Abstract
Azoles are widely used antifungals; however, their efficacy is compromised by fungistatic activity and selection of resistant strains during treatment. Recent studies demonstrated roles for the protein kinase C and calcium signaling pathways in modulating azole activity. Here we explored a role for the signaling pathway mediated by cyclic AMP (cAMP), which is synthesized by the regulated action of adenylate cyclase (encoded by CDC35 in Candida albicans and CYR1 in Saccharomyces cerevisiae) and cyclase-associated protein (encoded by CAP1 and SRV2, respectively). Relative to wild-type strains, C. albicans and S. cerevisiae strains mutated in these genes were hypersusceptible to fluconazole (>4- to >16-fold-decreased 48-h MIC), itraconazole (>8- to >64-fold), or miconazole (16- to >64-fold). Similarly, they were hypersusceptible to terbinafine and fenpropimorph (2- to >16-fold), which, like azoles, inhibit sterol biosynthesis. Addition of cAMP to the medium at least partially reversed the hypersusceptibility of Ca-cdc35 and Sc-cyr1-2 mutants. An inhibitor of mammalian adenylate cyclase, MDL-12330A, was tested in combination with azoles; a synergistic effect was observed against azole-susceptible and -resistant strains of C. albicans and five of six non-C. albicans Candida species. Analysis of cAMP levels after glucose induction in the presence and absence of MDL-12330A confirmed that it acts by inhibiting cAMP synthesis in yeast. RNA analysis suggested that a defect in azole-dependent upregulation of the multidrug transporter gene CDR1 contributes to the hypersusceptibility of the Ca-cdc35 mutant. Our results implicate cAMP signaling in the yeast azole response; compounds similar to MDL-12330A may be useful adjuvants in azole therapy.
Serious fungal infections have increased in recent years as a consequence of the growing number of people who are immunocompromised in association with AIDS, aggressive therapies for cancer and autoimmune disease, or organ and tissue transplantation. The primary pathogen is Candida albicans, normally a commensal of the oral cavity and gastrointestinal tract of humans. This opportunistic yeast causes mucosal infection or less commonly invasive disease that is life threatening unless treated. There are presently three major classes of antifungal agents in clinical use: azoles, allylamines, and polyenes (15). Azoles such as fluconazole, itraconazole, and miconazole inhibit biosynthesis of the membrane component ergosterol by blocking the action of lanosterol demethylase (encoded by ERG11). Allylamines such as terbinafine and morpholines such as fenpropimorph are also sterol biosynthesis inhibitors (SBIs), while polyenes such as amphotericin B directly interact with the cell membrane, causing electrolyte leakage and subsequent cell death. Echinocandins such as caspofungin represent a new class of antifungals that target cell wall biosynthesis.
Of all antifungals, azoles are the most commonly used, but their efficacy is limited by lack of fungicidal activity as well as development of clinical resistance. Several mechanisms have been described for the azole resistance of C. albicans, including increased expression of ERG11 and of genes encoding multidrug transporters (encoded by CDR1, CDR2, and MDR1) (14, 29, 33, 36). However, little is known about the regulatory pathways responsible for this increased expression. Relatedly, the signaling pathways that mediate phenotypic adaptation to antifungal treatment are just beginning to be understood. Recent studies have implicated the calcium-calmodulin-calcineurin (7, 11, 24, 26) and protein kinase C (PKC)-cell integrity (T. D. Edlind, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1844, 2001) signaling pathways in modulating azole activity.
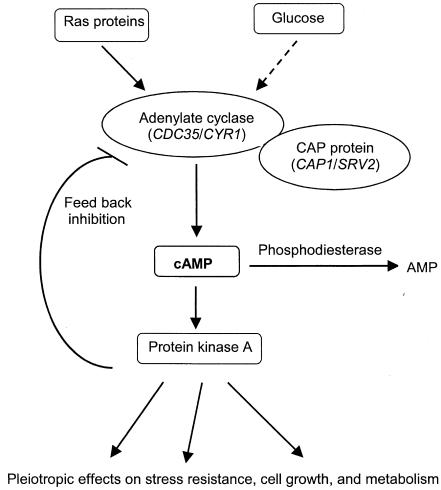
In this study we have examined the relationship of azole susceptibility to the evolutionary conserved cyclic AMP (cAMP)-protein kinase A (PKA) signaling pathway (Fig. 1). The central messenger molecule of this pathway, cAMP, is produced from ATP by the regulated action of adenylate cyclase (encoded by CDC35 in C. albicans and CYR1 in Saccharomyces cerevisiae) and the cyclase-associated protein (CAP) (encoded by CAP1 and SRV2, respectively) (10, 13, 19, 35). Adenylate cyclase is under dual regulation by membrane-bound Ras protein and by glucose via a G-protein-coupled receptor system (5, 22). The cAMP binds to the regulatory subunit of PKA, liberating the catalytic subunit, which then phosphorylates target proteins involved in stress resistance, cell growth, and intermediary metabolism (28, 35). Basal cAMP levels in cells are maintained by phosphodiesterases, which hydrolyze cAMP to AMP. The cAMP-PKA pathway has been implicated in virulence, morphogenesis, and development in various fungi (1, 10). More relevant to this work, it has been implicated in dicarboximide and aromatic hydrocarbon fungicide resistance in the phytopathogen Ustilago maydis (27, 30) and polymixin B and fluconazole resistance in S. cerevisiae (4, 21).
