Abstract
Necrotic enteritis (NE) is a worldwide poultry disease caused by the alpha toxin-producing bacterium Clostridium perfringens. Disease risk factors include concurrent coccidial infection and the dietary use of cereal grains high in nonstarch polysaccharides (NSP), such as wheat, barley, rye, and oats. Outbreaks of NE can be prevented or treated by the use of in-feed antibiotics. However, the current debate regarding the prophylactic use of antibiotics in animal diets necessitates a better understanding of factors that influence intestinal colonization by C. perfringens as well as the pathophysiological consequences of its growth. We report a study with a chick model of NE, which used molecular (16S rRNA gene [16S rDNA]) and culture-based microbiological techniques to investigate the impact of the macrolide antibiotic tylosin phosphate (100 ppm) and a dietary NSP (pectin) on the community structure of the small intestinal microbiota relative to colonization by C. perfringens. The effects of tylosin and pectin on mucolytic activity of the microbiota and C. perfringens colonization and their relationship to pathological indices of NE were of particular interest. The data demonstrate that tylosin reduced the percentage of mucolytic bacteria in general and the concentration of C. perfringens in particular, and these responses correlated in a temporal fashion with a reduction in the occurrence of NE lesions and an improvement in barrier function. The presence of pectin did not significantly affect the variables measured. Thus, it appears that tylosin can control NE through its modulation of C. perfringens colonization and the mucolytic activity of the intestinal microbiota.
Clostridium perfringens is a low G+C gram-positive anaerobic spore-forming bacterium and a widely distributed pathogen (19, 30). It is found commonly in soil and sewage and as a member of the normal microbial community in the gastrointestinal tract of animals and humans. Conditions that promote excessive growth of C. perfringens in the chicken intestine lead to toxin production that causes mucosal lesions and the clinical disease necrotic enteritis (NE). The disease occurs most commonly in broiler chicks (2 to 5 weeks of age) but is also observed in older pullets and layers (6, 29, 36). Disease outbreaks have been minimized to a significant extent through the use of in-feed antibiotics. However, the incidence of NE in broiler flocks has increased in Western Europe since the implementation of a ban on the growth promotion use of avoparcin in 1997 and of tylosin, spiramycin, virginiamycin, and zinc bacitracin in 1999 (8). The incidence of the disease may have been exacerbated by the use of wheat and barley as basal ingredients in European poultry diets. Dietary grains rich in water-soluble nonstarch polysaccharides (NSP) such as wheat, barley, or rye increase intestinal viscosity through enhanced mucus production (16, 24, 38). These changes may compromise mucosal barrier function due to increased microbial growth in general and that of C. perfringens in particular. Clearly, the current debate regarding the prophylactic use of antibiotics in animal diets necessitates a better understanding of factors that influence intestinal colonization by C. perfringens as well as the pathophysiological consequences of its growth.
As such, we report a study with a chick model of NE that examined the effects of tylosin and highly methylated citrus pectin (as a water soluble and fermentable NSP) on the community structure of the small intestinal microbiota relative to colonization by C. perfringens. The effects of tylosin on mucolytic activity of intestinal bacteria and its relationship to C. perfringens colonization and epithelial barrier function were of particular interest.
MATERIALS AND METHODS
Clostridial enteritis infection model.
Ross 508 broiler chicks (0 to 24 days of age) were used and were fed from day 0 with one of four diets: a basal diet of wheat and barley with (WBT) or without (WB) tylosin (Elanco Animal Health, Greenfield, Ind.) (provided at a therapeutic dose [100 μg/kg of diet] to control the population and activities of gram-positive gut bacteria [4, 41]) or a basal diet of wheat, barley, and highly methylated citrus (HMC) pectin (type RU 301; Contined BV, Bennekom, The Netherlands) (methoxylation > 60%) with (WBPT) or without (WBP) 100 μg of tylosin/kg of diet. The experimental diets included barley (21%), wheat (30%), extracted soybean meal (17.5%), full-fat soybeans (5%), dietary fat sources (8%), canola meal (5%), maize gluten meal (4%), and soy isolate (3%). The dietary nutrient composition was calculated to meet the bird's nutrient requirements (feed evaluation table, ISBN 90-72839-13-7, Bureau of Livestock Feedstuffs, CVB, Lelystad, The Netherlands, 2000 [http:www.pdv.nl/cvb]). To facilitate the onset of NE, all birds were inoculated in the crop at 10 days of age with Eimeria acervulina (Central Veterinary Laboratory, Weybridge, England) (∼200,000 oocysts per ml; 1 ml per bird) (1). All birds were then subsequently inoculated in the crop on days 14, 15, and 16 with C. perfringens type A (code GD 5.11.53) (∼108 CFU/ml; 1 ml per bird). Feed was withdrawn for 8 h prior to each inoculation to minimize the occurrence of regurgitation.
Pathological parameters.
On days 15, 16, 17, 20, and 24, 12 birds per treatment were killed by injection in the wing vein with a euthasate (T61; Intervet, Mechelen, Belgium) containing embutramide (200 mg/ml), mebenzonium iodide (50 mg/ml), and tetracaine hydrochloride (5 mg/ml). The abdomen was opened, and the gastrointestinal tract was exposed. The gastrointestinal tract was segmented into the duodenum and jejunum (the segment from the gizzard to the 10 cm preceding Meckel's diverticulum). The coccidiosis lesions were visually scored according to the method of Johnson and Reid (12). Necrotic lesions were scored on a scale from 0 to 3 (0, no dot lesions; 1, one to five small [<1-mm diameter] dot lesions; 2, more than five small dot lesions but fewer than five larger [1- to 2-mm diameter] ones; 3, more than five larger lesions and erosive zones).
