Abstract
Weathering of the As-rich pyrite-rich tailings of the abandoned mining site of Carnoulès (southeastern France) results in the formation of acid waters heavily loaded with arsenic. Dissolved arsenic present in the seepage waters precipitates within a few meters from the bottom of the tailing dam in the presence of microorganisms. An Acidithiobacillus ferrooxidans strain, referred to as CC1, was isolated from the effluents. This strain was able to remove arsenic from a defined synthetic medium only when grown on ferrous iron. This A. ferrooxidans strain did not oxidize arsenite to arsenate directly or indirectly. Strain CC1 precipitated arsenic unexpectedly as arsenite but not arsenate, with ferric iron produced by its energy metabolism. Furthermore, arsenite was almost not found adsorbed on jarosite but associated with a poorly ordered schwertmannite. Arsenate is known to efficiently precipitate with ferric iron and sulfate in the form of more or less ordered schwertmannite, depending on the sulfur-to-arsenic ratio. Our data demonstrate that the coprecipitation of arsenite with schwertmannite also appears as a potential mechanism of arsenite removal in heavily contaminated acid waters. The removal of arsenite by coprecipitation with ferric iron appears to be a common property of the A. ferrooxidans species, as such a feature was observed with one private and three collection strains, one of which was the type strain.
Weathering of sulfide-rich rocks results in the formation of highly acidic and heavy metal-laden effluents. At the abandoned Pb-Zn mining site of Carnoulès (southeastern France), the pyrite-rich tailings are subject to bioleaching. Consequently, the Reigous spring, which collects the seepage waters from the waste materials, is acid (pH 3) and contains high levels of solubilized metals (Fe, Zn, and Pb) but also extremely high arsenic (As) concentrations (250 mg liter−1 on average) (26, 27). The extremely high contents of this metalloid are lowered by 2 to 3 orders of magnitude between the bottom of the tailing dam and few hundred meters downstream. The presence in the sediments of bacteria coated with Fe-As-rich material suggested that arsenic attenuation was due to precipitation mechanisms mediated by microorganisms (24, 25, 26, 27). Among them, Acidithiobacillus ferrooxidans was proposed to be involved in the removal of soluble arsenic (9, 26, 27, 35). The role of microorganisms in arsenic attenuation in this ecosystem was evaluated by two different approaches: (i) an A. ferrooxidans strain was isolated from the effluent and its role in arsenic oxidation and/or precipitation was determined (this paper); (ii) arsenite oxidizing bacteria, one of which belongs to the Thiomonas genus, were isolated and characterized (K. Duquesne, A. Yarzabal, J. Ratouchniak, D. Muller, D. Lièvremont, M-C. Lett, and V. Bonnefoy, unpublished results).
A. ferrooxidans is an acidophilic chemolithoautotrophic gram-negative bacterium commonly encountered in acid mine drainage. This bacterium is resistant to heavy metals and metalloids at concentrations in the milligram per liter range, which is considered toxic for other microorganisms (22, 44). In particular, bioleaching of arsenopyrite by A. ferrooxidans has already been described, suggesting that this bacterium is tolerant to arsenic (12, 16, 17, 32, 34, 45). Indeed, the arsenic resistance genes are present on the chromosome of at least four A. ferrooxidans strains (7). The arsenic resistance system detoxifies the cell by active arsenite extrusion from the cytoplasm, lowering the intracellular concentration of this metalloid (7, 10). This system includes an arsenite transmembrane efflux pump (ArsB) as well as an arsenate reductase enzyme which converts arsenate to arsenite (ArsC) (7, 10).
In this paper, we show that A. ferrooxidans is not merely tolerant to arsenic but is also involved in arsenic precipitation.
MATERIALS AND METHODS
Chemical analysis of field samples.
