doi: 10.1038/bjc.1965.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo E. H., Burger P. C., Vogel F. S. The melanocytotoxicity of gamma-L-glutaminyl-4-hydroxybenzene: a study of the pigmented cells in the mouse eye. Am J Pathol. 1981 Jan;102(1):40–48. [PMC free article] [PubMed] [Google Scholar]
- MASON H. S., INGRAM D. J., ALLEN B. The free radical property of melanins. Arch Biochem Biophys. 1960 Feb;86:225–230. doi: 10.1016/0003-9861(60)90409-4. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Salmon S. E. Inhibition of human melanoma colony formation by retinoids. Cancer Res. 1979 Oct;39(10):4055–4057. [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Salmon S. E. Modulation of clonogenic human melanoma cells by follicle-stimulating hormone, melatonin, and nerve growth factor. Br J Cancer. 1981 Jan;43(1):111–115. doi: 10.1038/bjc.1981.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Soehnlen B. J., Saxe D. F., Casey W. J., Salmon S. E. In vitro clonal assay for human metastatic melanoma cells. Stem Cells. 1981;1(1):61–72. [PubMed] [Google Scholar]
- Mizrahi A., Vosseller G. V., Yagi Y., Moore G. E. The effect of dissolved oxygen partial pressure on growth, metabolism and immunoglobulin production in a permanent human lymphocyte cell line culture. Proc Soc Exp Biol Med. 1972 Jan;139(1):118–122. doi: 10.3181/00379727-139-36092. [DOI] [PubMed] [Google Scholar]
- Richter A., Sanford K. K., Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J Natl Cancer Inst. 1972 Dec;49(6):1705–1712. doi: 10.1093/jnci/49.6.1705. [DOI] [PubMed] [Google Scholar]
- Steel G. G., Adams K. Stem-cell survival and tumor control in the Lewis lung carcinoma. Cancer Res. 1975 Jun;35(6):1530–1535. [PubMed] [Google Scholar]
- Stephens T. C., Peacock J. H., Steel G. G. Cell survival in B16 melanoma after treatment with combinations of cytotoxic agents: lack of potentiation. Br J Cancer. 1977 Jul;36(1):84–93. doi: 10.1038/bjc.1977.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit K. M., Endresen L., Rugstad H. E., Fodstad O., Pihl A. Comparison of two soft-agar methods for assaying chemosensitivity of human tumours in vitro: malignant melanomas. Br J Cancer. 1981 Oct;44(4):539–544. doi: 10.1038/bjc.1981.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Pihl A. Cultivation of human melanomas in soft agar. Factors influencing plating efficiency and chemosensitivity. Int J Cancer. 1981 Sep 15;28(3):329–334. doi: 10.1002/ijc.2910280312. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Timing of assays: an important consideration in the determination of clonogenic cell survival both in vitro and in vivo. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1213–1220. doi: 10.1016/0360-3016(79)90641-2. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Forseth B., Metelmann H. R., Harris G., Rowan S., Coltman C. A., Jr Direct cloning of human malignant melanoma. Cancer. 1982 Aug 15;50(4):696–701. doi: 10.1002/1097-0142(19820815)50:4<696::aid-cncr2820500413>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]


