Abstract
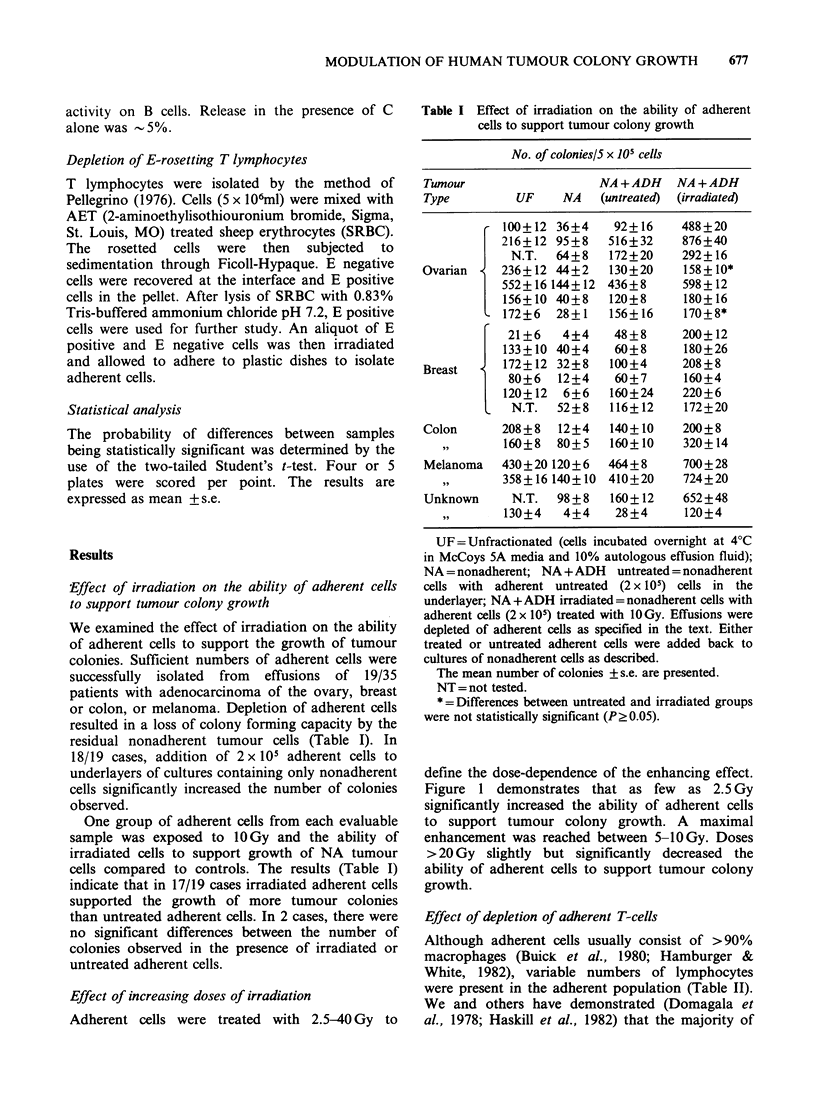
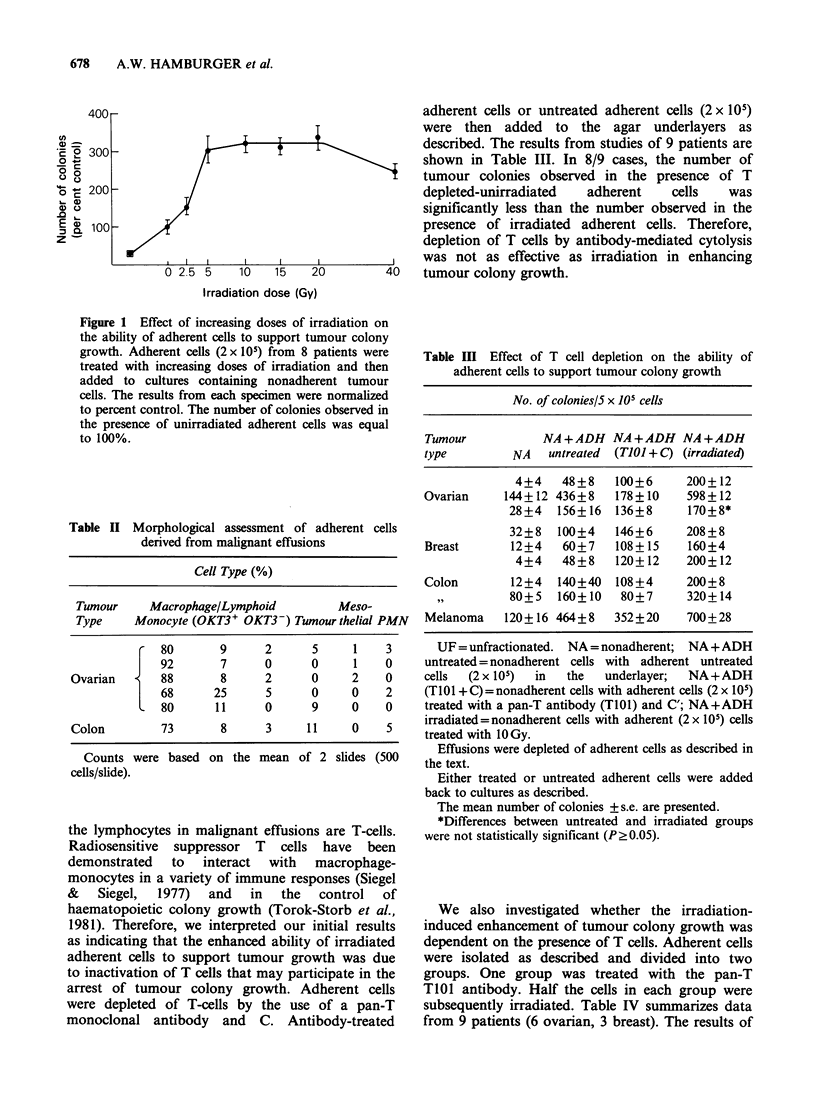
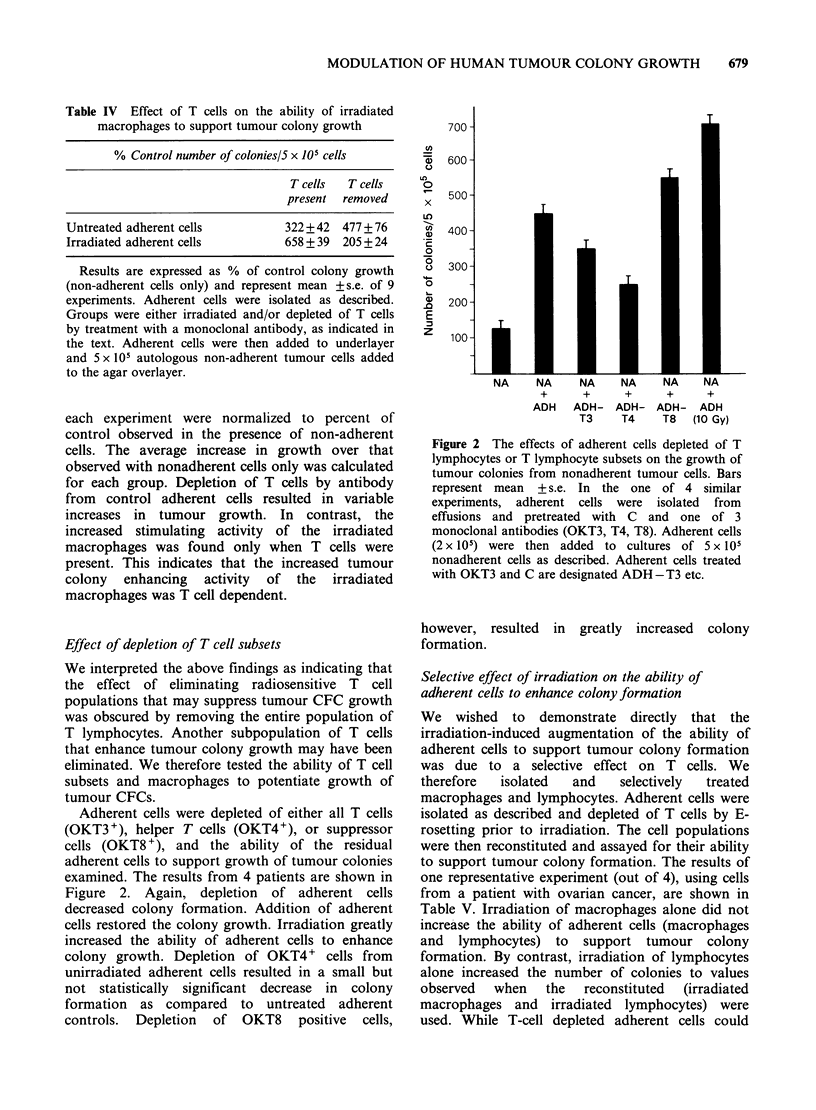
The ability of human tumour cells to form colonies in soft agar is enhanced by the presence of autologous phagocytic/adherent cells. We investigated the effect of irradiation on the ability of the adherent cells to support human tumour colony formation. Relatively low doses of irradiation significantly increased the growth enhancing ability of adherent cells in 17/19 cases. The possibility that the enhancement was mediated by inactivation of radiosensitive contaminating lymphocytes was explored. Depletion of T lymphocytes from unirradiated adherent cells by a monoclonal antibody and complement resulted in little overall change in tumour colony growth. However, elimination of only the suppressor subset (OKT8+) of T lymphocytes resulted in increased colony growth relative to control values obtained with unirradiated adherent cells. In contrast, depletion of T lymphocytes from irradiated adherent cells by a pan T monoclonal antibody and complement decreased colony formation. Thus, the ability of irradiated macrophages to enhance tumour colony growth appeared to be mediated by a T lymphocyte. The effect of irradiation on isolated populations of macrophages and T lymphocytes was also examined. The enhanced ability of irradiated adherent cells to support tumor colony growth appeared to have been due to treatment of T lymphocytes alone. The results indicate that both adherent macrophages and lymphocytes may influence the growth of clonogenic human tumour cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. C., Jr, Rigas V. D., Bennett R. M., Vandenbark A. A., Garewal H. S. Interaction of lactoferrin, monocytes, and T lymphocyte subsets in the regulation of steady-state granulopoiesis in vitro. J Clin Invest. 