FIG. 1.
Simplified model of the yeast cAMP signaling pathway (35).
MATERIALS AND METHODS
Strains, media, and drugs.
The C. albicans and S. cerevisiae strains used in this study are listed in Table 1. Additional Candida strains were obtained from the American Type Culture Collection, T. White (Seattle, Wash.), and J. Rex (Houston, Tex.). Strains were cultured in yeast extract-peptone-dextrose (YPD) (1% yeast extract, 2% peptone, and 2% dextrose) liquid or agar medium. For cAMP assays, YPG medium, containing 3% glycerol in place of dextrose, was used. The following compounds were obtained from the indicated suppliers: fluconazole (Pfizer, New York, N.Y.), itraconazole (Janssen, Titusville, N.J.), terbinafine (Novartis, East Hanover, N.J.), fenpropimorph (Crescent Chemical, Hauppauge, N.Y.), caspofungin (Merck, Rahway, N.J.), miconazole, amphotercin B, MDL-12330A, and cAMP (Sigma). Fluconazole and caspofungin were dissolved in normal saline (0.85%), and the remaining drugs were dissolved in dimethyl sulfoxide.
TABLE 1.
Strains used in this study
| Strain | Designation | Genotype | Source |
|---|---|---|---|
| C. albicans | |||
| SC5314 | Ca-SC5314 | URA3/URA3 | D. Harcus |
| CAF2-1 | Ca-CAF2-1 | SC5314 URA3/ura3::imm434 | D. Sanglard |
| CAI4 | Ca-CAI4 | SC5314 ura3::imm434/ura3::imm434 | D. Harcus |
| CR216 | Ca-cdc35 | CAI4 cdc35::hisG-URA3-hisG/cdc35::hisG | D. Harcus |
| CAC1-1A | Ca-cap1 | CAI4 cap1::hisG-URA3-hisG/cap1::hisG | P. Sundstrom |
| S. cerevisiae | |||
| BY4743 | Sc-BY | MATa/αhis3Δ/his3Δ leu2Δ/leu2ΔMET15/met15ΔLYS2/lys2Δ ura3Δ/ura3Δ | ResGen |
| 37316 | Sc-srv2 | BY4743 srv2::KanMX/srv2::KanMX | ResGen |
| W303-1A | Sc-W | MATa leu2 ura3 trp1 his3 ade2 can1 | A. Hudson |
| YJN246 | Sc-cyr1-2 | MATαleu2 ura3 trp1 his3 can1 cyr1-2 | J. Nickels |
Broth microdilution assays.
A single colony from fresh culture was suspended in 1 ml of YPD and was incubated 2 to 3 h with aeration. Cells were then counted and diluted in medium to 5 × 103 cells/ml. Aliquots of 100 μl were distributed to a 96-well, flat-bottomed plate, except the first well of each row, which received 200 μl. Drug (0.5 to 1 μl) was added to the first well from the appropriate stock to obtain the desired concentration and was then serially twofold diluted to the remaining wells except the final row, which served as drug-free control. Plate contents were incubated at 35°C for C. albicans and at 30°C for S. cerevisiae. Absorbance at 630 nm was read with a microplate reader (Bio-Tek Instruments, Winooski, Vt.). MICs (i.e., concentrations inhibiting growth by ≥80% relative to control) were determined at the indicated times. To observe the effect of cAMP and MDL-12330A on antifungal susceptibility, these were combined (at the desired concentration) with the inoculum before dispensing into the wells.
cAMP extraction and measurement.
cAMP was extracted from YPG-grown cells under highly glucose-inducible conditions in the presence or absence of MDL-12330A following published procedures (1, 12) with certain modifications. In brief, C. albicans strain SC5314 was grown overnight in YPG medium, centrifuged, and resuspended in fresh YPG medium to a density of 107 cells/ml. A 5-ml aliquot was removed and was collected by filtration through nitrocellulose (25-mm diameter; 0.45-μm pore size). The remaining culture was divided into two equal parts, and 25 μg of MDL-12330A/ml was added to one part, while the other part served as untreated control. Both cultures were incubated for 30 min. At 0 min, glucose was added to 100 mM and 5-ml aliquots were rapidly processed as per above at the indicated times. Filters from all time points were inverted into 35-mm-diameter plastic petri dishes containing 1 ml of ice-cold 1 M formic acid saturated with n-butanol. After 5 min the liquid, containing eluted cAMP, was transferred to a microcentrifuge tube. Filters were treated again with 0.5 ml of formic acid-butanol, and the eluates were pooled together; cellular debris was removed by centrifugation for 1 min. Solvent was evaporated under vacuum, and the dried pellets were stored at −70°C. Prior to assaying, pellets were dissolved in 50 mM sodium acetate (pH 4.8), and cAMP levels in each sample were estimated by quantitative enzyme immunoassay by utilizing the Biotrak cAMP enzyme immunoassay kit (Amersham, Piscataway, N.J.).
