Abstract
A common bar soap and tap water solution was able to demonstrate a 30-fold human immunodeficiency virus (HIV) inactivation and a 57 to 87% reduction in viable peripheral blood mononuclear cells in a mixture of cervicovaginal lavage fluid and seminal fluid. These observations indicate that soap and water might be used to inactivate HIV and HIV-infected cells in genital secretions.
Postcoital genital cleansing with soap and water may be effective in preventing human immunodeficiency virus (HIV) infection (9), but the direct effect of a soap solution on HIV has not been reported. HIV can be inactivated by a variety of chemicals and surfactants (6, 7) as well as by an antimicrobial hand wash product (3). However, these agents may be irritating to the genital mucosa and difficult to obtain. We report here the effects of common soap and water on HIV as well as white blood cells, which may also play a key role in HIV transmission (1, 4, 11-13).
A commercial bar soap (Ivory: tallow, palm kernel oil, coconut oil, and trace chemicals; Johnson & Johnson) was completely dissolved in warm tap water at several concentrations. The 1-g/200 ml and the 1-g/400 ml solutions resembled standard hand washing conditions according to surveyed laboratory personnel. For these studies, a more dilute solution was used (1 g/1,000 ml) to ascertain possible conditions in the environment.
The direct virucidal action of genital secretions was first measured. HIV type 1 strain SF33 (HIV-1SF33) (10) at 1,275 50% tissue culture infective doses was centrifuged (12,000 × g for 2 h at 4°C) and resuspended in control medium (RPMI 1640 containing 10% fetal calf serum and antibiotics), cervicovaginal lavage fluid (CVL), or a 1:1 mixture of CVL with seminal fluid (SF). HIV centrifugation did not have a notable effect on virus infectivity (data not shown). These virus suspensions were then evaluated for infectivity after exposure to an equal volume of soap and tap water, tap water alone, or control medium for 2 or 6 min. For all virus assays, serial 10-fold dilutions were made with control medium and were inoculated onto 106 phytohemagglutinin-stimulated, Polybrene-treated normal peripheral blood mononuclear cells (PBMCs) separated from blood provided by the Blood Centers of the Pacific (San Francisco, Calif.). The supernatants from triplicate cultures at days 7, 11, and 14 were assayed for viral reverse transcriptase (RT) (2). On day 7, 2.5 × 105 phytohemagglutinin-stimulated PBMCs were added to each RT-negative well.
To determine the effect of soap and water on cell viability, phytohemagglutinin-stimulated PBMCs in either control medium or CVL-SF were exposed to an equal volume of soap and water, tap water, or control medium for 2 or 6 min. The number of viable cells was immediately determined by trypan blue (Sigma) staining. To assess the effect of genital secretions on cell survival, PBMCs were resuspended in control medium, CVL, SF, or CVL-SF. The number of viable cells was then determined at several culture time points.
When HIV was exposed to control medium, CVL alone, SF alone, or CVL-SF, no appreciable effect on viral infectivity was noted until the 6 h time point, when a 10-fold-decreased infectivity of the virus in CVL was observed (data not shown). By 12 h, the CVL had caused a 1,000-fold decrease in virus infectivity. In contrast, HIV in control medium or CVL-SF did not show a 10-fold-decreased infectivity until 12 h after incubation, and at 24 h a 1,000-fold dilution of virus still resulted in a productive infection. Due to the cytotoxic effect of SF on PBMCs, measurements of infectious HIV in this genital fluid were limited to dilute concentrations (>10−3). Under these conditions, HIV infectivity could be found at the 105 dilution until the 1-h time point and at the 104 dilution up to the 12-h time point (data not shown). After an incubation period of 96 h, no infectious virus could be detected in any environment. The mean pHs of the SF and CVL were found to be 7.9 and 4.9, respectively. The pH of the CVL-SF mixture was between 7.4 and 7.9 (mean, 7.75) and did not differ substantially from that of the control medium.