1981 Jul;68(1):56–63. doi: 10.1172/JCI110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar B. A., Miller F. R., Heppner G. H. In situ lymphoid cells of mouse mammary tumors. III. In vitro stimulation of tumor cell survival by lymphoid cells separated from mammary tumors. J Immunol. 1978 Jun;120(6):1887–1891. [PubMed] [Google Scholar]
- Buick R. N., Fry S. E., Salmon S. E. Effect of host-cell interactions on clonogenic carcinoma cells in human malignant effusions. Br J Cancer. 1980 May;41(5):695–704. doi: 10.1038/bjc.1980.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D. J., Churchill W. H. Cytotoxicity of human macrophages for tumor cells. Enhancement by human lymphocyte mediators. J Clin Invest. 1979 May;63(5):977–984. doi: 10.1172/JCI109398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagala W., Emeson E. E., Koss L. G. Distribution of T-lymphocytes and B-lymphocytes in peripheral blood and effusions of patients with cancer. J Natl Cancer Inst. 1978 Aug;61(2):295–300. [PubMed] [Google Scholar]
- Gabizon A., Leibovich S. J., Goldman R. Contrasting effects of activated and nonactivated macrophages and macrophages from tumor-bearing mice on tumor growth in vivo. J Natl Cancer Inst. 1980 Nov;65(5):913–920. [PubMed] [Google Scholar]
- Gupta S., Fernandes G., Nair M., Good R. A. Spontaneous and antibody-dependent cell-mediated cytotoxicity by human T cell subpopulations. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5137–5141. doi: 10.1073/pnas.75.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E., Kim M. B., Trent J. M., Soehnlen B. J., Alberts D. S., Schmidt H. J. Direct cloning of human ovarian carcinoma cells in agar. Cancer Res. 1978 Oct;38(10):3438–3444. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., White C. P. Interaction between macrophages and human tumor clonogenic cells. Stem Cells. 1982;1(4-5):209–223. [PubMed] [Google Scholar]