RNA analysis.
Log-phase cultures (106 cells/ml) were exposed to itraconazole (0.25 μg/ml) at 30°C with shaking for the indicated times, and RNA was extracted as described previously (20, 34). Briefly, 5 × 107 cells from each culture were collected by centrifugation, resuspended in sodium acetate-EDTA buffer, and stored at −70°C. RNA was extracted by vortexing in the presence of glass beads, sodium dodecyl sulfate, and buffer-saturated phenol at 65°C for 10 to 15 min. Samples were cooled on ice and centrifuged, and the aqueous phase was transferred to tubes containing 2.5 volumes of ethanol and 0.1 volume of 3 M sodium acetate. Precipitated RNA was recovered by centrifugation, dried, rapidly dissolved in water, and denatured in formaldehyde-SSPE (1× SSPE is 0.18 NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) buffer with incubation for 15 min at 65°C. Either 40 μl (for ACT1 probing) or 220 μl (for other probes) of denatured RNA was applied to nylon membrane by using a slot blot apparatus. Membranes were rinsed in SSPE, UV cross-linked, and hybridized to randomly primed 32P-labeled PCR products specific for the C. albicans ACT1, CDR1, or ERG11 gene. RNA levels were quantified by densitometry of moderately exposed autoradiographs; CDR1 or ERG11 mRNA levels were normalized to ACT1 mRNA controls.
RESULTS
Adenylate cyclase and CAP mutants are hypersusceptible to SBIs.
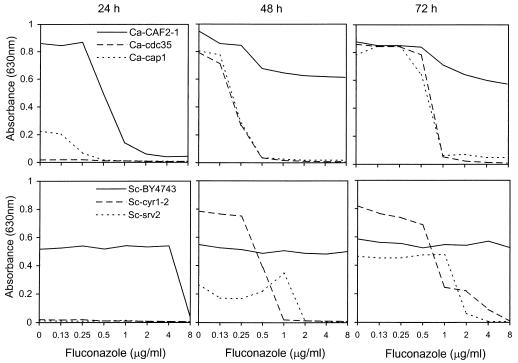
To examine the role of the cAMP pathway in yeast susceptibility to antifungals, broth microdilution assays were used. C. albicans and S. cerevisiae adenylate cyclase mutants Ca-cdc35 (homozygous deletion) and Sc-cyr1-2 (weakened, temperature-sensitive allele) and CAP mutants Ca-cap1 and Sc-srv2, respectively, were tested in parallel with wild-type strains for in vitro susceptibility to five SBI and two non-SBI antifungal agents. Representative dose-response curves for fluconazole are shown in Fig. 2; MIC (≥80% inhibition) data for all antifungals are summarized in Table 2. Note that the growth rates of these strains differ (compare drug-free controls in Fig. 2) due to the variably deleterious effects of the adenylate cyclase or CAP mutations. Consequently, data are presented for 24, 48, and 72 h to allow comparisons across as well as within time points.
FIG. 2.
Broth microdilution assays of fluconazole activity versus wild-type and mutant strains of C. albicans (top row) and S. cerevisiae (bottom row). Absorbance was recorded at the indicated times (24, 48, and 72 h). Note that there was undetectable growth of the Ca-cdc35, Sc-srv2, and Sc-cyr1-2 mutants at 24 h.
TABLE 2.
