Abstract
The efficacies of liposomal amphotericin B (LAmB) and amphotericin B deoxycholate (AmB) were compared in a diabetic murine model of hematogenously disseminated Rhizopus oryzae infection. At 7.5 mg/kg of body weight twice a day (b.i.d.), LAmB significantly improved overall survival compared to the rates of survival in both untreated control mice (P = 0.001) and mice treated with 0.5 mg of AmB per kg b.i.d. (P = 0.047). These data indicate that high-dose LAmB is more effective than AmB in treating murine disseminated zygomycosis.
Zygomycosis is a frequently fatal infection that occurs in patients with elevated available levels of iron in serum, such as those treated with deferoxamine, or in patients immunocompromised by diabetic ketoacidosis, organ transplantation, or neutropenia (2, 12). The therapy for invasive zygomycosis includes reversal of the underlying predisposing factors, emergent surgical debridement, and antifungal chemotherapy (5, 10, 12). Although prospective clinical studies are lacking, amphotericin B deoxycholate (AmB) remains the antifungal therapy of choice for invasive zygomycosis (5, 12), largely because of a historical lack of alternative cidal therapies. Because the fungus is relatively resistant to AmB, high doses are required, frequently resulting in nephrotoxicity and other adverse effects (12). Even when surgical debridement is combined with high-dose AmB, the mortality associated with zygomycosis exceeds 50% (12). This mortality rate approaches 100% in patients with disseminated zygomycosis, possibly because surgery to remove the infected foci is not feasible (2). These data emphasize the critical need for more effective antifungal chemotherapy for this lethal infection.
The lipid formulations of AmB allow the administration of higher drug doses due to their limited toxicities (1, 4). Scattered case reports have demonstrated successful outcome in patients with zygomycosis treated with lipid-associated AmB (7, 8). Since diabetic ketoacidosis represents a major risk factor for the development of zygomycosis infection (5, 12), we used a diabetic mouse model to compare the efficacy of high doses of liposomal AmB (LAmB) against that of AmB in treating hematogenously disseminated zygomycosis caused by Rhizopus oryzae, the most common etiologic pathogen of zygomycosis (11).
(This work was presented in part at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002.)
R. oryzae 99-880 was obtained from the Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio. This strain was isolated from a brain abscess of a diabetic patient with rhinocerebral zygomycosis. Spores were collected by flooding potato dextrose agar plates (PDA) with 7 ml of endotoxin-free phosphate-buffered saline (PBS) containing 0.01% Tween 80 and gently scrapping the aerial mycelium.
Male BALB/c mice (≥24 g) were rendered diabetic with a single intraperitoneal (i.p.) injection of 210 mg of streptozocin per kg of body weight in 0.2 ml of ice-cold citrate buffer 10 days prior to fungal challenge (13). Glycosuria, as determined by the use of keto-Diastix reagent strips, was confirmed in mice 7 days after streptozocin treatment. Suspensions of R. oryzae spores in 0.2 ml of endotoxin-free PBS were injected into the lateral tail vein. AmB (Fungizone) and LAmB (Fujisawa Healthcare, and Gilead Sciences) were administered via the lateral tail vein in 5% glucose solution, with the first dose starting 24 h postinfection. Survival data were analyzed by the nonparametric log-rank test. Median survival times were compared by using the nonparametric Steel test for multiple comparisons. Comparisons with P < 0.05 were considered significant.
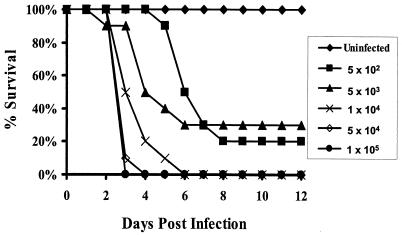
Preliminary studies indicated that inocula of ≥5 × 103 spores would not be suitable for subsequent efficacy studies, because the majority of the deaths at these high inocula occurred within 4 days of infection, which would not allow time to administer a complete 4-day course of treatment (Fig. 1). Consequently, an inoculum of 103 spores was chosen for antifungal efficacy experiments. Intravenous challenge of diabetic mice with R. oryzae resulted in hematogenous dissemination of the infection to all organs examined, including brain, kidneys, lungs, liver, spleen, and heart, as indicated by detection of fungal growth from organs cultured on PDA plates.
