Abstract
Rifampin is a front-line antibiotic for the treatment of tuberculosis. Infections caused by rifampin- and multidrug-resistant Mycobacterium tuberculosis strains are difficult to treat and contribute to a poor clinical outcome. Rifampin resistance most often results from mutations in rpoB. However, some drug-resistant strains have rpoB alleles that encode the phenotype for susceptibility. Similarly, non-M. tuberculosis mycobacteria exhibit higher levels of baseline resistance to rifampin, despite the presence of rpoB alleles that encode the phenotype for susceptibility. To identify other genes involved in rifampin resistance, we generated a library of Mycobacterium smegmatis mc2155 transposon insertion mutants. Upon screening this library, we identified one mutant that was hypersensitive to rifampin. The transposon insertion was localized to the arr gene, which encodes rifampin ADP ribosyltransferase, an enzyme able to inactivate rifampin. Sequence analysis revealed differences in the arr alleles of M. smegmatis strain mc2155 and previously described strain DSM 43756. The arr region of strain mc2155 contains a second, partial copy of the arr gene plus a novel insertion sequence, IS1623.
Mycobacterial infections, including tuberculosis (TB) and leprosy, are bacterial diseases of global importance. The World Health Organization estimates that the worldwide incidences of TB increased 0.4% in 2001, to 8.5 million new cases (28). Control of TB is complicated by its ease of transmission, difficulties in administering the long-course chemotherapy regimens, and the appearance of strains that are multidrug resistant (MDR), which is defined as resistance to the two front-line anti-TB drugs, isoniazid and rifampin. Rifampin is a broad-spectrum antibiotic that inhibits bacterial DNA-dependent RNA polymerase activity. Resistance to rifampin is most often caused by mutations in rpoB, which encodes the β subunit of RNA polymerase (20, 25). In rifampin-resistant clinical isolates of Mycobacterium tuberculosis, an estimated 96% of the rpoB mutations map to an 81-bp region (codons 507 to 533) near the middle of the gene (20). Mutations at codons 531, 526, and 516 are the most common (13). A variety of assays that detect these sequence polymorphisms have been developed (3, 23) and allow the rapid determination of the drug susceptibilities of clinical M. tuberculosis isolates. In addition, an estimated 90% of rifampin-resistant clinical isolates are also isoniazid resistant, such that rifampin resistance is a positive indicator of MDR TB. However, about 4% of rifampin-resistant clinical isolates of M. tuberculosis have no mutations in the 81-bp core region or elsewhere in the rpoB gene (3, 8, 14, 20). In addition, some mycobacteria, particularly atypical mycobacteria, such as Mycobacterium smegmatis, Mycobacterium avium, and Mycobacterium intracellulare, are resistant to rifampin, even though they possess sensitive RNA polymerase (9, 14, 20). These findings indicate that other genes can contribute to rifampin resistance. To identify such genes, we generated a library of M. smegmatis mc2155 transposon insertion mutants and screened it for clones exhibiting enhanced rifampin sensitivity. In this report, we describe clone RHS 234, which exhibits a 16-fold increase in rifampin sensitivity.
MATERIALS AND METHODS
Bacterial strains, phage, and plasmids.
M. smegmatis strain mc2155 (22) was the target for transposon mutagenesis. The φMycoMar mariner transposon phage was provided by E. J. Rubin (Harvard University) (21). Escherichia coli strain DH5α pir116 was kindly supplied by G. J. Phillips (Iowa State University) (16). This strain supports replication of R6Kγ ori and was used during isolation of plasmids containing the φMycoMar transposon. Mycobacterium marinum strain 1218R was provided by L. Barker (National Institute of Allergy and Infectious Diseases). E. coli strain DH5α was used for routine manipulation of plasmid DNA.
E. coli strains were grown on Luria-Bertani broth or agar (Difco). Mycobacteria were routinely grown in Middlebrook 7H9 broth or Middlebrook 7H11 agar (Difco) supplemented with 10% oleic acid, bovine serum albumin (fraction V), dextrose, and catalase (OADC; Difco). In preparation for genomic DNA isolation, mycobacteria were grown in Middlebrook 7H9 broth supplemented with 0.1% Tween 80 and 10% albumin, dextrose, and catalase (Difco). The following antibiotics (Sigma) were added at the indicated concentrations: ampicillin, 50 μg/ml; kanamycin, 25 μg/ml for mycobacteria and 50 μg/ml for E. coli; and hygromycin, 75 μg/ml for mycobacteria and 150 μg/ml for E. coli.