Susceptibilities of C. albicans and S. cerevisiae wild-type and mutant strains to SBI and non-SBI antifungals
| Antifungal | Time (h) | MIC
|
||||||
|---|---|---|---|---|---|---|---|---|
|
C. albicans
|
S. cerevisiae
|
|||||||
| Ca-CAF2-1 | Ca-cdc35 | Ca-cap1 | Sc-BY4743 | Sc-srv2 | Sc-W303-1A | Sc-cyr1-2 | ||
| Fluconazole | 24 | 1 | UGa | 0.25 | 8 | UG | 8 | UG |
| 48 | >8 | 0.5 | 0.5 | >8 | 2 | >8 | 1 | |
| 72 | >8 | 0.5 | 0.5 | >8 | 2 | >8 | 4 | |
| Itraconazole | 24 | 0.12 | UG | <0.06 | 4 | UG | 2 | UG |
| 48 | >4 | <0.06 | <0.06 | >4 | 0.5 | >4 | 0.25 | |
| 72 | >4 | <0.06 | <0.06 | >4 | 1 | >4 | 1 | |
| Miconazole | 24 | 0.008 | UG | <0.004 | 0.31 | UG | 0.008 | UG |
| 48 | >0.25 | <0.004 | <0.004 | 0.06 | <0.004 | 0.06 | <0.004 | |
| 72 | >0.25 | 0.008 | <0.004 | 0.12 | <0.008 | 0.06 | 0.008 | |
| Terbinafine | 24 | 0.5 | UG | <0.25 | 2 | UG | 0.5 | UG |
| 48 | 8 | 0.25 | 1 | >16 | 0.25 | 4 | 2 | |
| 72 | 8 | 0.5 | 2 | >16 | 0.5 | 16 | 4 | |
| Fenpropimorph | 24 | 0.25 | UG | 0.06 | 0.5 | UG | 0.12 | UG |
| 48 | 0.5 | 0.25 | 0.25 | >0.5 | 0.03 | 0.5 | 0.25 | |
| 72 | >0.5 | 0.5 | 0.25 | >0.5 | 0.06 | 0.5 | 0.5 | |
| Amphotericin B | 24 | 0.12 | UG | 0.12 | 0.12 | UG | 0.125 | UG |
| 48 | 0.25 | 0.06 | 0.5 | 0.5 | <0.06 | 0.25 | 0.25 | |
| 72 | 0.25 | 0.25 | 0.5 | 0.5 | 0.06 | 0.5 | 0.5 | |
| Caspofungin | 24 | 0.06 | UG | NDb | 0.12 | UG | 0.25 | UG |
| 48 | 0.12 | 0.06 | ND | 0.25 | 0.25 | >0.25 | 0.25 | |
| 72 | 0.25 | 0.12 | ND | 0.25 | 0.25 | >0.25 | >0.25 | |
UG, undetectable growth.
ND, not done.
The effects of the adenylate cyclase and CAP mutations were most apparent at 48 and 72 h, when “trailing” growth in the presence of azoles was pronounced for the wild-type strains (Fig. 2). Consequently, comparing MICs obtained at 48 h revealed that both the C. albicans and S. cerevisiae mutations resulted in significant hypersusceptibility to azoles (Table 2). Both Ca-cdc35 and Ca-cap1 mutants were >16-fold hypersusceptible to fluconazole, >64-fold to itraconazole, and >64-fold to miconazole. Similarly, for Sc-cyr1-2 and Sc-srv2 mutants, these figures were >4- to >8-fold hypersusceptible to fluconazole, >8- to >16-fold to itraconazole, and >16-fold to miconazole.
It is unlikely that this azole hypersusceptibility was simply due to the impaired growth of the mutant strains, since this would predict that any drug at partially inhibitory concentrations would be synergistic with azoles, which is not the case. Nevertheless, we tested this directly by comparing azole susceptibility of Ca-CAI4 in YPD versus YPD plus uridine (80 μg/ml). Although this ura3/ura3 strain grew to fivefold-lower cell density in the unsupplemented medium (measured at 20 h), the MICs of fluconazole, itraconazole, and miconazole were identical or slightly higher in unsupplemented versus supplemented medium. Thus, impaired growth does not lead to azole hypersusceptibility.
The hypersusceptibility of these mutants was not limited to azoles but was also extended to nonazole SBIs. The Ca-cdc35 and Ca-cap1 mutants demonstrated 32- and 8-fold decreased terbinafine MICs, respectively. For Sc-srv2 MIC decreased >64-fold; however, for Sc-cyr1-2, this decrease was only twofold. With fenpropimorph, the C. albicans mutants and Sc-cyr1-2 demonstrated twofold-decreased MICs, but the decrease was > 16-fold for Sc-srv2.
With the non-SBIs amphotericin B and caspofungin, variable or relatively minor effects of the adenylate cyclase and CAP mutations were observed. Specifically, Ca-cdc35 and Sc-srv2 mutants demonstrated, respectively, 4- and >8-fold decreased MICs for amphotericin B; however, there was little or no change with Ca-cap1 and Sc-cyr1-2. There was similarly little or no effect of the mutations tested on caspofungin activity.
cAMP reverses the growth defect and SBI hypersusceptibility of adenylate cyclase mutants.