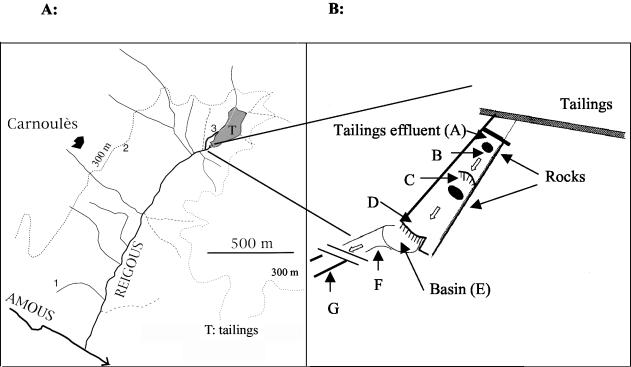
Three sampling stations were set in two standing pools receiving seepage waters from the quarries (stations 1 and 2) and one from the drainage channel of the tailings (station 3). Seven other sampling stations were located along the Reigous creek where no addition of seepage waters was detected, at 0, 2, 12, 29, 32, 38, and 120 m from the mine waste effluent outlet, corresponding to stations A to G, respectively (Fig. 1). Measurements of pH, total soluble iron (Fe), and total soluble arsenic (As), soluble arsenite as As(III), and ferrous iron as Fe(II) were made as previously described (9).
FIG. 1.
Schematic map of the Carnoulès acid mine drainage system, showing the locations of the different sampling points.
Strains and culture conditions.
A. ferrooxidans ATCC 33020, ATCC 19859, and ATCC 23270 strains were obtained from the American Type Culture Collection. The isolation of A. ferrooxidans strain BRGM1 was described previously (29). A. ferrooxidans was grown at 30°C with vigorous shaking on liquid Fe(II) or sulfur (S0) medium. S0 medium consisted of 1% (wt/vol) S0 and 25% (vol/vol) solution for basal salts containing (NH4)2SO4, 0.2 g/liter; KH2PO4, 3 g/liter; MgSO4 · 7H2O, 0.5 g/liter; and CaCl2 · 2H2O, 0.16 g/liter, adjusted to pH 3.5 with H2SO4. Fe(II) medium consisted of 3.5% (wt/vol) FeSO4 · 7H2O adjusted to pH 1.6 with H2SO4, and 25% (vol/vol) solution for basal salts containing (NH4)2SO4, 0.4g/liter; KH2PO4, 0.4 g/liter; MgSO4 · 7H2O, 0.4 g/liter; and trisodium citrate (C6H5Na3O7 · 2H2O), 0.3 g/liter, adjusted to pH 3 with H2SO4. Trisodium citrate was used to buffer the Fe(II) medium to avoid the pH increase which leads to ferric [Fe(III)] precipitates known to inhibit A. ferrooxidans growth. At the concentration used, which did not interfere with cell growth, the pH increased after 4 days of growth from 1.6 to 1.8, instead of 2.4 in the original medium, and consequently no visible iron precipitates were formed by that time (4).
As(III), Fe(II)-As(III), and S0-As(III) media consisted, respectively, of 25% (vol/vol) solution for basal salts (pH 3) and Fe(II) and S0 media supplemented with 200 mg of sodium arsenite/liter. A. ferrooxidans was detected and isolated on DOP solid medium, which was designed for acidophilic chemolithoautotrophic iron or thiosulfate oxidizers able to grow at pH 4.2 (28). DOP medium was prepared as follows: solution A (200 ml) consisting of basal salts containing (NH4)2SO4, 22.5 g/liter; KCl, 0.75 g/liter; and MgSO4 · 7H2O, 3.75 g/liter, adjusted to pH 4.6 with H2SO4; solution B (200 ml) was a nonacidified solution of agar (Pastagar B from Bio-Rad) (12.5 g/liter); solution C (4 ml), a thiosulfate solution (Na2S2O3 · 5H2O, 200 g/liter); solution D (3.2 ml), a ferrous sulfate solution (FeSO4 · 7H2O, 250 g/liter); solution E (0.8 ml), a 50 mM diaminopimelic acid solution; and solution F (2 ml), an 8-mg/ml leucine solution. Solutions were sterilized by filtration (D), autoclaving (A, B, E, and F), or three consecutive autoclavings (C). All solutions were mixed and dispensed into petri plates.
Microbial analysis of field samples and A. ferrooxidans strain isolation.