- Haskill J. S., Proctor J. W., Yamamura Y. Host responses with solid tumors. I. Monocytic effector cells within rat sarcomas. J Natl Cancer Inst. 1975 Feb;54(2):387–393. [PubMed] [Google Scholar]
- Haskill S., Becker S., Fowler W., Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br J Cancer. 1982 May;45(5):728–736. doi: 10.1038/bjc.1982.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman E. S., Decker J. M., Muchmore A. V. In vitro cellular regulation of monocyte function: evidence for a radiosensitive suppressor. J Reticuloendothel Soc. 1981 Nov;30(5):373–380. [PubMed] [Google Scholar]
- Mantovani A., Peri G., Polentarutti N., Bolis G., Mangioni C., Spreafico F. Effects on in vitro tumor growth of macrophages isolated from human ascitic ovarian tumors. Int J Cancer. 1979 Feb;23(2):157–164. doi: 10.1002/ijc.2910230204. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Polentarutti N., Peri G., Shavit Z. B., Vecchi A., Bolis G., Mangioni C. Cytotoxicity on tumor cells of peripheral blood monocytes and tumor-associated macrophages in patients with ascites ovarian tumors. J Natl Cancer Inst. 1980 Jun;64(6):1307–1315. doi: 10.1093/jnci/64.6.1307. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Theofilopoulos A. N. Isolation of human T and B lymphocytes by rosette formation with 2-aminoethylisothiquronium bromide (AET) -treated sheep red blood cells with monkey red blood cells. J Immunol Methods. 1976;11(3-4):273–279. doi: 10.1016/0022-1759(76)90120-4. [DOI] [PubMed] [Google Scholar]
- Prehn R. T. Immunostimulation of the lymphodependent phase of neoplastic growth. J Natl Cancer Inst. 1977 Oct;59(4):1043–1049. doi: 10.1093/jnci/59.4.1043. [DOI] [PubMed] [Google Scholar]