Permeability measurements.
A 5-cm-long segment anterior to Meckel's diverticulum and a 5-cm-long segment starting at 1 cm preceding the ileocecal junction were taken for permeability measurements from 6 of the 12 birds sampled on days 17, 20, and 24. The samples were transported in ice-cold HEPES-buffered, phenol red-free Dulbecco's modified Eagle's medium (DMEM). Each intestinal section was rinsed by flushing intestinal contents with an ice-cold DMEM buffer solution before the segment was cut longitudinally and laid open, exposing the luminal surface. The mucosal layer was carefully stripped (using the blunt edge of a sterile razor blade) from the muscle layer. Samples of the mucosal layer were punched with a 9-mm-diameter steel punch and placed in flat sheets that separated the 1.5-ml mucosal and 1.5-ml serosal compartments in Ussing chambers (effective absorptive-tissue surface area, 0.196 cm2). The mucosal and serosal compartments were filled with 1.25 ml of DMEM buffer solution, and both compartments were aerated (O2/CO2 ratio, 95/5) at a temperature of 37°C. Radiolabeled markers [14C]GlySar (Zeneca Cambridge Research Biochemicals, Northwich, United Kingdom) (glycylsarcosine dipeptide derivative; 10 μmol · liter−1) and [3H]mannitol (ICN Biochemicals, Zoetermeer, The Netherlands) (10 μmol · liter−1) were added to the mucosal compartment. GlySar is transported mainly by a transcellular route with an H+-coupled peptide carrier, while [3H]mannitol is transported mainly by a paracellular route (5, 13). Fluids in both compartments were circulated by gas lifting. At the indicated time points (15, 45, 75, and 105 min), 0.5-ml samples were taken from the serosal compartments and the volume was reconstituted with DMEM. 3H- and 14C-labeled compounds were analyzed using a Wallac 1409 liquid scintillation counter. Permeability coefficients were determined on basis of the appearance of the probe in the serosal compartment according to the formula Pms = P/(A · C0), where Pms is the permeability coefficient from the mucosal to serosal side (in centimeters per second), P is the permeability rate (in mole seconds per liter), A is the exposed intestinal area (in square centimeters), and C0 is the initial mucosal concentration of the test substance (in moles per millimeter).
Sample collection for DNA extraction.
Three 5-cm-long sections were collected from the jejunum and ileum of 6 of the 12 birds from which samples were taken on days 17, 20, and 24 for molecular analysis. From one 5-cm-long section, the luminal contents were collected into a cryogenic vial. A second 5-cm-long section was cut longitudinally and laid open, exposing the luminal surface. A sterile glass slide was used to remove and discard luminal contents, and a clean glass slide was used to scrape the mucus layer into a sterile cryogenic vial. Similarly, mucus was scraped from a third 5-cm-long section from both intestinal segments and placed in a 1-ml cryoprotective mixture of water and glycerol (50/50) in another cryogenic vial. All samples were flash frozen in liquid nitrogen and stored at −80°C until further analysis was performed.
Genomic DNA extraction and PCR-DGGE analysis.
Genomic DNA was obtained using previously described phenol-based extraction methods (37). For 16S rRNA gene (16S rDNA) PCR-denaturant gradient gel electrophoresis (PCR-DGGE) analysis, each DNA sample was amplified using primers (534R and 341F) specific for conserved sequences flanking the variable V3 region of 16S rDNA, as described previously (21, 22, 32). A 40-nucleotide G+C clamp was added to the 5′ end of primer 341F. To minimize spurious products, touchdown PCR was performed as described by Muyzer and Smalla (21).
After agarose gel electrophoresis was used for visual confirmation of the 193-bp PCR product, DGGE was performed using a Bio-Rad (Hercules, Calif.) D-code system as described previously (31). To separate PCR fragments, 35 to 60% linear DNA-denaturing gradients were formed (using a Bio-Rad Gradient Former) in 8% polyacrylamide gels. Products were electrophoresed for 2 h at 60°C and 150 V and then for 1 h at 200 V. Additionally, bacterial 16S rDNA ladders representing known bacterial species were loaded to standardize band migration and gel curvature among gels (32). After electrophoresis, gels were silver stained and scanned using a GS-710 calibrated imaging densitometer (Bio-Rad). Amplicons were considered for analysis when the intensity of the image was at least 2% higher than the background intensity.
When antibiotic-dependent differences in banding profiles were observed, individual 16S-V3 rDNA bands were excised, reamplified as previously described, cloned using a TOPO TA cloning kit (InVitrogen, Carlsbad, Calif.), and sequenced using an automated sequencing system (Applied Biosystems, Foster City, Calif.) at the W. M. Keck Center for Comparative and Functional Genomics (University of Illinois). Sequence data were analyzed using Sequencher 3.0 software (Gene Codes, Ann Arbor, Mich.), and a BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) search was performed for phylogenetic identification. Positive identifications of unknown sequences were considered significantly similar when sequences were 97 to 99% identical to BLAST database sequences.
Estimates of microbial richness and diversity.
Discovery Series Diversity Database software (Bio-Rad) (version 2.1) was used to analyze banding patterns by measuring the migration distance and intensity of the bands within each gel lane (32). This information was then used to analyze banding patterns via measurement of community diversity parameters, including band number, Sorenson's pairwise similarity coefficient (Cs), and Ward's algorithm (18, 34).
