Abstract
We implemented a simple, sensitive, objective, and rapid cellular assay to reveal the antifungal activity of a novel class of glucan synthase inhibitors. The assay, especially useful for early drug discovery, measures the transformation of Candida albicans from the yeast form to the hyphal form. Test compounds were ranked by potency (50% inhibitory concentration) and efficacy (percent inhibition of germ tube formation); the intra-assay coefficients of variation for these parameters were 17 and 5%, respectively. The germ tube formation assay proved useful for the early-stage antifungal characterization of a novel class of glucan synthase inhibitors discovered at Pharmacia. Drug concentrations required in this assay to inhibit germ tube formation were lower for 90% of the novel compounds than the concentrations required to determine MICs. The method may have utility for other mechanistic classes of antifungal compounds during the hit-to-lead transition of drug discovery.
Perceptive selection of chemistry templates from among the screening hits of biochemical assays is critical to success during the hit-to-lead transition of drug discovery. Particularly desirable is a demonstration of biological activity for screening hits (and related analogs) in the target cell or organism. Heavy reliance is often placed on MIC assays employing the National Committee for Clinical Laboratory Standards (NCCLS) methodology for the evaluation of potential antifungal drugs (8); however, this is a stringent test devised and optimized for antifungal agents with proven clinical activity (3). We discovered a novel class of glucan synthase inhibitors, many of which did not yield a defined MIC, resulting in incomplete data for assessing structure-activity relationships. The need for a more inclusive biological data set on this class of compounds provoked our implementation of a simple and quantitative germ tube formation assay.
The rationale for the germ tube formation assay with Candida albicans was distilled from an extensive literature. C. albicans is a dimorphic fungus, capable of growth in a yeast or filamentous form. The yeast-to-hyphal transition begins with the formation of a germ tube, the initial stage of hyphal formation, making this transition a potentially useful end point for an antifungal drug assay (9). The end point could be particularly relevant for glucan synthase inhibitors, since there is extensive synthesis of cell wall during the formation of hyphae (7, 10). Indeed, Hawser and Islam (6) first demonstrated that inhibition of germ tube formation could be more sensitive to drug intervention than inhibition of growth, permitting the assessment of nonidealized antifungal drugs. The adherence of C. albicans germ tubes to plastic provides for the simple removal of yeast cells and objective quantitation of germ tubes via cellular staining (1, 12). These observations provide a compelling basis for the germ tube formation assay. We set out to implement a cell-based assay that would facilitate the biological evaluation of compounds during the hit-to-lead transition of drug discovery. This paper describes our use of a facile colorimetric assay for the objective measurement of hyphal growth in C. albicans.
MATERIALS AND METHODS
Strains and media.
C. albicans ATCC strain 31711 was used in this study. Cells were maintained on YPD agar plates, and fresh streaks were made monthly from −80°C freezer stocks. YPD contains 2% Bacto Peptone (Difco, Detroit, Mich.), 1% Bacto yeast extract (Difco), and 2% d-glucose (Mallinckrodt, Paris, Ky.) per liter of water. RPMI 1640 medium with l-glutamine (Gibco BRL, Rockville, Md.) was used in the germ tube formation assay.
Antifungal agents and chemicals.
Caspofungin acetate was purchased as the formulated therapeutic agent, Cancidas. Cilofungin was obtained from Eli Lilly. Fluconazole, ketoconazole, and amphotericin B were purchased from Sigma Chemical Co. (St. Louis, Mo.). All remaining compounds were synthesized at Pharmacia Corporation. Compounds were dissolved to a concentration of 20 mM in 100% dimethyl sulfoxide (Sigma) until needed with the exception of caspofungin, which was made up in 1× phosphate-buffered saline (Gibco BRL) at a concentration of 10 mg/ml. All compounds were stored at −20°C until needed.
Candida growth conditions.