Water samples were collected from stations 1 to 3, A to C, and E and transported to the laboratory. An aliquot of each water sample was (i) spread on DOP medium and incubated at 30°C for 3 weeks and (ii) inoculated into Fe(II) medium, shaken for 5 to 7 days at 30°C. A rust-colored colony obtained on DOP medium with the sample from station C (Table 1) was inoculated into Fe(II) medium, grown for 5 days at 30°C, and again spread on DOP medium. This cycle was repeated once more. The isolated colony will be referred to as strain CC1 (Carnoulès, station C, sample 1).
TABLE 1.
Analysis of samples collected at the Carnoulès mine in September 2000
| Station | Chemical analyses
|
Microbiological analyses
|
|||||
|---|---|---|---|---|---|---|---|
| pH | Fe (mg/liter) | As (mg/liter) | Growth on Fe(II) medium | Colony color on DOP medium | Rusticyanin detected | rus gene amplified | |
| 1 | 1.93 | 1,100 | 24.4 | + | Rusty + white | + | + |
| 2 | 3.55 | 1 | <0.002 | − | White | − | − |
| 3 | 2.7 | 200 | 6.4 | + | Rusty + white | + | + |
| A | 2.88 | 1,650 | 298 | + | Rusty | + | + |
| B | 2.87 | 1,600 | 297 | + | Rusty | + | + |
| C | 2.93 | 1,600 | 277 | + | Rusty | + | + |
| D | 3.1 | 1,500 | 240 | ||||
| E | 3.31 | NDa | 190 | + | Rusty + white | + | + |
| F | 3.34 | 1,100 | 175 | ||||
| G | 3.15 | 800 | 75.7 | ||||
ND, not determined.
DNA analysis.
A. ferrooxidans genomic DNA was prepared with the NucleoSpin Tissue kit from Macherey-Nagel according to the manufacturer's instructions. The 465-bp fragment of the rusticyanin gene of A. ferrooxidans was amplified with the oligonucleotides RUSNM and RCX (29). Almost the entire 16S rRNA gene was amplified with oligonucleotides D1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and P2 (5′-ACGGCTACCTTGTTACGACTT-3′), derived from the fD1 and rP2 oligonucleotides designed for most eubacteria (47). The PCR product was cloned into the EcoRV site of the Bluescript SK+ vector from Stratagene. The intergenic sequence between the 16S rRNA and 23S rRNA genes was amplified with oligonucleotides 23-01 and 23-07, designed for thiobacilli (41). Sequences were determined by automated multicapillary electrophoresis at Genome Express (Meylan, France).
Phylogenetic analysis.
Essentially complete sequences of 16S rDNA were determined for strains CC1, ATCC 19859, and BRGM1. These sequences were aligned with the corresponding sequences of A. ferrooxidans type strain ATCC 23270 (accession number AJ278718) and ATCC 33020 (accession number AJ278719), Acidithiobacillus thiooxidans type strain ATCC 19377 (accession number Y11596), Acidithiobacillus caldus type strain DSM 8584 (accession number Z29975), and, as the outgroup, Escherichia coli K-12 (accession number EO5133). Phylogenetic analyses were performed by with the ClustalX program (43) with the nucleotides that were unambiguous. Bootstrap analysis of 100 data were carried out to determine the statistical confidence of the branching points in the tree. The intergenic region sequences between the 16S and 23S rDNA genes were determined for strains CC1, ATCC 19859, and BRGM1 and aligned with the corresponding sequences of A. ferrooxidans strains ATCC 33020 (accession number AJ278724) and ATCC 23270 (accession number AJ278725). A phylogenetic tree was constructed as described above with the unambiguous nucleotide sequences.
Chemical analysis of A. ferrooxidans cultures under laboratory conditions.