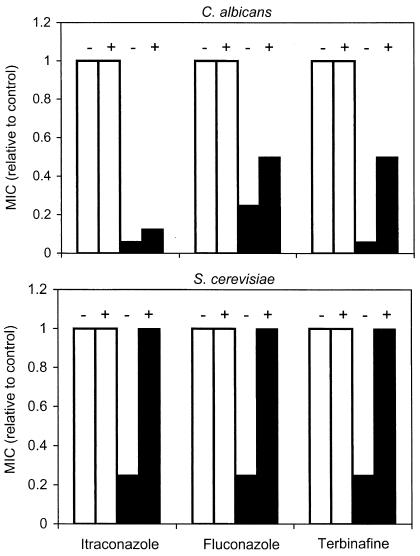
If, as expected, the SBI hypersusceptibility of cAMP pathway mutants results from deficient levels of intracellular cAMP, then addition of extracellular cAMP should overcome this defect and restore normal susceptibility. Consequently, the Ca-cdc35 and Sc-cyr1-2 assays described above were repeated in the presence of 0 to 10 mM cAMP. Addition of cAMP reversed the growth defects of both mutants: at 20 h there was a sixfold increase in cell density for both strains, although this required 10 mM cAMP with Ca-cdc35 but only 1 mM cAMP with Sc-cyr1-2. Furthermore, addition of cAMP to these concentrations conferred partial-to-complete reversal of the SBI hypersusceptibility of these adenylate cyclase mutants, while having no effect on the susceptibility of wild-type strains (Fig. 3). Specifically, addition of 1 mM cAMP to Sc-cyr1-2 cultures increased the itraconazole, fluconazole, and terbinafine MICs fourfold to values equal to those obtained for the control. With the Ca-cdc35 mutant, addition of 10 mM cAMP increased MICs two- to eightfold to values that were 50% of the control strain MICs for fluconazole and terbinafine; the effects on itraconazole susceptibility were more limited.
FIG. 3.
Effects of extracellular cAMP on SBI susceptibilities of C. albicans (top) and S. cerevisiae (bottom) wild-type strains (open bars) and adenylate cyclase mutants (filled bars). Broth microdilution assays were performed as indicated without (−) or with (+) added cAMP (10 mM for C. albicans and 1 mM for S. cerevisiae). Absorbance was recorded and MICs were determined after 42 (C. albicans) or 30 (S. cerevisiae) h; results are expressed relative to the MIC for the wild-type strain without added cAMP.
MDL-12330A inhibits adenylate cyclase in yeast and enhances SBI activity.
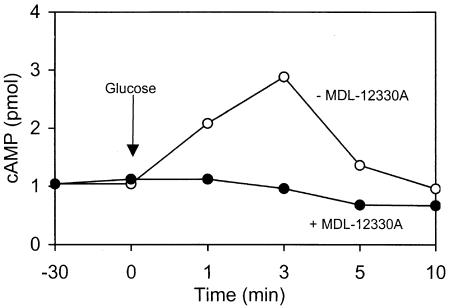
Specific inhibitors are commonly used tools in studies of cAMP signaling in various cells. MDL-12330A is a known inhibitor of mammalian adenylate cyclase (16), but its effects on the yeast enzyme have not been reported. To test this, an established model for cAMP induction in yeast by glucose was employed (12, 25). Ca-SC5314 cells cultured in glycerol-containing medium were exposed to 100 mM glucose both in the presence and absence of 25 μg of MDL-12330A/ml. In its absence, glucose induced a spike in cAMP levels within 3 min, which returned to basal level by 10 min (Fig. 4); similar effects have been reported for S. cerevisiae (12, 25). Preincubation of cells with MDL-12330A completely inhibited the glucose induction of cAMP synthesis (Fig. 4).
FIG. 4.
Cellular cAMP levels in Ca-SC5314 after addition of 100 mM glucose (at 0 min) in the absence (open circles) or presence (filled circles) of 25 μg of MDL-12330A/ml (added at −30 min).
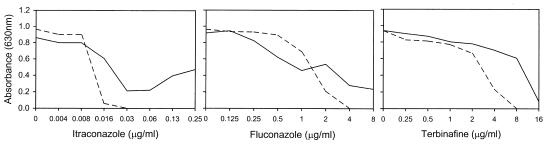
Therefore, the effects of MDL-12330A on SBI activity were examined to determine if they correlated with mutation in adenylate cyclase. At a concentration of 10 μg/ml, MDL-12330A had no effect on Ca-SC5314 growth by itself and minimal effect on its SBI susceptibility at early (24-h) time points (data not shown) but greatly reduced the trailing growth characteristic of SBIs at 48 h (Fig. 5). Consequently, in the presence of MDL-12330A, the itraconazole, fluconazole, and terbinafine 48-h MICs for C. albicans SC5314 were reduced >8-fold, >4-fold, and 2-fold, respectively.
FIG. 5.
Broth microdilution assays demonstrating effects of adenylate cyclase inhibitor MDL-12330A on itraconazole, fluconazole, and terbinafine activity versus Ca-SC5314. Solid line, no added inhibitor; dashed line, 10 μg of inhibitor/ml. Absorbance was recorded at 48 h.
The combination of MDL-12330A with itraconazole was further studied with a set of six fluconazole-susceptible and six fluconazole-resistant C. albicans strains, as well as with 11 strains representing six additional Candida species and S. cerevisiae. MDL-12330A (10 μg/ml) reduced the 48-h MICs of itraconazole in all fluconazole-susceptible (reduction of >8- to >250-fold) and fluconazole-resistant (reduction of 4- to >16-fold) strains tested. In non-C. albicans Candida species, the most significant effect was observed with Candida tropicalis (>1,000-fold decrease in 48-h MIC), consistent with its high degree of trailing growth. This was followed by Candida krusei (16-fold), Candida guilliermondii (eightfold), Candida glabrata (two- and eightfold), and Candida lusitaniae (one- and fourfold). For the two strains of Candida parapsilosis tested, antagonism of itraconazole activity was observed (two- and eightfold increases in 48-h MICs).
