Abstract
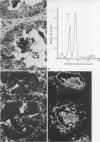
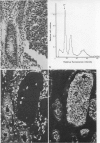
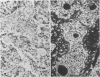
Epithelial expression of HLA-DR determinants and CEA was studied by immunofluorescence in tissue sections from 33 large bowel carcinomas of different histological grade and clinico-pathological stage; flow cytometric DNA measurements were performed in 31 of the tumours. Well-differentiated carcinomas showed a strikingly patchy staining, particularly for HLA-DR and all except one had a near-diploid DNA content. The latter feature might reflect cancer development at an early stage where no distinctly aneuploid DNA clone had as yet become a predominant subline. With decreasing degree of differentiation, the epithelial antigen expression became more homogeneous for individual tumours and the proportion of distinctly aneuploid DNA profiles increased. In the poorly differentiated group of carcinomas, epithelial staining was quite uniform, both for HLA-DR determinants and for CEA, and those tumours studied for DNA content were of the aneuploid variety. These observations are in agreement with the clonal proliferation theory of tumour development proposed by Nowell in 1976.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Rognum T. O. Evaluation of tissue preparation methods and paired immunofluorescence staining for immunocytochemistry of lymphomas. Histochem J. 1983 Jul;15(7):655–689. doi: 10.1007/BF01002987. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Ting A., Fabre J. W. Anomolous expression of HLA-DR antigens on human colorectal cancer cells. J Immunol. 1982 Aug;129(2):447–449. [PubMed] [Google Scholar]
- Hämmerling G. J. Tissue distribution of Ia antigens and their expression on lymphocyte subpopulations. Transplant Rev. 1976;30:64–82. [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Bigotti A., Pellegrino M. A., Ferrone S. Changes in Ia-like antigen expression on malignant human cells. Immunogenetics. 1981;12(3-4):409–413. doi: 10.1007/BF01561680. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Recio P., Bussey H. J. The pathology and prognosis of carcinoma of the rectum in the young. Proc R Soc Med. 1965 Oct;58(10):789–790. doi: 10.1177/003591576505801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Orjasaeter H., Elgjo K., Hognestad J. Immunohistochemical study of secretory component, secretory IgA and carcinoembryonic antigen in large bowel carcinomas. Pathol Res Pract. 1980 Dec;170(1-3):126–145. doi: 10.1016/s0344-0338(80)80161-0. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Thorud E., Elgjo K., Brandtzaeg P., Orjasaeter H., Nygaard K. Large-bowel carcinomas with different ploidy, related to secretory component, IgA, and CEA in epithelium and plasma. Br J Cancer. 1982 Jun;45(6):921–934. doi: 10.1038/bjc.1982.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T., Elgjo K., Brandtzaeg P., Orjasaeter H., Bergan A. Plasma carcinoembryonic antigen concentrations and immunohistochemical patterns of epithelial marker antigens in patients with large bowel carcinoma. J Clin Pathol. 1982 Sep;35(9):922–933. doi: 10.1136/jcp.35.9.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H., Solheim B. G., Brandtzaeg P., Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12(1):77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Stave R., Brandtzaeg P. Fluorescence staining of gastric mucosa. A study with special reference to parietal cells. Scand J Gastroenterol. 1977;12(7):885–891. doi: 10.3109/00365527709181735. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Nogueira N., Witmer M. D., Tydings J. D., Mellman I. S. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. J., Herlyn M. F., Elder D. E., Clark W. H., Steplewski Z., Koprowski H. Expression of DR antigens in freshly frozen human tumors. Hybridoma. 1982;1(2):161–168. doi: 10.1089/hyb.1.1982.1.161. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Jensen G., Nissen N. I. Limits of detection of nuclear DNA abnormalities by flow cytometric DNA analysis. Results obtained by a set of methods for sample-storage, staining and internal standardization. Cytometry. 1983 Mar;3(5):332–339. doi: 10.1002/cyto.990030505. [DOI] [PubMed] [Google Scholar]
- Wolley R. C., Schreiber K., Koss L. G., Karas M., Sherman A. DNA distribution in human colon carcinomas and its relationship to clinical behavior. J Natl Cancer Inst. 1982 Jul;69(1):15–22. [PubMed] [Google Scholar]