A colony of C. albicans ATCC 31711 was used to inoculate 5 ml of YPD in a 14-ml Falcon tube (Becton Dickinson, Lincoln Park, N.J.). To obtain a saturated culture containing synchronized cells, the culture was incubated for at least 48 h at 25°C without shaking. Hawser et al. (5) have shown that these culture conditions facilitate complete conversion to germ tubes. The culture was centrifuged for 5 min to pellet the cells, and the pellet was then resuspended in 10 ml of RPMI 1640 medium. Cells were diluted to a concentration of 2 × 106 cells/ml in RPMI 1640 medium, and three 50-μl aliquots were dispensed into a 96-well flat-bottom polystyrene plate (Costar 3595; Corning Life Sciences, Corning, N.Y.). Compounds were freshly prepared by diluting the test compounds into RPMI 1640 medium at concentrations of 0 to 400 μM with dimethyl sulfoxide at a final concentration of 2.5%. Three 50-μl aliquots were dispensed into three wells on a 96-well plate for each compound at each concentration. After the plate was briefly shaken on a Mini-Orbital Shaker (Bellco Biotechnology, Vineland, N.J.), the plate was placed in a 37°C incubator (Queue cell culture incubator; Queue Systems Inc., Packersburg, W.Va.) for 4 h without shaking.
Crystal violet staining of C. albicans.
Plates were removed from the incubator and processed by the procedure of Abe et al. (1). The medium in the plates was discarded by inverting the plates. The C. albicans cells were washed once by immersion in 70% ethanol, which was discarded, and then 200 μl of 0.25% sodium dodecyl sulfate (SDS) (Gibco BRL) was added to each well. The SDS was discarded, and the plates were washed three times by immersion in distilled water. Germ tubes attached to the wells were stained for 15 min with 100 μl of 0.02% crystal violet (Sigma) dissolved in phosphate-buffered saline. The crystal violet stock solution was filtered through a 0.22-μm-pore-size filter prior to use to remove precipitated dye particles. The dye solution was removed by inverting the plates, and the plates were washed three times with water, once with 0.25% SDS, and twice more with water. After the plates were dried, 200 μl of isopropanol (EM Science, VWR International) containing 0.04 N HCl (EM Science) and 50 μl of 0.25% SDS were added to the wells and mixed briefly on the orbital shaker. The absorbance at 590 nm was determined by using a SpectramaxPlus plate spectrophotometer and SoftMax Pro version 2.4.1 software (Molecular Devices, Sunnyvale, Calif.). Fifty percent inhibitory concentrations (IC50s) were determined by using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, Calif.). Efficacy values were calculated by using the following equation: [1 − (curve minimum/curve maximum)] × 100.
MIC determinations.
MICs using the NCCLS protocol were determined against a panel of fungi including several Candida and Aspergillus species and strains.
RESULTS
During assay development, several critical parameters were identified, including growth conditions for cells in the yeast form, cell inoculum density, and staining conditions. RPMI 1640 medium with l-glutamine was chosen for the assay, because it effectively induces germ tube formation (1) and is readily available commercially. In addition, the NCCLS recommends RPMI 1640 for MIC determinations. Culturing C. albicans in polystyrene microplates resulted in germ tube attachment to the surface of each well, in agreement with previous reports (1, 12). Incubation times of up to 24 h were examined, and results were qualitatively identical at all time points. We desired a rapid assay and elected a 4-h incubation time at 37°C. We selected this temperature for two reasons. First, temperature is known to be a powerful inducer of hyphal growth (9). Second, 37°C is the body temperature of the human host, and this experimental condition would help simulate responses of the organism in the host environment.
The use of synchronized, stationary yeast cells is critical for obtaining germ tube induction frequencies of greater than 99%, as first shown by Hawser et al. (5). Synchronized C. albicans cells were obtained by culturing in YPD at 25°C for a minimum of 48 h without agitation. Our experiments confirmed the observations of Hawser et al., as we achieved germ tubes on greater than 99% of untreated cells in all experiments. We determined that an inoculum of 106 cells/ml consistently yielded optical densities at 450 nm of 0.3 to 0.4 (data not shown). Finally, we found that the reported staining conditions of 0.02% crystal violet for 15 min (1) were suitable for this application. One observation we did make was the necessity of filtering the prepared crystal violet stock solution through a 0.22-μm-pore-size filter. Even at a concentration of 0.02%, some of the dye remains in a crystalline state. Without filtration, these crystals occasionally were transferred into the wells and significantly altered the absorbance readout.