Generation and screening of M. smegmatis φMycoMar insertion library.
Propagation of the φMycoMar transposon phage and preparation of phage lysates have been described previously (21). For phage infection, M. smegmatis strain mc2155 cells were grown to late-log phase in Middlebrook 7H9 broth without antibiotics. Cells were pelleted, washed twice with mycobacteriophage buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10 mM MgSO4, 2 mM CaCl2), and then resuspended in the same buffer. Phage was added at a multiplicity of infection of 10:1, and the cells and phage were incubated at 37°C for 3 h to allow infection to occur. The bacteria were then plated on Middlebrook 7H11 agar supplemented with kanamycin and incubated at 30°C. Kanamycin-resistant (i.e., transposon-containing) M. smegmatis colonies were patched onto Middlebrook 7H11 agar to obtain a library of 7,680 clones (i.e., 80 plates × 96 colonies per plate). To screen this library, clones were replica plated onto Middlebrook 7H11 agar supplemented with 12 μg of rifampin per ml, which is about one-third the MIC for wild-type M. smegmatis mc2155. Clones that failed to grow were deemed rifampin hypersensitive. A broth dilution method was used to determine the MIC and to confirm the rifampin hypersensitivities of these clones.
Localization of the φMycoMar insertion.
Chromosomal DNA of the rifampin-hypersensitive clone was isolated by standard methods (2). Total chromosomal DNA was cleaved with BamHI (MBI Fermentas), a restriction endonuclease that does not cut within the φMycoMar element. As such, digestion generates a restriction fragment containing the kanamycin resistance cassette and R6Kγ ori of the MycoMar element plus flanking chromosomal DNA. Self-ligation of this restriction fragment generates a plasmid that can replicate in E. coli strains producing the π protein. Digested DNA was self-ligated with T4 DNA ligase (Gibco) and transformed into competent E. coli DH5α pir116. Plasmid DNA was isolated from Kmr E. coli transformants. Oligonucleotide primers MAR1 5′-CCCGAAAAGTGCCACCGTGAAAAGCCC-3′ and MAR2 5′-CGCTTCCTCGTGCTTTACGGTATCG-3′ were used to determine the DNA sequence of the φMycoMar-chromosomal junction. These DNA sequences were compared to the sequences in the GenBank database and the M. smegmatis mc2155 genome database at the Institute for Genomic Research (http://www.tigr.com/) by using the BLASTN algorithm. Nucleotide sequences were also analyzed with Vector NTI Suite software (Informax).
Cloning of arr gene.
The arr gene of strain mc2155 was amplified by PCR from its genomic DNA by using forward primer 5′-TTGCACGAGTCCGGTCAT-3′ and reverse primer 5′-TCCACTCATCCTGGTTCTGG-3′. Vector pDrive (Qiagen) was used to clone the PCR products. To construct pARR, a PvuII fragment containing arr was cloned into E. coli-Mycobacterium shuttle vector pNBV1 (10), which contains a hygromycin resistance cassette. Plasmids pARR and pNBV1 were transformed into mycobacterial cells by electroporation by a standard protocol. Transformants were selected on Middlebrook 7H11 agar containing hygromycin. The rifampin sensitivities of the transformants were determined by a broth dilution method in Middlebrook 7H9 supplemented with OADC. The cultures were grown in the dark because it has been reported that Arr activity is inhibited by light (4).
RESULTS
Isolation and characterization of rifampin-hypersensitive mutant RHS 234.