Exposure of HIV to soap and water (1 g/1,000 ml) for 2 or 6 min decreased viral infectivity by more than 1,000-fold (Table 1). When the virus was in a CVL-SF mixture, the virucidal activity of the 1-g/1,000 ml soap and water mixture was completely eliminated. However, with a 1-g/200 ml soap solution, viral infectivity was reduced by more than 30-fold after either 2 or 6 min of exposure (data not shown).
TABLE 1.
Inactivation of HIV in control medium and genital fluids by soap and water
| Exposure mediuma | Exposure time (min) | Decreased infectivity (fold)b
|
||
|---|---|---|---|---|
| Control medium | CVL | CVL and SF | ||
| Control medium | 2 | |||
| 6 | ||||
| Water | 2 | 10 | 0 | 3 |
| 6 | 0 | 10 | 0 | |
| Soap and water (1 g/1,000 ml) | 2 | >1,000 | >1,000 | 0 |
| 6 | >1,000 | >1,000 | 0 | |
Virus (HIV-1SF33 at 1,275 50% tissue culture infective doses) in control medium or genital fluids was exposed to a soap and water solution (1 g/1,000 ml), tap water alone, or control medium. Serial 10-fold dilutions were made and used to infect PBMCs.
Infectivity was defined as the maximum dilution at which a productive infection could be detected based on the RT assay. The decreased infectivity (n-fold) is the ratio of the infectivity of the virus exposed to soap and water or water alone to that of the virus exposed to the control medium. Results reported are based on averages of results from two to three experiments.
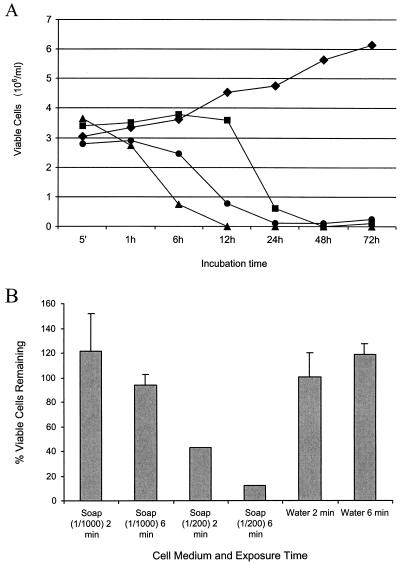
To evaluate the potential effect of genital fluids on HIV-infected cells, PBMCs were added to the different fluids. Whereas PBMCs cultured in the RPMI 1640 cell culture medium showed vigorous growth, those in the CVL or SF mixtures demonstrated substantially decreased viability (Fig. 1A). Compared to PBMCs in control medium, a ≥80% reduction in cell viability occurred by 6 h for PBMCs in CVL, by 12 h for PBMCs in SF, and by 24 h for PBMCs in CVL-SF. When PBMCs resuspended in CVL-SF were exposed to soap and water (1-g/200 ml), there was a 57% reduction (P < 0.05) in the number of viable cells after 2 min and an 87% reduction (P < 0.05) after 6 min compared with numbers of viable PBMCs in control medium (Fig. 1B). PBMCs in CVL-SF mixed with water or a relatively dilute soap and water solution (1-g/1,000 ml) demonstrated no substantial decrease in cell viability.
FIG. 1.
Effect of genital fluids and soap and water on PBMCs. (A) PHA-stimulated PBMCs were resuspended in control medium (♦), CVL alone (▴), SF alone (•), or a 1:1 mixture of CVL and SF (▪). The number of viable cells remaining was determined at various time points by trypan blue staining. (B) PBMCs in CVL-SF were exposed to an equal volume of soap and water (1 g/1,000 ml, 1 g/200 ml), tap water alone, or control medium for either 2 or 6 min. Cell viability was determined by trypan blue staining immediately after the end of the exposure period. The percentage of viable cells remaining was calculated by comparing the number of viable cells in each environment with the number of viable cells seen in control medium at the corresponding exposure time.