The band number corresponds to the number of individual bands in a single gel lane. Cs, sometimes referred to as the Dice coefficient, is a similarity index used to compare banding compositions of different ecosystems (17, 20, 28, 31). Cs values were determined by the formula Cs = [2j/(a + b)] × 100, where a represents the number of PCR-DGGE bands in lane 1, b represents the number of PCR-DGGE bands in lane 2, and j represents the number of common PCR-DGGE bands (11, 18, 20). Thus, two identical profiles create a Cs value of 100%, whereas completely different profiles (no common bands) result in a Cs value of 0%. The banding pattern for each sample was compared to those of other members in the same treatment group (i.e., an intragroup comparison), with results indicating the relative homogeneity of microbial populations within a treatment, and was also compared to those of members of all other groups (i.e., an intergroup comparison), with results indicating the extent to which treatment (diet or antibiotic) affected the composition of microbial populations at each intestinal site.
Ward's algorithm was used to construct a dendrogram of bacterial community structure for each day. Ward's algorithm is defined as
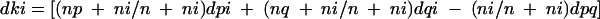 |
where p and q represent two clusters that are joined within a single cluster; k represents the index of the cluster formed by joining clusters p and q; i represents the index of any remaining clusters other than cluster p, q, or k; np represents the number of samples in the pth cluster; nq represents the number of clusters in the qth cluster; n represents the number of clusters in the kth cluster formed by joining the pth and the qth clusters (n = np + nq); and dpq represents the distance between cluster p and cluster q, as discussed by Sneath and Sokal (34) and presented in the Diversity Database manual (Bio-Rad).
Analysis of C. perfringens colonization by real-time qPCR.
The colonization of C. perfringens as a percentage of total bacteria in mucosal and luminal samples collected from the jejunum and ileum was analyzed via real-time quantitative PCR (qPCR) as described by Deplancke et al. (7). DNA isolates (as templates) and C. perfringens-specific 16S rDNA primers were used for PCR amplification in a GeneAmp 5700 sequence detection system (Applied Biosystems) (40). To assess the total concentration of bacterial DNA, 16S-V3 rDNA fragments were amplified with the 534R and 341F primers without the additional G+C clamp as described above. PCR protocols for both C. perfringens-specific and bacterial primer sets were identical to those used for PCR-DGGE. For both primer sets, a standard curve was generated with C. perfringens genomic DNA. The DNA concentrations were 10, 100, and 1,000 pg/μl, and these concentrations were plotted against the PCR cycle threshold (Ct) value. All standards and unknowns from mucosal and luminal contents from the jejunum and ileum were expressed as the average percentage of C. perfringens rDNA relative to total eubacterial rDNA.
Cultivation of mucolytic bacteria.
Diet and antibiotic modulation of mucolytic bacteria was analyzed via a mucolysis assay developed by Deplancke et al. (7) to evaluate possible mechanisms yielding C. perfringens a selective advantage in the intestine. To determine the ratio of mucolytic versus total mucosa-associated bacteria (MAB), diluted mucosal samples from birds (n = 6 birds/treatment) were inoculated under anoxic conditions onto nutrient agar of M10 medium containing multiple carbon sources, including mucin (25). After 5 days of anaerobic growth at 37°C, a total of 20 colonies from the jejunum and ileum of each bird (n = 120 colonies/treatment) at each day (days 17, 20, and 24; 1,440 total colonies) were randomly isolated from the plates and transferred to 24-well plates containing 300 μl of modified anaerobic basal medium 10 containing pig gastric mucin (mucin type II from porcine stomach with 1% bound sialic acids; Sigma M-2378)/well as the sole carbohydrate source. Growth in the mucin-limiting medium was assessed after 2 days of anaerobic growth by measurement of the optical density at 650 nm in a Vmax plate reader (Molecular Devices, Sunnyvale, Calif.). The number of colonies that grew on the mucin-limiting medium are expressed as a percentage of the 20 colonies per bird collected from the habitat-simulating medium. Additionally, the mucolytic potential of 20 C. perfringens colonies was examined in the same medium.
Statistics.
The occurrence of necrotic lesions, cellular permeability, Cs values, qPCR, and mucolytic potential were subjected to analysis of variance using SAS software (version 6.09) (The SAS Institute, Cary, N.C.). The partitioned sources of variation included diet and day and their interactions. Specific treatment comparisons were made using Fisher's protected least significant difference test with an assigned P value of <0.05. Dendrograms representing clustering patterns of microbial profiles were generated with Diversity Database software (version 2.2.0) (Bio-Rad) and Ward's algorithm (16). Pathological indices and data derived from molecular and culture-based techniques were evaluated by Spearman correlation analysis (SAS) to determine correlations to the onset of NE.
RESULTS
Pathological parameters of infection.
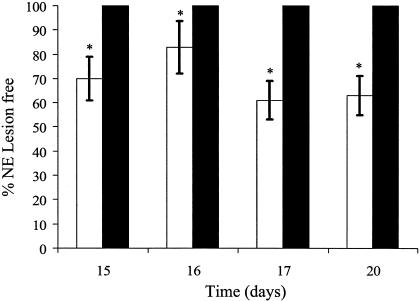
Coccidiosis-related lesions were observed in all birds tested (all of which had been inoculated with E. acervulina) independent of the treatment used (data not shown). Coccidial lesion scores were greatest on day 15 (average score, 2.5), and lesions became less severe in birds sacrificed at later ages (scores of 2.0, 1.6, and 1.0 on days 16, 17, and 20, respectively). For samples from all sampling days, necrotic lesions associated with C. perfringens infection were present only in birds not treated with tylosin (Fig. 1). The inclusion of pectin did not affect (P > 0.05) the occurrence of NE lesions in samples from any sampling day.
FIG. 1.
Percentages of birds (n = 24/treatment) free of NE lesions per treatment at days 15, 16, 17, and 20 as determined by visual scoring of duodenal and jejunal sections of birds fed a control diet (i.e., without tylosin [□]) or a diet including tylosin (▪). The effects of HMC pectin were not significant (P > 0.05); therefore, samples were pooled by day according to tylosin treatment. *, P < 0.05.