A. ferrooxidans was cultivated in Fe(II)-As(III), S0-As(III), or As(III) medium with vigorous shaking at 30°C. In each case, samples were removed at regular intervals and frozen until further analysis. Concentrations of dissolved As(III) and As were determined as described (9). The experiments were performed in triplicate from independent cultures. The variation between the values obtained in the three independent experiments never exceeded 5%. The Fe(II) concentration was determined by the o-phenanthroline method (36). Cellular growth on Fe(II) medium cannot be followed by densitometric reading because Fe(III) interferes. Therefore, as an estimate of cellular growth, the total protein content was determined. When necessary, 3% (vol/vol) chloroform was added to kill the cells. The efficiency of this treatment was checked by microscopic observation and by spreading on DOP plates. The Fe(II)-As(III) and S0-As(III) abiotic experiments were conducted in the same way as the biotic samples. The photochemical oxidation of As(III) by oxygen and iron at low pH was tested by shaking the Fe(II)-As(III) medium under white light (between 400 and 800 nm) at 50 μM photons m−2 s−1.
Mineralogical analyses.
The precipitates formed in Fe(II)-As(III) medium in abiotic conditions or after 5 days of A. ferrooxidans growth and in the abiotic Fe(III)-As(III) medium were collected by centrifugation at 13,000 rpm for 30 min and air dried at room temperature. These solid phases were characterized by powder X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and X-ray absorption near-edge structure spectroscopy (XANES). XRD data were collected with a Phillips PW1710 difractometer with Co-Kα radiation operating at 40 kV, 30 mA in step scan mode, between 3 and 120° 2θ with a 0.04° 2 θ step, and a counting time of 8 s per step. FTIR data were recorded with a Nicolet Magna 300 spectrometer. XANES data were recorded at the As-K edge at room temperature in transmission mode on the D44 bending-magnet beamline at the Laboratoire pour l'Utilisation du Rayonnement Électromagnétique (LURE, Orsay, France). A Si(511) double-crystal monochromator yielded an energy resolution of approximately 0.3 eV at the As-K edge (11,859 eV). Energy was calibrated with a double-transmission set-up, allowing simultaneous recording of the As-K edge spectrum of the samples and the Au-K edge spectrum of an Au foil.
The first “white-line” feature of the Au edge at 11,947 eV was used as the reference energy point. As K-XANES data were interpreted by comparison with model compound spectra, including scorodite (FeAsO4 · 2H2O), pharmacosiderite [KFe4(AsO4)3(OH)4 · 6-7H2O], beudantite [PbFe3(AsO4)(SO4)(OH)6], parasymplesite [Fe3(AsO4)2 · 8H2O], arsenolite (As2O3), and tooeleite [Fe6(AsO3)4(SO4)(OH)4 · 4H2O]. Our data set also includes synthetic amorphous Fe(III)-As(III) and Fe(III)-As(V) hydrous coprecipitates as described previously (35). A sorption sample containing 5% (wt/wt) As(III) on schwertmannite was also studied for comparison. The schwertmannite was prepared following the procedure of Barham (2) with hydrogen peroxide. This solid was rinsed three times with deionized water, and As(III) was adsorbed at pH 3 in sulfuric acid medium. The sample was recovered by centrifugation after 24 h of reaction.
Protein analysis.
Arsenite oxidase activity was determined as previously described (1). Briefly, reduction of 2,6-dichlorophenolindophenol (DCIP) (60 μM) in the presence of crude cell extracts was followed at 600 nm in 50 mM MES (2-[N-morpholino]ethanesulfonic acid, pH 6) containing 2 mM phenazine methosulfate. The assay was initiated by the addition of 250 μM sodium arsenite. The protein content was determined by the Bradford method (Bio-Rad protein assay) after hydrolysis of the cells in 0.4 N NaOH at 100°C for 10 min. Bovine serum albumin was used as the standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting were carried out as previously described (49).
Nucleotide sequence accession numbers.
The sequences of the 16S rDNA and of the intergenic sequence between the 16S and 23S rDNAs of strains CC1, BRGM1, and ATCC 19859 have been deposited in GenBank under accession numbers AJ457804 (16S, CC1), AJ457805 (intergenic sequence, CC1), AJ457806 (16S, BRGM1), AJ457807 (intergenic sequence, BRGM1), AJ457808 (16S, ATCC 19859), and AJ457809 (intergenic sequence, ATCC 19859).
RESULTS
Field investigations.
The pH and the concentrations of dissolved Fe and As in the samples collected at the different stations (Fig. 1 and Materials and Methods) are given in Table 1. The pH was low in all the samples analyzed (Table 1). Concentrations of dissolved Fe and As were high and ranged from 200 to 1,650 mg liter−1 and from 6.4 to 298 mg liter−1, respectively, in all the samples but sample 2 (Table 1).