In addition to the widespread use of soap solutions to clean female barrier contraceptives after their removal from the vaginal canal, several recent studies have indicated that the practice of vaginal cleansing with soap, detergents, or disinfectants is common in the developing world (5, 8). The present study demonstrates that soap and water solutions should be effective in inactivating HIV and HIV-infected cells associated with barrier contraceptives or cells that are present in the vaginal canal. For these experiments, Ivory bar soap was chosen because of its relatively simple ingredients and its accessibility to women internationally. However, soap and water needs to be further evaluated for its effects on the vaginal flora if used as a douche in conjunction with female barrier contraceptives. In addition, in developing countries, access to clean water is imperative for these simple approaches to be effective in preventing HIV transmission.
Acknowledgments
We thank Alan Landay for providing the CVL specimens.
J. Z. Li is a recipient of the Doris Duke Charitable Foundation Clinical Research Fellowship.
REFERENCES
- 1.Belec, L., C. Tevi-Benissan, X. S. Lu, T. Prazuck, and J. Pillot. 1995. Local synthesis of IgG antibodies to HIV within the female and male genital tracts during asymptomatic and pre-AIDS stages of HIV infection. AIDS Res. Hum. Retrovir. 11:719-729. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman, A. D., B. Banapour, and J. A. Levy. 1985. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology 147:326-335. [DOI] [PubMed] [Google Scholar]
- 3.Lavelle, G. C., S. L. Gubbe, J. L. Neveaux, and B. J. Bowden. 1989. Evaluation of an antimicrobial soap formula for virucidal efficacy in vitro against human immunodeficiency virus in a blood-virus mixture. Antimicrob. Agents Chemother. 33:2034-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy, J. A. 1988. The transmission of AIDS: the case of the infected cell. JAMA 259:3037-3038. [PubMed] [Google Scholar]
- 5.Martin, H. L., B. A. Richardson, P. M. Nyange, L. Lavreys, S. L. Hillier, B. Chohan, K. Mandaliya, J. O. Ndinya-Achola, J. Bwayo, and J. Kreiss. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 180:1863-1868. [DOI] [PubMed] [Google Scholar]
- 6.Martin, L. S., J. S. McDougal, and S. L. Loskoski. 1985. Disinfection and inactivation of the human T lymphotropic virus type III/Lymphadenopathy-associated virus. J. Infect. Dis. 152:400-403. [DOI] [PubMed] [Google Scholar]
- 7.Polsky, B., P. A. Baron, J. W. Gold, J. L. Smith, R. H. Jensen, and D. Armstrong. 1988. In vitro inactivation of HIV-1 by contraceptive sponge containing nonoxynol-9. Lancet i:1456. [DOI] [PubMed] [Google Scholar]
- 8.Reed, B. D., K. Ford, and D. N. Wirawan. 2001. The Bali STD/AIDS study: association between vaginal hygiene practices and STDs among sex workers. Sex. Transm. Infect. 77:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siraprapasiri, T., S. Thanprasertsuk, A. Rodklay, S. Srivanichakorn, P. Sawanpanyalert, and J. Temtanarak. 1991. Risk factors for HIV among prostitutes in Chiangmai, Thailand. AIDS 5:579-582. [PubMed] [Google Scholar]
- 10.Tateno, M., and J. A. Levy. 1988. MT-4 plaque formation can distinguish cytopathic subtypes of the human immunodeficiency virus (HIV). Virology 167:299-301. [DOI] [PubMed] [Google Scholar]
- 11.Umapathy, E., T. Simbini, T. Chipata, and M. Mbizvo. 2001. Sperm characteristics and accessory sex gland functions in HIV-infected men. Arch. Androl. 46:153-158. [DOI] [PubMed] [Google Scholar]
- 12.Wolff, H., K. Mayer, G. Seage, J. Politch, C. R. Horsburgh, and D. Anderson. 1992. A comparison of HIV-1 antibody classes, titers, and specificities in paired semen and blood samples from HIV-1 seropositive men. J. Acquir. Immune Defic. Syndr. 5:65-69. [PubMed] [Google Scholar]
- 13.Xu, C., J. A. Politch, L. Tucker, K. H. Mayer, G. R. Seage III, and D. J. Anderson. 1997. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J. Infect. Dis. 176:941-947. [DOI] [PubMed] [Google Scholar]