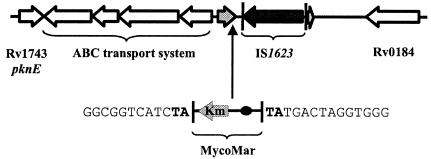
One rifampin-hypersensitive clone, RHS 234, was isolated from the transposon insertion library of M. smegmatis mc2155. This clone could not grow on a Middlebrook 7H11 agar plate containing 12 μg of rifampin per ml. The hypersensitivity of RHS 234 to rifampin was confirmed by a broth dilution method; the MIC for the strain was 2 μg/ml, which is 16-fold lower than that for the wild-type strain (Table 1). RHS 234 did not show altered sensitivity to other antibiotics, including isoniazid, ethambutol, erythromycin, tetracycline, chloramphenicol, and β-lactams (data not shown). The transposon insertion in RHS 234 was localized as described in Materials and Methods. Upon comparison, our DNA sequencing data matched the M. smegmatis mc2155 genome sequence data available at TIGR (http://www.tigr.org). The transposon insertion was localized to a TA dinucleotide near the end of an open reading frame that exhibits homology to several arr genes in GenBank, including arr from M. smegmatis DSM 43756 (GenBank accession number AF001493) (5, 19) (Fig. 1 and 2). The arr gene encodes rifampin ADP ribosylase, an enzyme that inactivates rifampin (12, 18). As such, disruption of arr in RHS 234 is consistent with the increased rifampin sensitivity of the mutant.
TABLE 1.
Rifampin MICs for mycobacterial strains
| Strain | MIC (μg/ml) |
|---|---|
| M. smegmatis mc2 155 | 32 |
| mc2155/pARR | 48 |
| mc2155/pNBV1 | 32 |
| M. smegmatis RHS 234 | 2 |
| RHS 234/pARR | 48 |
| RHS 234/pNBV1 | 2 |
| M. marinum 1218R | 4 |
| 1218R/pARR | 128 |
| 1218R/pNBV1 | 4 |
FIG. 1.
arr region of M. smegmatis mc2155. The arr gene and its partial duplication, IS1623, and flanking genes are depicted. The vertical arrow indicates the location of insertion of the φMycoMar element to generate rifampin-hypersensitive mutant RHS 234. The nucleotide sequence flanking the φMycoMar insertion is shown, with the duplicated AT dinucleotide indicated in boldface.
FIG. 2.
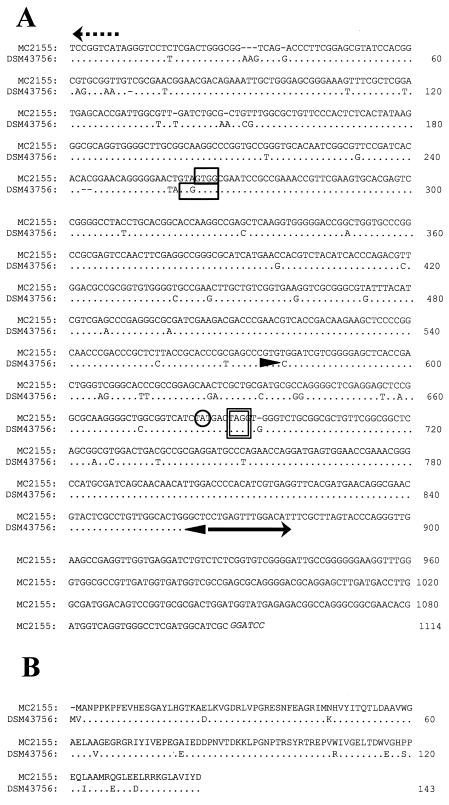
Sequence alignment of arr alleles from M. smegmatis mc2155 and DSM 43756. (A) Nucleotide sequences of arr regions. Translation start (GTG) and stop (TAG) codons for arr alleles are indicated by boxes. The TA dinucleotide site of MycoMar insertion is circled. The 15-bp inverted repeat unit of IS1623 is marked with a solid arrow. Arrowheads mark the boundaries of the direct repeat region flanking IS1623. The translational start site for the ABC-type permease adjacent to arr is indicated with a broken arrow. (B) Amino acid sequences of the arr gene products.
Complementation of rifampin hypersensitivity in M. smegmatis RHS 234.