Bacterial growth and iron oxidation were observed in Fe(II) liquid medium inoculated with samples 1, 3, A, B, C, and E (Table 1), suggesting the presence of iron oxidizers such as Leptospirillum ferrooxidans, L. ferriphilum, or Acidithiobacillus ferrooxidans. The presence of A. ferrooxidans in these samples was indicated by (i) the observation of rust-colored colonies, that is, iron oxidizers, on DOP plates (Table 1), the pH of which prevents the growth of leptospirilli (13, 38), (ii) detection by Western immunoassay experiments of rusticyanin, a protein involved in iron oxidation (23, 49) and described so far only in A. ferrooxidans (Table 1), and (iii) detection by PCR of the rus gene, encoding rusticyanin (Table 1). White colonies were also obtained on DOP plates spread with samples 1, 2, 3, and E (Table 1), suggesting the presence of thiosulfate oxidizers, like Acidithiobacillus thiooxidans and A. caldus or bacteria from the Thiomonas genus.
Isolation and characterization of an A. ferrooxidans strain from the Reigous spring.
A bacterium likely to belong to the A. ferrooxidans species, that is, an iron oxidizer tolerant to pH 4.2, was isolated from sample C after streaking twice on DOP solid medium. The isolated strain was identified by morphological, physiological, and molecular analyses.
Under a light microscope, the isolated colony, now referred to as strain CC1, was composed exclusively of motile, gram-negative rods, a morphology that is consistent with the Acidithiobacillus genus.
Strain CC1 was able to grow at pH 3.5 in liquid basal salts medium supplemented with either S0 or Fe(II) as the sole energy source and with no organic carbon source. Strain CC1 is therefore a chemolithoautotrophic microorganism thriving at low pH, presenting the same physiological properties as A. ferrooxidans.
Rusticyanin, a specific A. ferrooxidans protein, was detected by immunodetection (data not shown), and the corresponding gene, rus, was amplified by PCR from strain CC1 genomic DNA (data not shown).
The sequences of the 16S rDNA (1,440 nucleotides) and of the intergenic region between the 16S and 23S rDNAs (626 nucleotides) were determined. The 16S rDNA sequence of strain CC1 had almost 100% identity with that of strains BRGM1, ATCC 33020, and ATCC 19859 (data not shown). The phylogenetic analysis showed unambiguously that indeed, strain CC1 belongs to the A. ferrooxidans species. The phylogenetic tree deduced from the intergenic sequences was also consistent with this conclusion (data not shown).
Arsenic removal by A. ferrooxidans strain CC1.
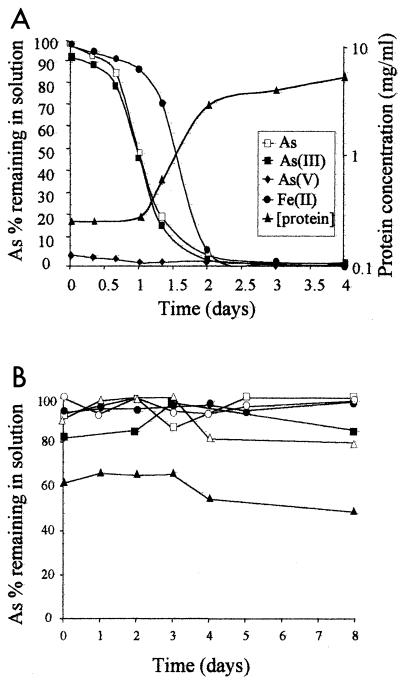
The concentrations of soluble As, As(III), and As(V) were determined during the course of growth of A. ferrooxidans strain CC1 in Fe(II)-As(III) medium. This medium was buffered to avoid a pH increase due to proton consumption during Fe(II) oxidation. The levels of dissolved As and As(III) decreased drastically after 2 days of growth, that is, during the exponential growth phase, while the level of dissolved As(V) was very low and did not change (Fig. 2A). In abiotic conditions, a small and immediate decrease in the concentration of dissolved As(III) but not of As was observed as soon as Fe(II) was added to the medium with or without light (Fig. 2B); this was likely due to the oxidation of As(III) in the presence of oxygen and Fe(II), as previously described (11, 15, 18, 21). The total and rapid removal of dissolved As and of As(III) seen in Fig. 2A was therefore due to the presence of A. ferrooxidans strain CC1 in the medium.