Azole hypersusceptibility of adenylate cyclase and CAP mutants correlates with reduced CDR1 upregulation.
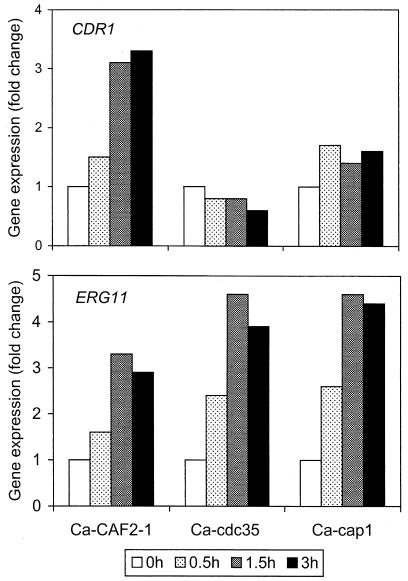
To examine potential molecular mechanisms for the SBI hypersusceptibility of the cAMP pathway mutants and related effects of MDL-12330A, RNA hybridization analysis was used to examine expression of two C. albicans genes known to affect SBI susceptibility. CDR1 encodes a multidrug transporter, and ERG11 encodes the azole target lanosterol demethylase. Both genes are commonly upregulated in azole-resistant clinical isolates (29, 36); furthermore, in vitro treatment of susceptible cells with SBIs leads to transient upregulation of these genes (34). Treatment of C. albicans Ca-CAF2-1 cultures with itraconazole (0.25 μg/ml) resulted in a threefold upregulation in CDR1 expression after 1.5 and 3 h; in contrast, CDR1 expression did not significantly change in the mutants Ca-cdc35 and Ca-cap1 (Fig. 6A). With respect to ERG11 (Fig. 6B), the two mutants actually exhibited slightly higher (four- to fivefold) upregulation than did their parent (threefold).
FIG. 6.
Comparative expression of CDR1 (top) and ERG11 (bottom) in itraconazole-treated cultures of the indicated C. albicans wild-type and mutant strains. Itraconazole (0.25 μg/ml) was added to cultures at 0 min, and aliquots were removed for RNA extraction at 0, 0.5, 1.5, and 3 h as indicated. Slot blot hybridization and densitometric analysis were used to quantitate gene expression as described in Materials and Methods. Expression was normalized to ACT1 RNA levels and was represented as change (n-fold) relative to the 0-h time point.
DISCUSSION
Recent studies have shown that yeasts respond to treatment with azoles and other SBIs by altering the expression of multiple genes that confer at least partial protection from these membrane-perturbing agents (2, 8, 9, 17, 18, 23, 34). The signaling pathways responsible for this antifungal response are beginning to be understood and include the calcium-calmodulin-calcineurin and PKC-cell integrity pathways (7, 11, 24, 26; Edlind, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother.). These pathways are linked to membrane stability or function via calcium channels and the PKC activator Rho1p, respectively. Here we examined a third conserved pathway that, similarly, is membrane associated: adenylate cyclase via the farnesylated-palmitoylated Ras proteins and CAP protein via the actin cytoskeleton (6, 19). The cAMP-PKA pathway was previously implicated in fungal resistance to the antibiotic polymyxin B (4), dicarboximide, aromatic hydrocarbon fungicides (27, 30) and, most pertinent to our studies here, fluconazole (21). It is not surprising that multiple mechanisms for responding to cell surface stress have evolved in unicellular organisms. It is interesting, however, that these mechanisms confer at least partial protection from the modern stresses of antifungal drugs.
The cAMP pathway in S. cerevisiae is complicated by the dual regulation of cAMP synthesis (glucose and Ras mediated) and by feedback inhibition (PKA activation of cAMP phosphodiesterase) (Fig. 1) (10, 35). Consequently, our initial studies focused on two central, interacting components of the pathway: adenylate cyclase responsible for cAMP synthesis and its associated protein, CAP. Relative to wild-type strains, C. albicans and S. cerevisiae mutants in the genes encoding these two proteins demonstrated pronounced hypersusceptibility to azoles and other SBIs. With C. albicans, this was most apparent after 48 h of incubation; i.e., the effect was primarily a reduction in trailing growth. Consistent with these findings, the hypersusceptibility was at least partially reversed by exogenous cAMP. Kontoyiannis and Rupp (21) previously showed that exogenous cAMP reversed the fluconazole hypersusceptibility of a cAMP-deficient S. cerevisiae strain, although interpretation of their results is potentially complicated by the multiple, offsetting mutations present in this strain (involving the adenylate cylase regulators Ras1p and Ras2p and one of two cAMP phosphodiesterases, Pde2p).