To confirm that the hypersensitive phenotype of strain RHS 234 was due to disruption of the arr gene and not a polar effect caused by the transposon insertion, we cloned the arr gene from wild-type strain mc2155 into a shuttle vector, pNBV1. The resulting plasmid, pARR, was transformed into RHS 234. Measurements of rifampin sensitivity showed that transformation with pARR, but not pNBV1, increased the MIC for strain RHS 234 from 2 to 48 μg/ml, which is slightly higher than that for the wild-type strain (32 μg/ml) (Table 1). Plasmids pARR and pNBV1 were also transformed into mc2155. Consistently, pARR increased the rifampin resistance of mc2155, with the MIC increasing from 32 to 48 μg/ml, whereas pNBV1 had no effect (Table 1).
Expression of arr increases rifampin resistance of M. marinum.
Homologs of arr were not found in the sequences of M. tuberculosis, M. leprae, M. avium, or M. marinum in genome databases. However, we were interested to know if arr could function in these pathogenic mycobacteria. M. marinum strain 1218R was transformed with pARR or pNBV1, and drug resistance was determined. Transformation with pARR, but not pNBV1, increased the rifampin MIC for M. marinum 32-fold, from 4 to 128 μg/ml. Expression of arr in E. coli DH5α also increased its resistance to rifampin >20-fold (data not shown).
M. smegmatis mc2155 contains a novel insertion sequence, IS1623.
The arr sequences of M. smegmatis strains mc2155 and DSM 43756 are highly homologous, but differences in both the promoter and the coding regions do exist (Fig. 2). Downstream of arr there is a significant sequence discrepancy (Fig. 1). Unlike DSM 43756, strain mc2155 contains a novel insertion sequence, IS1623. IS1623 comprises a 1,635-bp transposase gene flanked by 15-bp inverted repeat and 290-bp direct repeat units. The direct repeat unit comprises genomic M. smegmatis DNA, including the carboxy-terminal portion of the arr gene product. This duplication likely results from insertion of IS1623 (Fig. 1). Searches for homology with sequences available in mycobacterial genome databases uncovered no copies of IS1623 in M. tuberculosis, M. bovis, M. avium, M. paratuberculosis, M. marinum, or M. leprae. Similarly, a search of M. smegmatis mc2155 revealed no additional copies of IS1623, but a weak match to another insertion sequence, IS1549 (17), was obtained.
DISCUSSION
Rifampin is a key drug in the fight against TB. However, rifampin-resistant and MDR M. tuberculosis strains are a growing threat to global health. Mutations in rpoB have been well documented and constitute a significant source of rifampin resistance in clinical M. tuberculosis isolates (20). Drug resistance can also be affected by rifampin modification. Bacteria, including some actinomycetes, are able to inactivate rifampin via glucosylation, phosphorylation, decolorization, and ribosylation (5, 24). Atypical mycobacteria, especially rapidly growing species such as M. smegmatis, are naturally more resistant to rifampin (20). In this report, we describe a mechanism of drug resistance in M. smegmatis, rifampin ribosylation. Although this mechanism has not been detected in slowly growing mycobacteria such as M. tuberculosis and M. avium, the data presented here suggest that a potential (future) mechanism for rifampin resistance development for the slowly growing mycobacterial pathogens may be transposon-mediated gene transfer.
To identify mycobacterial genes that contribute to rifampin resistance, a library of insertion mutants was generated with M. smegmatis mc2155 and the φMycoMar transposon. Screening of this library uncovered clone RHS 234, which is 16-fold more sensitive to rifampin than the wild type. Insertion of the φMycoMar element was localized to the arr gene, which encodes rifampin ADP ribosylase.
The M. smegmatis mc2155 arr gene product exhibits more than 50% identity to ADP ribosyltransferases from Streptomyces coelicolor, Pseudomonas aeruginosa (26), Klebsiella pneumoniae (1), E. coli, and other enterobacteria (6, 7, 15). In the enterobacteria, rifampin ribosylation, mediated by arr-2 gene cassettes, is a clinically important mechanism of drug resistance. The arr-2 gene cassettes are part of plasmid-borne integrons and are associated with other resistance determinants such as the VEB-1 extended-spectrum β-lactamase (26) and ACC-1 cephalosporinase (1). The origin of the M. smegmatis arr gene is unknown. However, integrons are key contributors to horizontal gene transfer; and mycobacteria, like pseudomonads and streptomycetes, are commonly found in soil.