FIG. 2.
Arsenic removal in biotic (A) and abiotic (B) conditions. (A) Growth of CC1 in Fe(II)-As(III) conditions and changes in the level of soluble arsenic, arsenite, arsenate, and ferrous iron in the culture medium. The arithmetic plots show relative concentrations of As (open squares), As(III) (solid squares), As(V) (solid diamonds), and Fe(II) (solid circles). The logarithmic plot shows growth curve of strain CC1 expressed as protein concentration (solid triangles). (B) Effect of growth conditions on the removal of arsenic from the medium. Concentrations of dissolved As (open symbols) and of As(III) (solid symbols) in abiotic As(III) conditions were determined with vigorous shaking (circles); with vigorous shaking and Fe(II) (triangles); and with vigorous shaking, Fe(II), and light (squares).
Several authors (24, 26, 27) have proposed that, in the Reigous spring, As(III) was oxidized by iron oxidizers, such as A. ferrooxidans, to As(V), which immediately coprecipitated with Fe(III). One possibility is then that strain CC1 oxidizes As(III) either to support its growth or to detoxify the surrounding medium. When strain CC1 from an Fe(II)-As(III) culture was inoculated into As(III) medium, no growth was observed even after 15 days at 30°C (data not shown), indicating that it did not oxidize As(III) to gain energy. No arsenite oxidase enzymatic activity was found with DCIP as the terminal electron acceptor in crude cell extracts of strain CC1 grown in Fe(II)-As(III) medium. Furthermore, the concentrations of dissolved As and As(III) did not change during the growth of strain CC1 in S0-As(III) medium for 8 days (data not shown). According to these results, we conclude that (i) strain CC1 has no arsenite oxidase activity and (ii) As and As(III) removal from the medium requires the presence of both iron and strain CC1.
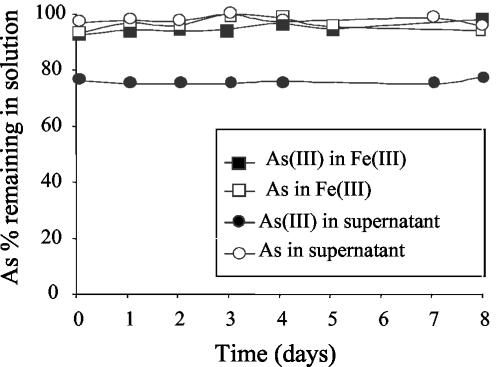
The disappearance of Fe(II) was followed as strain CC1 growth proceeded. The disappearance of dissolved As(III) and As from the medium coincided with the oxidation of Fe(II) to Fe(III) (Fig. 2A). The relative redox potentials of iron and arsenic suggested that Fe(III) should oxidize As(III) at low pH. However, the concentrations of dissolved As(III) and As remained constant over 8 days in As(III)-Fe(III) abiotic medium (Fig. 3), suggesting that removal of arsenic from the medium was not due to oxidation by the Fe(III) produced by the bacteria in the extracellular medium. In agreement with our results, it was previously reported that Fe(III) alone cannot oxidize As(III); instead, pyrite was needed (3, 32, 33, 39). The presence of Fe(II) and oxygen is indeed required in order to catalyze the oxidation of As(III) by iron (21). This reaction was observed in our abiotic As(III)-Fe(II) experiment, where a small fraction of As(III) oxidized to As(V) and precipitated with newly formed Fe(III) (see next section).
FIG. 3.
Effect of excreted products from CC1 cells on arsenic removal. Concentrations of soluble As (open symbols) and As(III) (solid symbols) in Fe(III) medium (squares) and in a filter-sterilized supernatant of strain CC1 grown on Fe(II)-As(III) medium supplemented with As(III) (circles).