FIG. 1.
Survival of diabetic mice infected with different inocula of R. oryzae. Mice were rendered diabetic by an intraperitoneal injection of 210 mg of streptozocin per kg of body weight 10 days prior to intravenous injection with R. oryzae (n = 10 mice per group).
Prior to evaluation of efficacy, we studied the toxicities of once-daily AmB at 1 mg/kg/day and LAmB at 15 mg/kg/day for 4 days in uninfected mice (treatment beyond 4 days was impossible due to profound sclerosis of the tail veins). The once-daily AmB regimen caused severe toxicity, resulting in 60% mortality in uninfected mice. This AmB-mediated toxicity was likely infusion related, because the mice expired within minutes of intravenous administration of the drug. In contrast, the once-daily LAmB regimen did not result in any deaths. Because of the toxicity of once-daily AmB, we chose to administer both drugs twice daily (b.i.d.) at the same total dose (i.e., AmB at 0.5 mg/kg b.i.d. and LAmB at 7.5 mg/kg b.i.d). This dosing regimen completely eliminated the deaths related to administration of AmB. A dose of 0.75-mg/kg b.i.d. AmB was still toxic to mice.
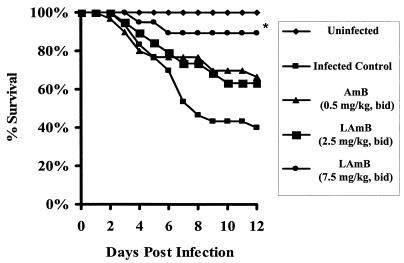
In infected animals, only high-dose LAmB (7.5 mg/kg b.i.d.) significantly increased the median survival time compared to infected, untreated control mice (>12 days versus 6 days; P = 0.01). Furthermore, while 90% of mice treated with high-dose LAmB (7.5 mg/kg b.i.d.) survived to day 12 postinfection (Fig. 2), only 40% percent of the untreated control mice (P = 0.001) and 67% of the mice treated with AmB survived to day 12 (P = 0.047 versus LAmB). Mice treated with 2.5- and 5-mg/kg b.i.d. LAmB had 12-day survival rates of 63 and 50%, respectively, which were not significantly different from those of untreated control or AmB-treated mice.
FIG. 2.
Survival of diabetic mice infected with 103 R. oryzae spores and treated with 0.5-mg/kg b.i.d. AmB or 2.5-, 5-, or 7.5-mg/kg b.i.d. LAmB. Drugs were given for a total of 4 consecutive days. The survival curve represents the sum of two separate experiments (n = 30 mice for untreated control or AmB treated, and n = 19 for LAmB treated). *, P < 0.05 when compared to infected untreated or AmB-treated mice.
AmB remains the only antifungal agent approved for the treatment of zygomycosis, although there have been no prospective trials to define the optimal antifungal therapy for this infection (5, 10, 12). The lipid formulations of AmB are significantly less nephrotoxic than AmB and can be administered at higher doses. Several case reports of patients with zygomycosis document successful treatment with LAmB up to 15 mg/kg/day (3, 6, 14). Although there is a developing consensus that high doses of lipid formulation AmB should be the initial antifungal therapy of choice for all patients with zygomycosis (9), until now there have been no data available to define the relative efficacies of the lipid formulations of AmB versus AmB for zygomycosis.