Based on previous studies (17, 32, 34), potential mechanisms for explaining the hypersusceptibility of cAMP pathway mutants include disruption of the SBI-induced transcriptional upregulation of multidrug transporter genes such as CDR1 or the lanosterol demethylase gene ERG11. Indeed, a defect in CDR1 upregulation in the itraconazole-treated Ca-cdc35 (adenylate cyclase) mutant was observed. Interestingly, in mammalian cells the cAMP pathway plays a similar role, regulating the expression of the MDR1 transporter responsible for resistance to cancer chemotherapeutics (31). Thus, at least one role for the cAMP pathway appears to be conserved from yeast to human, although further similarities between the regulatory mechanisms remain to be described. Somewhat paradoxically, ERG11 upregulation increased in the Ca-cdc35 mutant; this could be a consequence of reduced CDR1 expression resulting in increased intracellular azole levels, which in turn results in increased ERG11 upregulation by a distinct signaling pathway. Genome-wide expression studies in which microarrays are employed would be useful in identifying relevant genes—other than CDR1—whose expression is altered in cAMP pathway mutants following SBI treatment.
In light of the SBI hypersusceptibility of adenylate cyclase mutants, it was clearly of interest to examine pharmacological inhibitors of this enzyme for similar effects. MDL-12330A has been widely used in mammalian systems but to our knowledge has not been shown previously to inhibit fungal adenylate cyclase. Analogous to the results obtained with the Ca-cdc35 and Ca-cap1 mutants, MDL-12330A enhanced the SBI susceptibility of wild-type C. albicans strains (fluconazole susceptible and resistant), particularly at later time points when MICs were inflated due to trailing growth. MDL-12330A also enhanced the SBI susceptibility of wild-type S. cerevisiae, consistent with the results obtained with Sc-cyr1-2 and Sc-srv2 mutants. Given the evolutionary distance between these two species (3), it is not surprising then that MDL-12330A enhanced itraconazole activity versus a diverse group of additional Candida species. Nevertheless, these results should be interpreted cautiously with respect to mechanism, because it is possible that MDL-12330A has targets other than adenylate cyclase in yeast. Indeed, at a concentration sixfold higher than that used in our interaction studies (10 μg/ml), MDL-12330A alone inhibited the growth of C. albicans wild-type and adenylate cyclase mutant strains equally (data not shown). With respect to clinical application, toxicity to human cells would presumably preclude the use of MDL-12330A in combination with antifungals; nevertheless, it should be possible to identify other adenylate cyclase inhibitors that are fungus specific.
It can be predicted from the studies reported here that mutations or drugs targeting other components of the cAMP pathway, such as the PKA regulatory and catalytic subunits, will have similar effects on the SBI susceptibility of yeast. Studies to examine this idea are under way.
Acknowledgments
We thank D. Harcus, D. Sanglard, P. Sundstrom, A. Hudson, J. Nickels, T. White, and J. Rex for generously providing strains.
This study was supported by National Institutes of Health grants AI46768 and AI47718.
REFERENCES
- 1.Bahn, Y.-S., and P. Sundstrom. 2001. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J. Bacteriol. 183:3211-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bammert, G. F., and J. M. Fostel. 2000. Genome-wide expression patterns in Saccharomyces cerevisiae: comparison of drug treatments and genetic alterations affecting biosynthesis of ergosterol. Antimicrob. Agents Chemother. 44:1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barns, S. M., D. J. Lane, M. L. Sogin, C. Bibeau, and W. G. Weisburg. 1991. Evolutionary relationships among pathogenic Candida species and relatives. J. Bacteriol. 173:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boguslawski, G., and J. O. Polazzi. 1987. Complete nucleotide sequence of a gene conferring polymyxin B resistance on yeast: similarity of the predicted polypeptide to protein kinases. Proc. Natl. Acad. Sci. USA 84:5848-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo, S., P. Ma, L. Cauwenberg, J. Winderickx, M. Crauwels, A. Teunissen, D. Nauwelaers, J. H. de Winde, M.-F. Gorwa, D. Colavizza, and J. M. Thevelein. 1998. Involvement of distinct G-proteins Gpa2 and Ras1 in glucose- and intracellular acidification-induced cAMP signaling in the yeast S. cerevisiae. EMBO J. 17:3326-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crechet, J.-B., E. Jacquet, A. Bernardi, and A. Parmeggiani. 2000. Analysis of the role of the hypervariable region of yeast Ras2p and its farnesylation in the interaction with exchange factors and adenyl cyclase. J. Biol. Chem. 275:17754-17761. [DOI] [PubMed] [Google Scholar]