Definition of the processing parameters permitted the initiation of validation experiments with known antifungal agents. Figure 1A shows the effect of two known glucan synthase inhibitors, cilofungin and caspofungin, on germ tube formation. These two echinocandins inhibit the yeast-to-hyphal transformation at very low compound concentrations with IC50s of 0.27 μM (0.28 μg/ml) and 0.24 μM (0.29 μg/ml), respectively. The value for cilofungin is much lower than reported values of 10 to 12 μg/ml for other echinocandins (5, 6). This difference may be due to several factors, including differences in the strain of C. albicans used, incubation time, cell inoculum, growth medium, and method for end point measurement.
FIG. 1.
Dose-response curves illustrating the IC50s obtained in the germ tube formation assay. (A) Values for known glucan synthase inhibitors, cilofungin (♦) and caspofungin (▪). (B) Values for compounds in a novel class of glucan synthase inhibitors, PNU143678E (♦), PNU144159E (▪), and PNU271965E (•).
Figure 1B shows the dose-response curves for three Pharmacia compounds from a novel class of glucan synthase inhibitors (PNU compounds). The compounds used for these studies are shown in Fig. 2. IC50s of 13.1 μM (5.5 μg/ml), 1.8 μM (0.9 μg/ml), and 2.46 μM (1.4 μg/ml) were obtained for PNU143678E, PNU144159E, and PNU271965E, respectively.
FIG. 2.
Structures of several Pharmacia (PNU) compounds identified as glucan synthase inhibitors.
Additional reference compounds, such as amphotericin B, fluconazole, ketoconazole, and voriconazole, were also tested in the assay (data not shown). An IC50 of 0.157 μg/ml (0.17 μM) was obtained for amphotericin B, and this value agrees well with literature values (5, 13). No inhibition of germ tube formation was seen with any of the azoles at doses of <100 μM (0 to 64 μg/ml). While others have reported that these azoles can inhibit germ tube formation at concentrations of 4 to 10 μg/ml (5, 13), those observations were made using entirely different growth parameters, media, and strains of C. albicans.
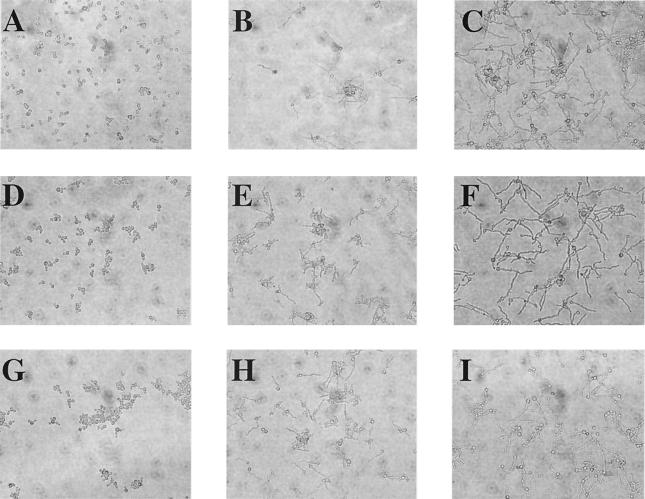
An important aspect of our validation of the methodology was the correlation of morphological changes with absorbance measurements. Microscopic observation of cells throughout these validation studies and many subsequent experiments with the novel class of glucan synthase inhibitors verified that the absorbance readings were an accurate representation of the incidence of germ tubes. Figure 3 shows micrographs of C. albicans cultures after treatment with glucan synthase inhibitors. All three compounds show dose-dependent inhibition of the yeast-to-hyphal transition.
FIG. 3.
Unstained C. albicans cells at ×20 magnification after treatment with glucan synthase inhibitors. (A to C) Caspofungin treatment at 0.31, 0.08, and 0 μM, respectively; (D to F) PNU143678E treatment at 100, 25, and 0 μM, respectively; (G to I) PNU144159E treatment at 12, 1.6, and 0 μM, respectively.