We now report that in murine disseminated zygomycosis, LAmB was significantly less toxic than AmB, allowing administration of 15-fold-higher doses of LAmB than AmB. Furthermore, high-dose LAmB (7.5 mg/kg b.i.d.) was more efficacious than AmB (0.5 mg/kg b.i.d.), significantly improving both the median survival time and overall survival of infected mice. Conversely, AmB mediated no survival benefit over that of untreated controls, and lower doses of LAmB (2.5 and 5 mg/kg b.i.d.) also did not significantly improve the survival of infected mice. Collectively, these data emphasize the critical importance of administration of high doses of drug for efficacy against R. oryzae and indicate that LAmB may be the preferred clinical treatment, given its diminished toxicity profile, which allows administration of doses ≥15-fold above those of AmB.
Acknowledgments
This study was supported in part by research and educational grants from Fujisawa Healthcare, Inc., and Gilead Sciences, Inc., and was conducted entirely at the biomedical research facilities of the Research and Education Institute at Harbor-UCLA Medical Center. A.S.I. is supported by a Burroughs Wellcome New Investigator Award in Molecular Pathogenic Mycology.
We would like to thank Jill Adler-More for helpful discussions and Helen Lee for excellent technical assistance.
REFERENCES
- 1.Adler-Moore, J., and R. T. Proffitt. 2002. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 49(Suppl. 1):21-30. [DOI] [PubMed] [Google Scholar]
- 2.Boelaert, J. R. 1994. Mucormycosis (zygomycosis): is there news for the clinician? J. Infect. 28(Suppl. I):1-6. [DOI] [PubMed] [Google Scholar]
- 3.Cagatay, A. A., S. S. Oncu, S. S. Calangu, T. T. Yildirmak, H. H. Ozsut, and H. H. Eraksoy. 2001. Rhinocerebral mucormycosis treated with 32 gram liposomal amphotericin B and incomplete surgery: a case report. BMC Infect. Dis. 1:22. [Online.] http://www.biomedcentral.com/1471-2334/1/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupont, B. 2002. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemother. 49(Suppl. 1):31-36. [DOI] [PubMed] [Google Scholar]
- 5.Edwards, J. E., Jr. 1989. Zygomycosis, p. 1192-1199. In P. D. Hoeprich and M. C. Jordan (ed.), Infectious disease, 4th ed. J. B. Lippincott Co., Philadelphia, Pa.
- 6.Ericsson, M., M. Anniko, H. Gustafsson, C. A. Hjalt, R. Stenling, and A. Tarnvik. 1993. A case of chronic progressive rhinocerebral mucormycosis treated with liposomal amphotericin B and surgery. Clin. Infect. Dis. 16:585-586. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, C. E., D. R. Couriel, and T. J. Walsh. 1997. Disseminated zygomycosis in a neutropenic patient: successful treatment with amphotericin B lipid complex and granulocyte colony-stimulating factor. Clin. Infect. Dis. 24:192-196. [DOI] [PubMed] [Google Scholar]
- 8.Herbrecht, R., V. Letscher-Bru, R. A. Bowden, S. Kusne, E. J. Anaissie, J. R. Graybill, G. A. Noskin, B. A. Oppenheim, E. Andres, and L. A. Pietrelli. 2001. Treatment of 21 cases of invasive mucormycosis with amphotericin B colloidal dispersion. Eur. J. Clin. Microbiol. Infect. Dis. 20:460-466. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim, A. S., J. E. Edwards, Jr., and S. G. Filler. 2003. Zygomycosis, p. 241-251. In W. E. Dismukes, P. G. Pappas, and J. D. Sobel (ed.), Clinical mycology. Oxford University Press, New York, N.Y.
- 10.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology, p. 524-559. Lea & Febiger, Philadelphia, Pa.
- 11.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugar, A. M. 1995. Agent of mucormycosis and related species, p. 2311-2321. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 13.Waldorf, A. R., N. Ruderman, and R. D. Diamond. 1984. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J. Clin. Investig. 74:150-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]