- 7.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Backer, M. D., T. Ilyina, X.-J. Ma, S. Vandoninck, W. H. M. L. Luyten, and H. Vanden Bossche. 2001. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob. Agents Chemother. 45:1660-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimster-Denk, D., J. Rine, J. Phillips, S. Scherer, P. Cundiff, K. DeBord, D. Gilliland, S. Hickman, A. Jarvis, L. Tong, and M. Ashby. 1999. Comprehensive evaluation of isoprenoid biosynthesis regulation in Saccharomyces cerevisiae utilizing the genome reporter matrix. J. Lipid Res. 40:850-860. [PubMed] [Google Scholar]
- 10.D'Souza, C. A., and J. Heitman. 2001. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25:349-364. [DOI] [PubMed] [Google Scholar]
- 11.Edlind, T., L. Smith, K. Henry, S. Katiyar, and J. Nickels. 2002. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signaling. Mol. Microbiol. 46:257-268. [DOI] [PubMed] [Google Scholar]
- 12.Fedor-Chaiken, M., R. J. Deschenes, and J. R. Broach. 1990. SRV2, a gene required for RAS activation of adenylate cyclase in yeast. Cell 61:329-340. [DOI] [PubMed] [Google Scholar]
- 13.Field, J., A. Vojtek, R. Ballester, G. Bolger, J. Colicelli, K. Ferguson, J. Gerst, T. Kataoka, T. Michaeli, and S. Powers. 1990. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell 61:319-327. [DOI] [PubMed] [Google Scholar]
- 14.Franz, R., S. L. Kelly, D. C. Lamb, D. E. Kelly, M. Ruhnke, and J. Morschhauser. 1998. Multiple mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guellaen, G., J. L. Mahu, P. Mavier, P. Berthelot, and J. Hanoune. 1977. RMI 12330 A, an inhibitor of adenylate cyclase in rat liver. Biochim. Biophys. Acta 13:465-475. [DOI] [PubMed] [Google Scholar]
- 17.Henry, K. W., J. T. Nickels, and T. D. Edlind. 2002. ROX1 and ERG regulation in Saccharomyces cerevisiae: implications for antifungal susceptibility. Eukaryot. Cell 1:1041-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernaez, M. L., C. Gil, J. Pla, and C. Nombela. 1998. Induced expression of the Candida albicans multidrug resistance gene CDR1 in response to fluconazole and other antifungals. Yeast 14:517-526. [DOI] [PubMed] [Google Scholar]
- 19.Hubberstey, A. V., and E. P. Mottillo. 2002. Cyclase-associated proteins: capacity for linking signal transduction and actin polymerization. FASEB J. 16:487-499. [DOI] [PubMed] [Google Scholar]
- 20.Katiyar, S. K., and T. D. Edlind. 2001. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei. Med. Mycol. 39:109-116. [DOI] [PubMed] [Google Scholar]
- 21.Kontoyiannis, D. P., and S. Rupp. 2000. Cyclic AMP and fluconazole resistance in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 44:1743-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraakman, L., K. Lemaire, P. Ma, A. W. R. H. Teunissen, M. C. V. Donaton, P. Van Dijck, J. Winderickx, J. H. de Winde, and J. M. Thevelein. 1999. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32:1002-1012. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy, S., V. Gupta, R. Prasad, S. L. Panwar, and R. Prasad. 1998. Expression of CDR1, a multidrug resistance gene of Candida albicans: transcriptional activation by heat shock, drugs, and human steroid hormones. FEMS Microbiol. Lett. 160:191-197. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2002. Potent synergism of the combination of fluconazole and cyclosporin in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mbonyi, K., L. V. Aelst, J. C. Arguelles, A. W. H. Jans, and J. M. Thevelein. 1990. Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 10:4518-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orth, A. B., M. Rzhertskaya, E. J. Pell, and M. Tien. 1995. A serine (threonine) protein kinase confers fungicide resistance in the phytopathogenic fungus Ustilago maydis. Appl. Environ. Microbiol. 61:2341-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan, X., and J. Heitman. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perea, S., J. L. Lopez-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillan, M. Martinez, D. Calabrese, D. Sanglard, and T. F. Patterson. 2001. Prevalence of molecular mechanisms of resistance to a azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramesh, M. A., R. D. Laidlaw, F. Durrenberger, A. B. Orth, and J. W. Kronstad. 2001. The cAMP signal transduction pathway mediates resistance to dicarboximide and aromatic hydrocarbon fungicides in Ustilago maydis. Fungal Genet. Biol. 32:183-193. [DOI] [PubMed] [Google Scholar]
- 31.Rohlff, C., and R. I. Glazer. 1995. Regulation of multidrug resistance through the cAMP and EGF signalling pathways. Cell. Signal. 7:431-443. [DOI] [PubMed] [Google Scholar]
- 32.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanglard, D., K. Kuchler, F. Ischer, J.-L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, W. L., and T. D. Edlind. 2002. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 46:3532-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thevelein, J. M., and J. H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904-918. [DOI] [PubMed] [Google Scholar]
- 36.White, T. C., S. Holleman, F. Dy, L. F. Mirels, and D. A. Stevens. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]