Reproducibility between assays is an essential requirement of any method used to study structure-activity relationships over time. Figure 4 shows the variation in IC50s and efficacy values for a subset of the novel class of glucan synthase inhibitors evaluated on multiple occasions in the germ tube inhibition assay. The data show that the consistency of calculated values for each parameter between assays was acceptable. The average intra-assay coefficients of variation for IC50 and efficacy estimates were 17 and 5%, respectively. These values can be placed into perspective by comparing them to the observed coefficients of variation for MIC assays at Pharmacia. Historical company data show that the average interassay coefficient of variation for amphotericin B and ketoconazole MICs with two strains of C. albicans was 17%.
FIG. 4.
Reproducibility of estimates for IC50 and efficacy for a subset of 11 compounds from a novel class of glucan synthase inhibitors evaluated on multiple occasions in the germ tube formation assay. Each triangle on the plots is the calculated value from a curve-fitting analysis of the dose-response curve. The average interassay coefficients of variation for IC50 and efficacy estimates were 17 and 5%, respectively.
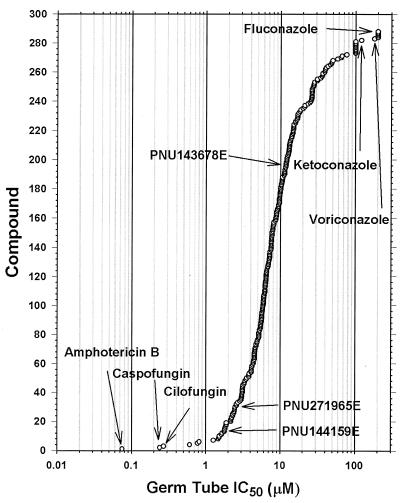
After the assay was validated with reference antifungal compounds, 475 PNU compounds from the novel class of glucan synthase inhibitors were assayed. Initially, a response curve at four drug concentrations (0 to 40 μM) was used to identify compounds with activity in the assay. The 288 active compounds were then analyzed in the full dose-response experiment described in Materials and Methods (11 drug concentrations). Figure 5 shows the IC50s determined for the active compounds tested over the full range of drug concentrations (0 to 200 μM). The echinocandins caspofungin and cilofungin are more potent inhibitors of germ tube formation than the low-molecular-weight glucan synthase inhibitors. Amphotericin B was the most potent compound, while fluconazole, ketoconazole, and voriconazole are among the least active antifungal agents in this assay.
FIG. 5.
IC50s for 288 compounds in the germ tube formation assay. The values for reference compounds (amphotericin B, caspofungin, cilofungin, fluconazole, ketoconazole, and voriconazole) are indicated by the arrows. The remaining compounds are from a novel class of glucan synthase inhibitors.
Efficacy values were calculated for 272 compounds, and they are plotted in Fig. 6. Compound efficacy was calculated using the maximum and minimum curve fit values for the IC50 curve using the following equation: [1 − (curve minimum/curve maximum)] × 100. The efficacy values obtained gave a measure of the extent to which each compound inhibited germ tube formation. Amphotericin B, despite having a low IC50 for inhibition of hyphal growth, is not among the most efficacious compounds in this assay (efficacy of 69%). Cilofungin was very efficacious (95%) at inhibiting germ tube formation, while caspofungin was less so (79%). Many of the low-molecular-weight glucan synthase inhibitors demonstrate an efficacy comparable to those of the echinocandins in the germ tube formation assay.
FIG. 6.
Efficacy values for inhibition of germ tube formation (n = 273 active compounds). The values for reference compounds (amphotericin B, caspofungin, and cilofungin) are indicated by arrows. The remaining compounds are from a novel class of glucan synthase inhibitors.