At day 17, paracellular and transcellular permeability results did not differ due to diet or tylosin treatment (data not shown). At day 20, levels of jejunal paracellular, but not transcellular, permeability were higher (P < 0.05) in birds fed the pectin-supplemented diet independent of tylosin treatment and higher (P < 0.05) in non-tylosin-treated birds than in those treated with antibiotics across all diet treatments (data not shown). This increase in paracellular permeability indicates an impaired intestinal barrier function, which facilitates the transport of antigenic or toxic substances across the mucosa.
Effects of diet and tylosin on community structure of the jejunal and ileal microbiota.
The average number of bands detected in each sample ranged from 10 to 22, depending on treatment and sampling day. Across treatments, fewer bands were detected in the jejunum versus the ileum (P < 0.05) at days 17, 20, and 24; however, band numbers between luminal and mucosal samples were similar (P > 0.05) for both sites. A gel image of ileal lumen V3-16S rDNA amplicons from day 20 is shown in Fig. 2A. Similar results were observed for jejunal lumen and mucosal and ileal mucosal samples at day 17. At day 20, fewer bands were observed in tylosin-treated versus non-tylosin-treated birds (P < 0.05) and band numbers did not differ for treatments with or without pectin. This result was observed for both the jejunum and the ileum. By day 24, the numbers of 16S rDNA bands amplified from the jejunum and ileum did not differ (P > 0.05) among treatments.
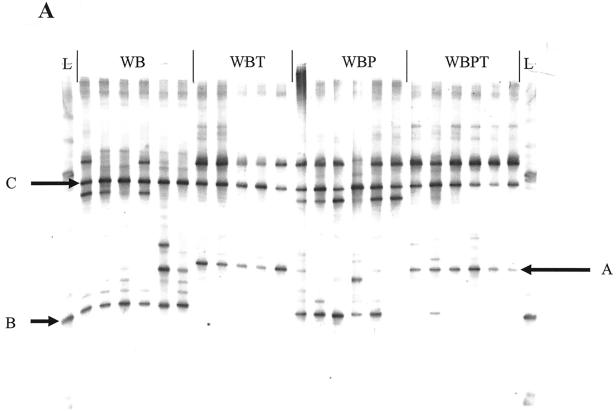
FIG. 2.
(A) DGGE profile generated from PCR-amplified V3-16S rDNA from day 20 ileal lumen contents of birds fed WB, WBT, WBP, or WBPT. Each lane represents one bird. Band A, Lactobacillus gasseri; band B, C. perfringens; band C, L. pontis; band L, reference ladder. (B) Dendrogram depicting antibiotic- and diet-associated effects on V3-16S rDNA banding patterns from day 20 ileal lumen contents of birds fed WB, WBT, WBP, or WBPT. The dendrogram was constructed using Ward's algorithm and Diversity Database software as described in Materials and Methods. Clusters of similar treatments are boxed, and dissimilar samples within clusters are indicated with arrows.
Individual banding patterns were subjected to cluster analysis using Ward's algorithm to determine the effects of diet and antibiotic treatment on microbial community profiles (34). At day 17, jejunal and ileal community profiles clustered according to antibiotic treatment but not by diet. Similar results were observed for day 20, except the jejunal profiles of birds fed tylosin and pectin (WBPT) formed a single tight cluster (Fig. 2B). By day 24, four distinct clusters were observed for the jejunum and ileum according to the four experimental treatments (data not shown). A temporal or developmental effect on microbial communities was observed when all samples from all days were analyzed concurrently in that three distinct clusters were observed for three sampling days (17, 20, and 24) independent of treatment (data not shown).
The effects of diet and tylosin on ileal lumen 16S rDNA banding patterns were further assessed by intragroup comparisons using Cs values, as presented in Table 1 (18). At days 17 and 20, luminal and mucosal intragroup Cs values were higher (P < 0.05) for tylosin-treated (WBT and WBPT) than for non-tylosin-treated (WB and WBP) birds independent of diet. This indicates that tylosin decreased the diversity of bacterial populations and that antibiotic treatment homogenized microbial community structure to a greater extent than diet. All intragroup Cs values decreased by day 24 regardless of treatment relative to the results for days 17 and 20, indicating that bacterial populations became more heterogeneous over time independent of treatment. This result was observed for both the jejunum and ileum.
TABLE 1.
Similarity percentages for 16S rDNA PCR-DGGE banding patterns from ileal lumen
| Comparison and treatment | % Similaritya
|
||
|---|---|---|---|
| Day 17 (SEM) | Day 20 (SEM) | Day 24 (SEM) | |
| Intragroup | |||
| WB | 81.4 (2.1b) | 81.5 (3.0b) | 70.2 (2.0d) |
| WBT | 90.4 (3.0c) | 87.6 (1.2c) | 74.9 (3.0d) |
| WBP | 81.2 (2.6b) | 82.5 (2.0b) | 69.2 (1.6d) |
| WBPT | 93.2 (1.5c) | 89.2 (0.9c) | 69.9 (1.5d) |
| Intergroup | |||
| ± Pectin − tylosin | 59.6 (2.0b) | 85.6 (2.6d) | 85.6 (2.6d) |
| ± Pectin + tylosin | 60 (2.6b) | 87.4 (2.0d) | 84.2 (1.2d) |
| ± Tylosin − pectin | 68 (2.5c) | 69.4 (1.5c) | 81.4 (1.3d) |
| ± Tylosin + pectin | 68.5 (3.0c) | 71.3 (3.0c) | 82.6 (3.0d) |
Cs values were used to compare average percent similarities among PCR-DGGE banding patterns (based on the average number of bands in common) as described in Materials and Methods. Values represent means ± standard errors of the means (n = 6). Values not sharing a common superscript are different (P < 0.05) within the intra- and intergroup comparisons.