To test the possible role of an extracellular electron shuttle excreted by the bacteria in the As removal process, we added As(III) to a filter-sterilized supernatant of a 3-day culture of strain CC1 grown on Fe(II)-As(III) medium, and we monitored the soluble As and As(III) concentrations. These concentrations remained unchanged over an 8-day period (Fig. 3). This result indicates that, in the biotic As(III)-Fe(II) experiments, the removal of As from the medium was not due to an extracellular electron shuttle excreted by the bacteria. We conclude that A. ferrooxidans strain CC1 did not oxidize As(III) to As(V) directly or indirectly.
Crystal chemistry of the solid phases.
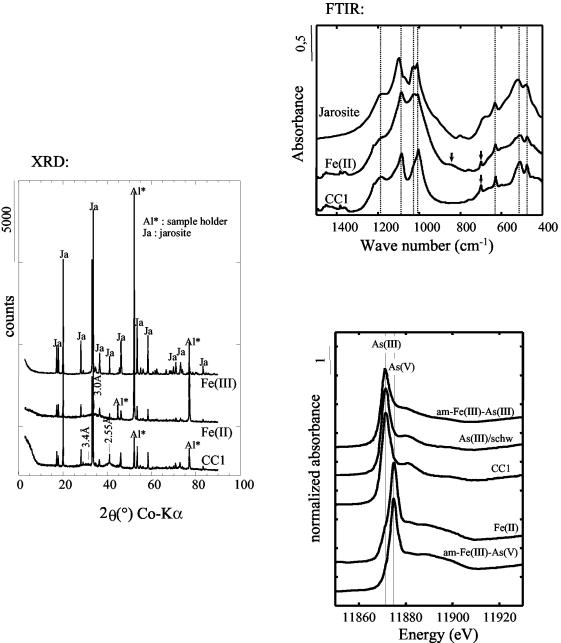
We investigated whether strain CC1 was able to precipitate As(III). Therefore, the solid phases formed in Fe(II)-As(III) medium with or without A. ferrooxidans strain CC1 and in the abiotic Fe(III)-As(III) medium were recovered and analyzed. XRD and FTIR analyses indicated that the three samples consisted essentially of jarosite [KFe3(OH)6(SO4)2] almost devoid of arsenic (Fig. 4).
FIG. 4.
XRD patterns and FTIR and As K-edge XANES spectra of the precipitates obtained from the Fe(II)-As(III)-CC1 (CC1), Fe(II)-As(III) abiotic [Fe(II)], and Fe(III)-As(III) abiotic [Fe(III)] experiments. As K-edge XANES spectra of reference compounds are reported for comparison, including 5% (wt/wt) As(III) adsorbed on schwertmannite [As(III)/schw], amorphous Fe(III)-As(III) mixed oxyhydroxide [am-Fe(III)-As(III)], and amorphous Fe(III)-As(V) mixed oxyhydroxide [am-Fe(III)-As(V)].
In the Fe(III) sample, almost pure jarosite was obtained. Arsenic was present in very small amounts (<1%) and could not be detected by As-XANES spectroscopy in our experimental conditions. In the CC1 sample and, to a lesser extent, in the Fe(II) sample, XRD data indicated the presence of a poorly ordered material. In the Fe(II) (abiotic) experiment, the poorly ordered phase exhibited a very broad and weak Bragg reflection around 3 Å (XRD in Fig. 4) that can be related to that observed in amorphous hydrous As(V)-Fe(III) precipitates (8, 35). XANES data indicated that As(V) dominated over As(III) in this sample (Fig. 4). The presence of As(V) in this sample is in agreement with the slight decrease in As(III) observed in the soluble fraction (Fig. 2B). The presence of As(V) in this precipitate could be related to the Fenton oxidation of As(III) in the presence of Fe(II) and oxygen in the absence of cells (21).