Rifampin ribosylation has been described as a mechanism of mycobacterial drug resistance in several fast-growing species, including M. smegmatis, Mycobacterium chelonae, and Mycobacterium parafortuitum (5, 12, 19). Homologs of arr are not present in M. tuberculosis, M. leprae, M. avium, or other slowly growing species for which genome data are available. Yet pARR, which contains an intact copy of the arr gene, is functional in M. marinum, increasing the rifampin MIC from 4 to 128 μg/ml.
The arr alleles of M. smegmatis mc2155 and DSM 43756 are not identical. However, within the coding region, most of the differences are conservative substitutions (Fig. 2). The region downstream of arr is not conserved. A novel insertion sequence is present in mc2155. IS1623 includes a 1,635-bp transposase gene that is flanked by 15-bp inverted repeat units. The IS1623 transposase exhibits homology to the transposases of M. smegmatis IS1549 (17), Mycoplasma mycoides and Mycoplasma hyopneumoniae IS1634 (27), and Thermoanaerobacter tengcongensis strain MB4T (Fig. 3). As noted by Plikaytis et al. (17), the C1 motif of these proteins resembles that of the IS4 class of transposases, but the potential N2 and N3 motifs are unique. Together, these transposons represent an emerging class of mobile genetic elements that generate large direct repeats upon transposition. Most insertion sequences are flanked by short direct repeat units of 4 to 9 bp (11). However, the direct repeat flanking IS1623 is 290 bp. This repeat region includes 118 bp of the arr gene. Although only a portion of arr is replicated here, a longer direct repeat would provide a mechanism for gene duplication.
FIG. 3.
Sequence alignment of transposases IS1623 and IS1549 from M. smegmatis mc2155 with IS1634 from M. hyopneumoniae (MH1634), M. mycoides (MM1634), and T. tengcongensis strain MB4T (TTMB4T). Consensus sequences for the putative N2, N3, and C1 regions are indicated.
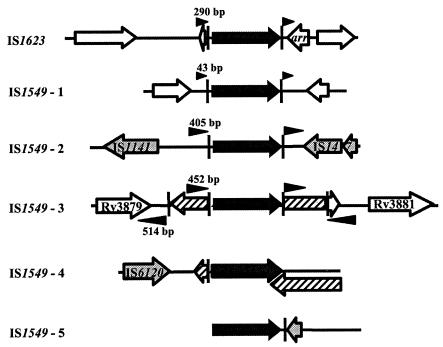
A BLASTN search of the M. smegmatis mc2155 genome sequence available at The Institute for Genomic Research (http://www.tigr.org/) revealed IS1549 elements at five different loci (Fig. 4). This is consistent with Southern blotting data suggesting the presence of five copies of IS1549 in M. smegmatis 607, the parent of strain mc2155 (17). Multiple copies of IS1549 are present at two of these loci. At four of the IS1549 loci, additional insertion sequence elements are also present. Although the M. smegmatis genome sequence data are incomplete, it was possible to identify the direct repeat units flanking four of the IS1549 elements. They range in size from 43 to 514 bp. Invariant trinucleotide spacers, CCT at the upstream flank and GGG at the downstream flank, are present between the direct repeat regions and the 11-bp inverted repeats of IS1549. This suggests that the inverted repeat units flanking IS1549 are composed of the imperfect 14-bp sequence 5′-CC(T/C)GACTTGGCTCA-3′. The inverted repeat flanking IS1623 is a similar 15-bp sequence, 5′-CCTGAGTTTGGACAT-3′. The partial duplication of arr associated with IS1623 and the proximity of IS1549 to other transposons suggests that these elements may be able to mediate horizontal gene transfer (Fig. 4). In other strains or in time, transposition of IS1623 (or another insertion sequence) could lead to duplication of the full arr gene and/or its incorporation into a mobile genetic element. Indeed, the entire arr gene would fit within the 514-bp direct repeat associated with one IS1549 insertion.
FIG. 4.