Pairwise Cs intergroup comparisons reveal treatment-specific differences in bacterial populations (Table 1). At day 17, Cs values for birds fed diets with or without pectin were lower than those treated with or without tylosin. By day 20, intergroup values of birds treated with or without tylosin did not change from day 17 (P > 0.05), while values for birds fed with or without pectin were significantly higher than those for birds in the groups fed with or without pectin at day 17 and with or without tylosin at days 17 and 20. At day 24, Cs values of birds fed with or without pectin did not change significantly from day 20 and those of birds treated with or without tylosin were higher (P < 0.05) than at day 20 but did not differ (P > 0.05) from those of birds fed with or without pectin. Therefore, pectin-induced alterations in microbial profiles were observed only at day 17, while tylosin-induced changes were observed at days 17 and 20. The increased intergroup Cs values for all samples at day 24 indicate that the effects of pectin and tylosin diminished during the week 4 of the study. Treatment and temporal responses for mucosal samples (data not shown) were similar to those for luminal samples from both the jejunum and ileum, as described above.
Identification of antibiotic-specific 16S rDNA PCR-DGGE bands.
Two 16S rDNA-V3 bands differentially affected by tylosin were excised from a day 17 PCR-DGGE gel containing ileal luminal samples and sequenced for phylogenetic identification (Fig. 2A). Band A was present at days 17, 20, and 24 in all birds fed tylosin (and in one bird fed a tylosin-free diet) independent of diet. Sequence analysis of band A identified it as representing Lactobacillus gasseri (GenBank accession no. AF375930; 99.3% similarity). In contrast, band B was present in all non-tylosin-treated birds at days 17 and 20, in two of five tylosin-treated birds at day 17, and in three of five birds at day 20. Sequence analysis identified band B as representing C. perfringens (GenBank accession no. NC003212; 98.2% similarity), and this sequence was 98.99% similar (two mismatches out of 198 bp) to the 16S rDNA sequence of the C. perfringens inoculum. Band C appeared in all gels regardless of treatment, intestinal segment, or day and was determined by sequence analysis to represent Lactobacillus pontis (GenBank accession no. X76329; 98.8% similarity) (Fig. 2A).
Quantification of C. perfringens in jejunal and ileal mucosa.
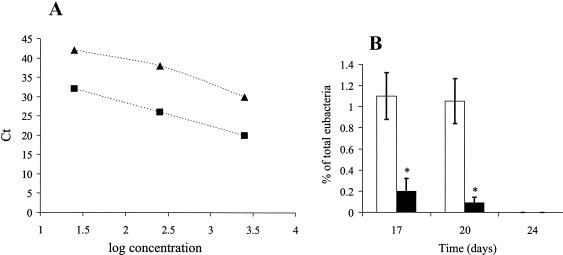
A real-time, qPCR-based method was used to specifically measure C. perfringens concentrations (Fig. 3). Both C. perfringens-specific and bacterial primers yielded one band of the correct size, namely, 279 bp for the C. perfringens-specific primers and 193 bp for the bacterium-specific primers, when visualized on an agarose gel (data not shown). The identity of these products was verified by sequence analysis. Regression values for the C. perfringens and bacterial primer sets (for log DNA concentrations plotted against Ct values) were both 0.99, as determined by serial dilution of C. perfringens DNA in the concentration range of 10 to 1,000 pg/μl (Fig. 3A). Total bacterial concentrations tended to be higher in birds fed a tylosin-free diet than in those treated with tylosin (data not shown), though this difference was not statistically significant. The results of investigations of the effects of pectin and tylosin on C. perfringens 16S rDNA concentrations (expressed as a percentage of total bacterial 16S rDNA in the ileal lumen) are presented in Fig. 3B. The concentrations of C. perfringens in luminal contents from the jejunum and ileum of birds fed a tylosin-free diet were higher (P < 0.05) at days 17 and 20 than those from the jejunum and ileum of tylosin-treated birds independent of diet. C. perfringens was not detected at day 24 regardless of treatment. The inhibitory effect of tylosin on C. perfringens colonization was greater in the ileal and jejunal mucosa than in the lumen (P < 0.05) (data not shown). Pectin did not significantly affect C. perfringens colonization at any day, regardless of intestinal segment or experimental treatment.
FIG. 3.
Real-time qPCR analysis of C. perfringens colonization. (A) Linear regression of total eubacterial (▴) and C. perfringens (▪) DNA concentrations versus Ct values. (B) The concentration of C. perfringens in the ileal lumen at days 17 and 20 expressed as a percentage of total eubacterial rDNA in birds (n = 12/treatment) fed a control diet (i.e., without tylosin [□]) or a diet including tylosin (▪). The effects of HMC pectin were not significant (P > 0.05); therefore, samples were pooled by day according to tylosin treatment. C. perfringens was not detectable at day 24. *, P < 0.05.
Relative proportion of mucolytic bacteria.