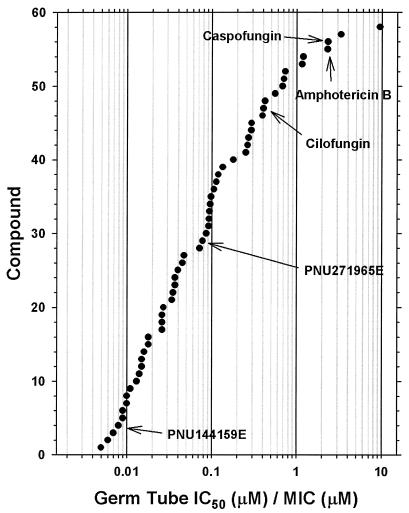
A prime objective in developing this assay was to provide a sensitive method of demonstrating whole-cell antifungal activity for a novel class of antifungal compounds. A total of 144 novel glucan synthase inhibitors were tested in both the MIC and full-dose germ tube inhibition assays. Only 39% of the compounds (56 of 144) yielded a definitive MIC for C. albicans ATCC 31711, whereas 140 of 144 (97%) gave defined IC50s in the germ tube inhibition assay with the same strain. Clearly, the germ tube inhibition assay allows the characterization of antifungal activity for a considerably larger proportion of compounds during the hit-to-lead transition of early-stage drug discovery. Figure 7 provides a further illustration of assay sensitivity for those compounds with both defined MICs and germ tube inhibition values. The ratio of germ tube IC50/MIC for all but six of these compounds was <1, showing that the quantity of drug required to inhibit germ tube formation by 50% was less than that inhibiting growth. Inhibition of germ tube formation is a more sensitive whole-cell end point than fungal growth when evaluating a novel class of nonidealized glucan synthase inhibitors.
FIG. 7.
The ratios for inhibition of germ tube formation (IC50) to MIC are plotted for the compounds showing activity in both assays (n = 58). Values for reference compounds amphotericin B, caspofungin, and cilofungin are indicated by arrows. The remaining compounds are from a novel class of glucan synthase inhibitors.
As an example of the utility of the germ tube assay in early drug discovery, compare the two PNU compounds, PNU143678E and PNU271965E. PNU143678E was an early hit in the glucan synthase inhibition assay. As shown in Fig. 5, PNU143678E does exhibit inhibitory activity in the germ tube assay. However, the compound did not have activity in more-stringent whole-cell assays. PNU143678E showed no antiproliferative activity or fungicidal activity at concentrations up to 200 μM, nor did it yield a NCCLS MIC for C. albicans (ATCC 90028 and ATCC 76485), Candida glabrata (ATCC 90030), Candida parapsilosis (ATCC 22019), Aspergillus fumigatus (ATCC 96918 and ATCC 64026), or Aspergillus terreus (ATCC 64029), although an MIC of 32 μg/ml was obtained for Candida krusei (ATCC 14243) with PNU143678E. In contrast, PNU271965E, a later-stage analog that also inhibited glucan synthase, showed increased potency in the germ tube assay as well as antiproliferative and fungicidal activity against C. albicans, C. krusei, and Aspergillus fumigatus. In addition, improved MIC activities were obtained against all fungal strains tested (MICs of 8 μg/ml for C. albicans ATCC 90028, 32 μg/ml for C. albicans ATCC 76485, 16 μg/ml for C. glabrata ATCC 90030, 0.25 μg/ml for C. krusei ATCC 14243, 32 μg/ml for C. parapsilosis ATCC 22019, 4 μg/ml for A. fumigatus ATCC 96918, 4 μg/ml for A. fumigatus ATCC 64026, and 32 μg/ml for Aspergillus terreus ATCC 64029). Although PNU271965E lacks the potency required for a clinical candidate, it illustrates the feasibility of using the germ tube assay to select compounds with improved characteristics within a chemical family during the early drug discovery process.
DISCUSSION
Inhibition of hyphal growth is an end point for an antifungal drug assay, especially during early drug discovery (2, 4, 9, 11). Inhibition of germ tube formation can be more sensitive to drug intervention than inhibition of growth, permitting the assessment of nonidealized antifungal drugs (6). The adherence of germ tubes to plastic provides for the simple separation of cells with divergent morphologies and objective quantitation via cellular staining (1, 12). The end point is particularly relevant for glucan synthesis inhibitors, since the activation of glucan synthase is associated with the extensive synthesis of cell wall during the formation of hyphae (7, 10). These observations provide a compelling basis for the germ tube assay.