Comparison of IS1623 and IS1549 insertions in the M. smegmatis mc2155 genome. Genes are depicted with arrows. Black arrows represent IS1623 and IS1549 transposases. Cross-hatched arrows represent IS1549 transposases that have been interrupted by a second copy of IS1549. Shaded arrows represent transposase genes from other insertion sequence elements. The inverted (vertical bars) and direct (arrowheads) repeat regions flanking the IS1623 and IS1549 insertions are indicated. The nucleotide lengths of the direct repeats are also shown.
Antibiotic resistance can result from modification of the drug target or modification of the drug itself. Both mechanisms cause rifampin resistance in mycobacteria: mutations in rpoB alter the drug target, and expression of arr leads to rifampin inactivation. Although arr has not been found in slowly growing mycobacteria, it can function in pathogenic species, including M. marinum. The spread of arr to M. tuberculosis would be devastating to global control efforts. Treatment of infections caused by drug-resistant strains is both difficult and expensive. Swift diagnosis and appropriate chemotherapy are essential for a positive clinical outcome but require resources that are often not available. However, the present study indicates that mycobacterial genomes do change. The arr loci of M. smegmatis strains DSM 43756 and mc2155 have diverged. The promoter and coding regions of the arr alleles exhibit a variety of nucleotide changes. More significantly, the insertion of IS1623 has resulted in the partial duplication of arr. The large direct repeats associated with IS1623 and IS1549 have the potential to duplicate entire genes. Multiple insertions of these elements and their association with other transposons provide a mechanism for mobilization of antibiotic resistance determinants, such as arr.
Acknowledgments
We thank E. J. Rubin, G. J. Phillips, and L. Barker for providing the transposon and bacterial strains.
This work was supported by Canadian Institutes of Health Research (CIHR) grant MOP-15107 and a grant from National Sanitarium Association of Canada (to J.L.). J.L. is a CIHR New Investigator.
REFERENCES
- 1.Arlet, G., D. Nadjar, J. L. Herrmann, J. L. Donay, P. H. Lagrange, and A. Philippon. 2001. Plasmid-mediated rifampin resistance encoded by an arr-2-like gene cassette in Klebsiella pneumoniae producing an ACC-1 class C β-lactamase. Antimicrob. Agents Chemother. 45:2971-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belisle, J. T., and M. G. Sonnenberg. 1998. Isolation of genomic DNA from mycobacteria. Methods Mol. Biol. 101:31-44. [DOI] [PubMed] [Google Scholar]
- 3.Caws, M., and F. A. Drobniewski. 2001. Molecular techniques in the diagnosis of Mycobacterium tuberculosis and the detection of drug resistance. Ann. N. Y. Acad. Sci. 953:138-145. [DOI] [PubMed] [Google Scholar]
- 4.Dabbs, E. R., and S. Quan. 2000. Light inhibits rifampicin inactivation and reduces rifampicin resistance due to a cloned mycobacterial ADP-ribosylation gene. FEMS Microbiol. Lett. 182:105-109. [DOI] [PubMed] [Google Scholar]
- 5.Dabbs, E. R., K. Yazawa, Y. Mikami, M. Miyaji, N. Morisaki, S. Iwasaki, and K. Furihata. 1995. Ribosylation by mycobacterial strains as a new mechanism of rifampin inactivation. Antimicrob. Agents Chemother. 39:1007-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girlich, D., T. Naas, A. Leelaporn, L. Poirel, M. Fennewald, and P. Nordmann. 2002. Nosocomial spread of the integron-located veb-1-like cassette encoding an extended-spectrum beta-lactamase in Pseudomonas aeruginosa in Thailand. Clin. Infect. Dis. 34:603-611. [DOI] [PubMed] [Google Scholar]
- 7.Girlich, D., L. Poirel, A. Leelaporn, A. Karim, C. Tribuddharat, M. Fennewald, and P. Nordmann. 2001. Molecular epidemiology of the integron-located VEB-1 extended-spectrum β-lactamase in nosocomial enterobacterial isolates in Bangkok, Thailand. J. Clin. Microbiol. 39:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrero, C., L. Stockman, F. Marchesi, T. Bodmer, G. D. Roberts, and A. Telenti. 1994. Evaluation of the rpoB gene in rifampicin-susceptible and -resistant Mycobacterium avium and Mycobacterium intracellulare. J. Antimicrob. Chemother. 33:661-663. [DOI] [PubMed] [Google Scholar]