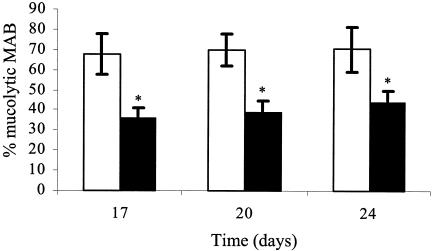
The ratio of mucolytic bacteria as a percentage of total MAB was assessed to examine the effects of diet and antibiotic treatment on bacterial mucolysis (Fig. 4). The percentage of mucolytic bacteria in the jejunum of tylosin-treated birds was significantly lower than that for nontreated birds at days 17 and 20 independent of pectin administration. By day 24, birds fed a WB diet harbored a higher (P < 0.05) percentage of culturable mucolytic bacteria than those fed all other diets. By day 24, tylosin-treated birds harbored significantly fewer mucolytic bacteria in the jejunum than those of the other treatment groups. Similar tylosin effects were observed in the ileum (data not shown). Further, the ileum harbored a higher percentage of mucolytic bacteria than the jejunum. The percentages of mucolytic bacteria from all treatments were similar across days 17, 20, and 24. Each of the C. perfringens colonies (n = 20; 100%) selected grew on the mucin-limiting medium (data not shown).
FIG. 4.
Percentage of mucolytic bacteria present in ileal mucosa of 17-, 20-, and 24-day-old birds (n = 12/treatment) fed a control diet (i.e., without tylosin [□]) or a diet including tylosin (▪). The effects of HMC pectin were not significant (P > 0.05); therefore, samples were pooled by day according to tylosin treatment. MAB colonies isolated from cultures grown on an intestinal habitat-simulating medium were transferred and grown anaerobically in habitat-simulating medium 10 (31) modified to contain mucin as the sole carbon and energy source as described in Materials and Methods. Bacterial growth was monitored by measurement of absorbance (optical density at 650 nm). *, P < 0.05.
Correlation analysis.
The occurrence of necrotic lesions was inversely correlated to tylosin treatment (r = −0.95; P < 0.01) and correlated positively to epithelial permeability (r = 0.87; P < 0.01), growth of mucolytic bacteria (r = 0.91; P < 0.01), and C. perfringens colonization as determined by qPCR (r = 0.81; P < 0.01). The proportion of mucolytic bacteria was inversely correlated to tylosin treatment (r = −0.94; P < 0.01). C. perfringens growth was positively correlated to the proportion of mucolytic bacteria (r = 0.91; P < 0.01) and inversely related to tylosin treatment (r = −0.91; P < 0.01). Paracellular permeability was inversely correlated to tylosin administration (r = −0.91; P < 0.01). Inclusion of dietary pectin was positively correlated to paracellular permeability at day 20 (data not shown) but not when all days were analyzed together. Sampling day did not correlate significantly with any of the other variables.
DISCUSSION
The present study demonstrates that tylosin administration quantitatively decreased the proportion of mucolytic bacteria in general and of C. perfringens specifically and that these responses correlated with an improvement in pathophysiological indices of disease in a chick model of NE. The data further indicate that mucolysis can serve as an initiating step of C. perfringens virulence and point to a possible link between bacterial mucolysis by other commensal bacteria in the chick intestine and compromised barrier function.
The molecular ecological data indicate that microbial populations of tylosin-treated birds were more homogeneous than and dissimilar to those of birds not treated with tylosin. Similarly, previous culture-based studies demonstrated that the oral administration of antibiotics causes a shift in intestinal microbial populations (3, 27). The effects of tylosin on microbial profiles diminished over the course of this study. Therefore, the tylosin-induced changes in community composition may reflect an overgrowth of gram-positive bacteria or the replacement of antibiotic-susceptible strains with resistant organisms (2, 23, 26). Indeed, the alteration of microbial profiles in response to tylosin reflects to a significant extent the differential presence of L. gasseri. Similar results have been demonstrated via PCR-DGGE and culture-based analyses by Knarreborg et al. (15) whereby they determined that lactobacilli and C. perfringens bacteria were the bacterial groups most strongly affected by antibiotic administration. The apparent inverse relationship between C. perfringens and L. gasseri may indicate a competitive interaction between these bacteria. Lactobacillus species appear to be particularly resistant to antibiotic administration (9). Also, L. gasseri has been shown to competitively exclude pathogenic bacteria via the production of bacteriocins and its ability to compete for limiting nutrients (14, 35, 42). Additional evidence of a competitive interaction between C. perfringens and L. gasseri would indicate that tylosin might further benefit the host by selecting for L. gasseri growth.
The mechanisms by which coccidiosis and the inclusion of NSP in the diet independently enhance the growth of C. perfringens and predispose to NE are not clear. Intriguingly, they both appear to increase intestinal mucus production (10, 16, 33, 39). We observed a positive correlation between the growth of mucolytic bacteria and (i) the growth of C. perfringens, (ii) the occurrence of necrotic lesions, and (iii) decreased intestinal epithelial barrier function. Moreover, the high percentage of mucolytic bacteria, particularly in the ileum, suggests that they might contribute to the onset of disease. Indeed, the proportion of mucolytic bacteria was also reduced in tylosin-treated birds at day 24, when C. perfringens was no longer detectable by qPCR, and this effect correlated with the preservation of intestinal barrier function. This result (combined with evidence that the chick small intestine is colonized by a potentially diverse population of mucolytic bacteria) demonstrates the crucial importance of phylogenetically identifying and studying these bacteria. Although the present data do not reveal the relative diversity of mucolytic bacteria in the chick intestine, the apparent prevalence of this activity indicates the importance of gaining this information. In comparisons of the diversity of mucolytic bacteria in the ileum of neonatal piglets as affected by enteral versus parenteral nutrition, Deplancke et al. (7) observed a wide range of 16S rDNA PCR-DGGE bands from mucin enrichment plates that were inoculated with mixed MAB samples. Therefore, the ability to use mucus as a nutrient source may be widespread among gut bacteria, at least in chicks and neonatal piglets, and may be a characteristic of species not traditionally associated with mucolysis. Intriguingly, Deplancke et al. (7) also found that C. perfringens was indeed differentially selected in parenterally fed piglets, which led us to initially explore the mucolytic potential of this opportunistic pathogen.