- Saiki O., Ralph P. Induction of human immunoglobulin secretion. II. T lymphocyte dependency and radiosensitivity of T-cell help for induction of B-cell differentiation by Staphylococcus aureus strain Cowan I. Cell Immunol. 1982 Jul 1;70(2):301–310. doi: 10.1016/0008-8749(82)90331-8. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Buick R. N. Preparation of permanent slides of intact soft-agar colony cultures of hematopoietic and tumor stem cells. Cancer Res. 1979 Mar;39(3):1133–1136. [PubMed] [Google Scholar]
- Schultz R. M., Chirigos M. A., Olkowski Z. L. Stimulation and inhibition of neoplastic cell growth by tumor promoter-treated macrophages. Cell Immunol. 1980 Aug 15;54(1):98–106. doi: 10.1016/0008-8749(80)90193-8. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Small M., Trainin N. Separation of populations of sensitized lymphoid cells into fractions inhibiting and fractions enhancing syngeneic tumor growth in vivo. J Immunol. 1976 Jul;117(1):292–297. [PubMed] [Google Scholar]
- Torok-Storb B., Hansen J. A. Modulation of in vitro BFU-E growth by normal Ia-positive T cells is restricted by HLA-DR. Nature. 1982 Jul 29;298(5873):473–474. doi: 10.1038/298473a0. [DOI] [PubMed] [Google Scholar]
- Torok-Storb B., Martin P. J., Hansen J. A. Regulation of in vitro erythropoiesis by normal T cells: evidence for two T-cell subsets with opposing function. Blood. 1981 Jul;58(1):171–174. [PubMed] [Google Scholar]
- Williams N., Jackson H., Ralph P., Nakoinz I. Cell interactions influencing murine marrow megakaryocytes: nature of the potentiator cell in bone marrow. Blood. 1981 Jan;57(1):157–163. [PubMed] [Google Scholar]
- Yron I., Wood T. A., Jr, Spiess P. J., Rosenberg S. A. In vitro growth of murine T cells. V. The isolation and growth of lymphoid cells infiltrating syngeneic solid tumors. J Immunol. 1980 Jul;125(1):238–245. [PubMed] [Google Scholar]