We were able to develop a simple, objective, and quantitative assay to measure the yeast-to-hyphal transition. The use of synchronized cells, RPMI 1640 medium, and plastic labware was likely responsible for the uniform cellular response described here (5). Crystal violet staining and a spectrophotometric quantitation eliminated the subjectivity and tediousness of visual scoring. The simplicity of the assay in conjunction with a 96-well assay format enhanced its capacity, permitting the evaluation of more compounds each week, and rapid turnaround of data for the medicinal chemist. Data are generated after 4 h as opposed to the 24 to 72 h required for other whole-cell assays, such as proliferation and MIC determinations. Most importantly, dose-response curves with replicates could be performed, giving us the opportunity to fully characterize responses and perform statistical analyses on multiple end points. We could calculate potencies (IC50s), determine efficacy, and evaluate slopes of the fitted curves of each compound for germ tube inhibition. The excellent day-to-day reproducibility in these responses facilitated comparisons between compounds synthesized at different times during the project.
Amphotericin B and the known glucan synthase inhibitors, cilofungin and caspofungin, had IC50s in the assay of 0.17, 0.27, and 0.24 μM, respectively. In contrast, fluconazole, ketoconazole, and voriconazole had minimal activity in this assay at concentrations of >100 μM. The sensitivity of the method may allow the demonstration of biological activity for compounds that are inactive in other whole-cell assays. The utility of the germ tube inhibition assay was amply demonstrated through our characterization of the novel class of glucan synthase inhibitors, but it may not be appropriate for all mechanistic classes of antifungal agents, as demonstrated by the azoles. Clearly, the germ tube assay was more sensitive, allowing the determination of a definitive IC50 for most of the compounds, as contrasted to the determination of a MIC for approximately one-third of the same compounds. Unfortunately, none of the novel glucan synthase inhibitors evaluated during our studies were as potent as the known echinocandin inhibitors of glucan synthase for inhibition of germ tube formation.
Acknowledgments
We thank Sara Morin and Debora Sweeney for the MIC data and the medicinal chemists involved in the project.
REFERENCES
- 1.Abe, S., T. Satoh, Y. Tokuda, S. Tansho, and H. Yamaguchi. 1994. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol. Immunol. 38:385-388. [DOI] [PubMed] [Google Scholar]
- 2.Brown, A. J. P., and N. A. R. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff, A., T. White, and M. A. Pfaller. 1999. Antifungal agents and susceptibility tests, p. 1640-1652. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 4.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279:1355-1358. [DOI] [PubMed] [Google Scholar]
- 5.Hawser, S., M. Francolini, and K. Islam. 1996. The effects of antifungal agents on the morphogenetic transformation by Candida albicans in vitro. J. Antimicrob. Chemother. 38:579-587. [DOI] [PubMed] [Google Scholar]
- 6.Hawser, S., and K. Islam. 1999. Comparisons of the effects of fungicidal and fungistatic antifungal agents on the morphogenetic transformation of Candida albicans. J. Antimicrob. Chemother. 43:411-413. [DOI] [PubMed] [Google Scholar]
- 7.Hector, R. F. 1993. Compounds active against cell walls of medically important fungi. Clin. Microbiol. Rev. 6:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 1995. Reference method for broth dilution antifungal susceptibility testing of yeast: tentative standard M27-T. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Odds, F. C. 1985. Morphogenesis in Candida albicans. Crit. Rev. Microbiol. 12:45-93. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd, M. G. 1987. Cell envelope of Candida albicans. Crit. Rev. Microbiol. 15:7-25. [DOI] [PubMed] [Google Scholar]
- 11.Soll, D. R. 1997. Gene regulation during high-frequency switching in Candida albicans. Microbiology 143:279-288. [DOI] [PubMed] [Google Scholar]
- 12.Tronchin, G., J. P. Bouchara, R. Robert, and J. M. Senet. 1988. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect. Immun. 56:1987-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van't Wout, J. W., I. Meynaar, I. Linde, R. Poell, H. Mattie, and R. Van Furth. 1990. Effect of amphotericin B, fluconazole, and itraconazole on intracellular Candida albicans and germ tube development in macrophages. J. Antimicrob. Chemother. 25:803-811. [DOI] [PubMed] [Google Scholar]