We propose (on the basis of the positive correlation between the presence of mucolytic bacteria and C. perfringens and the onset of NE as affected by the presence of tylosin) that conditions that result in increased mucus production by the host can select for mucolytic bacteria and thereby serve as an initiating step in NE pathogenesis. This hypothesis is supported by evidence that distinct intestinal insults (such as coccidiosis and highly viscous dietary feedstuffs that predispose to NE) commonly result in an increase in host mucus production. Because C. perfringens may be particularly mucolytic (7), its growth would be favored by a proinflammatory-mediated increase in mucus production. Furthermore, the enhanced fluid secretion and rate of passage of digesta that characterize intestinal inflammatory responses might select for bacteria capable of rapid growth, such as C. perfringens (8- to 10-min generation time) (5). Together, these responses would enable C. perfringens to outgrow other nonmucolytic commensals. The enzymatic basis of mucolysis by C. perfringens will need to be defined before this hypothesis can be evaluated fully.
In summary, the present data demonstrate that tylosin decreases C. perfringens colonization early in chick development and can thereby decrease the occurrence of NE during this critical period. The data also support the hypothesis that intestinal conditions that favor enhanced mucus production can provide a selective advantage for C. perfringens. Decreasing mucolysis-mediated C. perfringens growth may prove to be beneficial for minimizing the onset of NE in birds. Furthermore, while the threat of C. perfringens infection diminishes with age, tylosin continues to suppress the endogenous mucolytic microbiota and decreases the occurrence of NE. This suggests the need to identify the extent and distribution of mucolytic bacteria within the intestinal microbiota as well as the molecular basis for this potentially pathogenic process.
Acknowledgments
We acknowledge Ceese Kwakernaak for his valuable assistance with the chick NE model and thank Roderick Mackie, Tom Shyrock, and Erwin Zoetendal for their review of the manuscript.
This work was funded in part by Elanco Animal Health.
REFERENCES
- 1.Al-Sheikhly, F., and A. Al-Saieg. 1980. Role of Coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 24:324-333. [PubMed] [Google Scholar]
- 2.Baquero, F., J. F. Barrett, P. Courvalin, I. Morrissey, L. Piddock, and W. J. Novick. 1998. Epidemiology and mechanisms of resistance among respiratory tract pathogens. Clin. Microbiol. Infect. 4(Suppl. 2):S19-S26. [PubMed] [Google Scholar]
- 3.Berg, R. D. 1981. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect. Immun. 33:854-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan, J., G. Moore, S. E. Poe, A. Zimmermann, G. Vessie, D. A. Barnum, and J. Wilson. 2001. Efficacy of in-feed tylosin phosphate for the treatment of necrotic enteritis in broiler chickens. Poult. Sci. 80:1451-1454. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, B. T. 1984. Tests of small intestinal permeability in clinical practice. J. Clin. Gastroenterol. 6:499-500. [PubMed] [Google Scholar]
- 6.Craven, S. E., N. J. Stern, J. S. Bailey, and N. A. Cox. 2001. Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing. Avian Dis. 45:887-896. [PubMed] [Google Scholar]
- 7.Deplancke, B., O. Vidal, D. Ganessunker, S. M. Donovan, R. I. Mackie, and H. R. Gaskins. 2002. Selective growth of mucolytic bacteria including Clostridium perfringens in a neonatal piglet model of total parenteral nutrition. Am. J. Clin. Nutr. 76:1117-1125. [DOI] [PubMed] [Google Scholar]
- 8.European Commission. May 2001, posting date. 2nd opinion on anti-microbial resistance. [Online.] European Commission, Brussels, Belgium. http://europa.eu.int/comm/food/fs/sc/ssc/out203_en.pdf.
- 9.Felten, A., C. Barreau, C. Bizet, P. H. Lagrange, and A. Philippon. 1999. Lactobacillus species identification, H2O2 production, and antibiotic resistance and correlation with human clinical status. J. Clin. Microbiol. 37:729-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ficken, M. D., and D. P. Wages. 1997. Necrotic enteritis, p. 261-264. In B. W. Calnek (ed.), Diseases of poultry, 10th ed. Mosby-Wolfe, London, United Kingdom.
- 11.Gillan, D. C., A. G. C. L. Speksnijder, G. Zwart, and C. Deridder. 1998. Genetic diversity of the biofilm covering Montacuta ferruginosa (Mollusca, Bivalvia) as evaluated by denaturing gradient gel electrophoresis analysis and cloning of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 64:3464-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, J., and W. M. Reid. 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 28:30-36. [DOI] [PubMed] [Google Scholar]
- 13.Juby, L. D., A. J. Rothwell, and A. T. R. Axon. 1989. Lactulose/mannitol test: an ideal screen for celiac disease. Gastroenterology 96:79-85. [DOI] [PubMed] [Google Scholar]
- 14.Kawai, Y., T. Saito, H. Kitazawa, and T. Itoh. 1998. Gassericin A; an uncommon cyclic bacteriocin produced by Lactobacillus gasseri LA39 linked at N- and C-terminal ends. Biosci. Biotechnol. Biochem. 62:2438-2440. [DOI] [PubMed] [Google Scholar]
- 15.Knarreborg, A., M. A. Simon, R. M. Engberg, B. B. Jensen, and G. W. Tannock. 2002. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 68:5918-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langhout, D. J. 1998. The role of the intestinal flora as affected by non-starch polysaccharides in broiler chicks. Ph.D. thesis. Wageningen Agricultural University, Wageningen, The Netherlands.
- 17.Leser, T. D., R. H. Lindecrona, T. K. Jensen, B. B. Jensen, and K. Møller. 2000. Changes in bacterial community structure in the colon of pigs fed different experimental diets and after infection with Brachyspira hyodysenteriae. Appl. Environ. Microbiol. 66:3290-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magurran, A. 1988. Ecological diversity and its measurement, p. 8-45. Princeton University Press, Princeton, N.J.