- 9.Hetherington, S. V., A. S. Watson, and C. C. Patrick. 1995. Sequence and analysis of the rpoB gene of Mycobacterium smegmatis. Antimicrob. Agents Chemother. 39:2164-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard, N. S., J. E. Gomez, C. Ko, and W. R. Bishai. 1995. Color selection with a hygromycin-resistance-based Escherichia coli-mycobacterial shuttle vector. Gene 166:181-182. [DOI] [PubMed] [Google Scholar]
- 11.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morisaki, N., H. Kobayashi, S. Iwasaki, K. Furihata, E. R. Dabbs, K. Yazawa, and Y. Mikami. 1995. Structure determination of ribosylated rifampicin and its derivative: new inactivated metabolites of rifampicin by mycobacterial strains. J. Antibiot. 48:1299-1303. [DOI] [PubMed] [Google Scholar]
- 13.Morlock, G. P., B. B. Plikaytis, and J. T. Crawford. 2000. Characterization of spontaneous, in vitro-selected, rifampin-resistant mutants of Mycobacterium tuberculosis strain H37Rv. Antimicrob. Agents Chemother. 44:3298-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musser, J. M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8:496-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naas, T., Y. Mikami, T. Imai, L. Poirel, and P. Nordmann. 2001. Characterization of In53, a class 1 plasmid- and composite transposon-located integron of Escherichia coli which carries an unusual array of gene cassettes. J. Bacteriol. 183:235-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platt, R., C. Drescher, S. K. Park, and G. J. Phillips. 2000. Genetic system for reversible integration of DNA constructs and lacZ gene fusions into the Escherichia coli chromosome. Plasmid 43:12-23. [DOI] [PubMed] [Google Scholar]
- 17.Plikaytis, B. B., J. T. Crawford, and T. M. Shinnick. 1998. IS1549 from Mycobacterium smegmatis forms long direct repeats upon insertion. J. Bacteriol. 180:1037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan, S., T. Imai, Y. Mikami, K. Yazawa, E. R. Dabbs, N. Morisaki, S. Iwasaki, Y. Hashimoto, and K. Furihata. 1999. ADP-ribosylation as an intermediate step in inactivation of rifampin by a mycobacterial gene. Antimicrob. Agents Chemother. 43:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan, S., H. Venter, and E. R. Dabbs. 1997. Ribosylative inactivation of rifampin by Mycobacterium smegmatis is a principal contributor to its low susceptibility to this antibiotic. Antimicrob. Agents Chemother. 41:2456-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramaswamy, S., and J. M. Musser. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuberc. Lung Dis. 79:3-29. [DOI] [PubMed] [Google Scholar]
- 21.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2001. Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl. Acad. Sci. USA 98:12712-12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 23.Soini, H., and J. M. Musser. 2001. Molecular diagnosis of mycobacteria. Clin. Chem. 47:809-814. [PubMed] [Google Scholar]
- 24.Tanaka, Y., K. Yazawa, E. R. Dabbs, K. Nishikawa, H. Komaki, Y. Mikami, M. Miyaji, N. Morisaki, and S. Iwasaki. 1996. Different rifampicin inactivation mechanisms in Nocardia and related taxa. Microbiol. Immunol. 40:1-4. [DOI] [PubMed] [Google Scholar]
- 25.Telenti, A., P. Imboden, F. Marchesi, D. Lowrie, S. Cole, M. J. Colston, L. Matter, K. Schopfer, and T. Bodmer. 1993. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 341:647-650. [DOI] [PubMed] [Google Scholar]
- 26.Tribuddharat, C., and M. Fennewald. 1999. Integron-mediated rifampin resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:960-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vilei, E. M., J. Nicolet, and J. Frey. 1999. IS1634, a novel insertion element creating long, variable-length direct repeats which is specific for Mycoplasma mycoides subsp. mycoides small-colony type. J. Bacteriol. 181:1319-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. 2003. Global tuberculosis control: surveillance, planning, financing. WHO Report. World Health Organization, Geneva, Switzerland.