- 19.McDonel, J. L. 1980. Clostridium perfringens toxins (type A, B, C, D, E). Pharmacol. Ther. 10:617-655. [DOI] [PubMed] [Google Scholar]
- 20.Murray, A. E., J. T. Hollibaugh, and C. Orrego. 1996. Phylogenetic compositions of bacterioplankton from two California estuaries compared by denaturing gradient gel electrophoresis of 16S rDNA fragments. Appl. Environ. Microbiol. 62:2676-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muyzer, G., and K. Smalla. 1998. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 73:127-141. [DOI] [PubMed] [Google Scholar]
- 22.Muyzer, G., T. Brinkhoff, U. Nübel, C. Santegoeds, H. Schäfer, and C. Wawer. 1998. Denaturant gradient gel electrophoresis in microbial ecology. p. 1-27. In A. Akkermans, J. D. van Elsas, and F. de Bruijn (ed.), Molecular microbial ecology manual, vol. 3.4.4. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 23.Onishi, H. R., D. R. Daoust, S. B. Zimmerman, D. Hendlin, and E. O. Stapley. 1974. Cefoxitin, a semisynthetic cephamycin antibiotic: resistance to beta-lactamase inactivation. Antimicrob. Agents Chemother. 5:38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riddell, C., and X. M. Kong. 1992. The influence of diet on necrotic enteritis in broiler chickens. Avian Dis. 36:499-503. [PubMed] [Google Scholar]
- 25.Roberton, A. M., and R. A. Stanley. 1982. In vitro utilization of mucin by Bacteroides fragilis. Appl. Environ. Microbiol. 43:325-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salyers, A. A., and N. B. Shoemaker. 1996. Resistance gene transfer in anaerobes: new insights, new problems. Clin. Infect. Dis. 23(Suppl. 1):S36-S43. [DOI] [PubMed] [Google Scholar]
- 27.Savage, D. C., and R. Dubos. 1968. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J. Exp. Med. 128:97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scala, D. J., and L. J. Kerkhof. 2000. Horizontal heterogeneity of denitrifying bacterial communities in marine sediments by terminal restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 66:1980-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shane, S. M., D. G. Koetting, and K. S. Harrington. 1984. The occurrence of Clostridium perfringens in the intestine of chicks. Avian Dis. 28:1120-1124. [PubMed] [Google Scholar]
- 30.Shoemaker, S. P., and M. D. Pierson. 1976. “Phoenix phenomenon” in the growth of Clostridium perfringens. Appl. Environ. Microbiol. 32:803-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson, J. M., V. J. McCracken, B. A. White, H. R. Gaskins, and R. I. Mackie. 1999. Application of denaturant gradient gel electrophoresis for the analysis of the porcine gastrointestinal microbiota. J. Microbiol. Methods 36:167-179. [DOI] [PubMed] [Google Scholar]
- 32.Simpson, J. M., V. J. McCracken, H. R. Gaskins, and R. I. Mackie. 2000. Denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA amplicons to monitor changes in fecal bacterial populations of weaning pigs following introduction of Lactobacillus reuteri strain MM53. Appl. Environ. Microbiol. 66:4705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smits, C. H. M. 1996. Viscosity of dietary fibre in relation to lipid digestibility in broiler chickens. PhD thesis. Wageningen Agricultural University, Wageningen, The Netherlands.
- 34.Sneath, P. H., and R. R. Sokal. 1973. Numerical taxonomy: the principles and practice of numerical classification. W. H. Freeman & Company, San Francisco, Calif.
- 35.Tahara, T., S. Yoshioka, R. Utsumi, and K. Kanatani. 1997. Isolation and partial characterization of bacteriocins produced by Lactobacillus gasseri JCM 2124. FEMS Microbiol. Lett. 148:97-100. [DOI] [PubMed] [Google Scholar]
- 36.Truscott, R. B., and F. Al-Sheikhly. 1977. Reproduction and treatment of necrotic enteritis in broilers. Am. J. Vet. Res. 38:857-861. [PubMed] [Google Scholar]
- 37.Tsai, Y. L., and B. H. Olson. 1992. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl. Environ. Microbiol. 58:2292-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Klis, J. D., and A. J. M. Jansman. 2002. Optimising nutrient digestion, absorption and gut barrier function in monogastrics: reality or illusion?, p. 15-36. In M. C. Blok, H. A. Vahl, L. de Lange, A. E. van de Braak, G. Hemke, and M. Hessing (ed.), Nutrition and health of the gastrointestinal tract. Wageningen Academic Publishers, Wageningen, The Netherlands.
- 39.Wagner, D. D., and O. P. Thomas. 1978. Influence of diets containing rye or pectin on the intestinal flora of chicks. Poult. Sci. 57:971-975. [DOI] [PubMed] [Google Scholar]
- 40.Wang, R. F., W. W. Cao, W. Franklin, W. Campbell, and C. E. Cerniglia. 1994. A 16S rDNA-based PCR method for rapid and specific detection of Clostridium perfringens in food. Mol. Cell. Probes 8:131-137. [DOI] [PubMed] [Google Scholar]
- 41.Watkins, K. L., T. R. Shryock, R. N. Dearth, and Y. M. Saif. 1997. In-vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin. Vet. Microbiol. 54:195-200. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, W. M., W. Liu, and D. Q. Wu. 2000. Isolation and characterization of a new bacteriocin from Lactobacillus gasseri KT7. J. Appl. Microbiol. 88:877-886. [DOI] [PubMed] [Google Scholar]