In the CC1 (biotic) experiment, the poorly ordered phase exhibited very broad Bragg reflections at 3.4 Å and 2.55 Å (XRD in Fig. 4) that can be related to the main reflections of schwertmannite, Fe8O8(OH)6SO4, i.e., the 310 and 212 reflections, respectively (5). In this sample, XANES analysis indicated that arsenic was essentially present as As(III) (Fig. 4), confirming that no As(III) oxidation was mediated by strain CC1. The absence of As(V) in the CC1 precipitate could be related to the fast Fe(II) oxidation, due to the A. ferrooxidans activity, which overcame the effect of the Fenton oxidation of As(III). The XANES spectrum of the CC1 sample also exhibited some similarities with that of tooeleite, a rare iron-arsenite mineral found in large amounts in the Carnoulès sediments (35). However, none of the Bragg reflections of this phase was observed in the XRD pattern of the CC1 sample, indicating that, if present, this phase should have extremely small coherent domain sizes (<3 nm) and is not dominant. Therefore, we propose that the removal of As and As(III) from the aqueous phase by strain CC1 was due to the massive oxidation of Fe(II) to Fe(III) and the subsequent coprecipitation of As(III) with Fe(III) in a poorly ordered phase that could be related to schwermannite. At the same time, the major Fe removal was due to the crystallization of jarosite.
pH effect on arsenic precipitation.
Arsenic disappearance from the medium, that is, As precipitation, coincided with Fe(III) production in the medium (Fig. 2A). Since Fe(II) oxidation consumes protons according to the reaction 4Fe2+ + O2 + 4H+→ 4Fe3+ + 2H2O, the pH of the medium rises substantially. Then, the arsenic precipitation could be due to pH increase. An argument against this hypothesis is that the medium that we used was buffered to avoid this problem. The pH indeed increased from 2.4 to only 2.6 after 1 day of growth of strain CC1 on Fe(II)-As(III) medium and then decreased to pH 2 after 8 days (data not shown). Furthermore, the filter-sterilized supernatant of a 3-day culture of strain CC1 on Fe(II) medium had no effect on As precipitation (Fig. 3). Nevertheless, we analyzed the pH effect on As precipitation by removing aliquots from a CC1 culture as growth proceeded on Fe(II) medium. After killing the cells with chloroform, which has no effect on the pH, As(III) was added. The pH and the concentrations of dissolved As(III), As(V), and As in these samples were determined throughout growth. As can be seen in Table 2, the As, As(III), and As(V) concentrations decreased slightly but remained high. Therefore, we conclude that As(III) precipitation was not due to a pH change in the medium and required live cells.
TABLE 2.
Arsenic removal by an Fe(II)-grown CC1 culture killed with chloroforma
| Time of growth on Fe(II) medium (h) | pH | % of initial As concn
|
||
|---|---|---|---|---|
| As | As(III) | As(V) | ||
| 0 | 2.4 | 100 | 81 | 9 |
| 24 | 2.57 | 96 | 64 | 0 |
| 40 | 2.48 | 94 | 64 | 0 |
| 48 | 2.4 | 83 | 73 | 0 |
| 72 | 2.35 | 85 | 74 | 2 |
Strain CC1 was grown on Fe(II) medium. Samples were removed throughout growth, treated with chloroform to kill the cells, supplemented with As(III), shaken at room temperature for 5 min, and frozen until analysis. The pH and the concentrations of soluble As, As(III), and As(V) were determined and expressed as a percentage of the soluble arsenic concentration at the beginning of the experiment.
Removal of arsenic from the medium by different A. ferrooxidans strains.
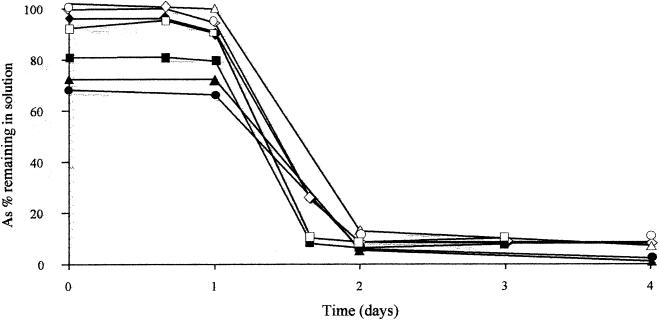
Different A. ferrooxidans strains (ATCC 19859, ATCC 33020, ATCC 23270, and BRGM1) were grown on Fe(II)-As(III) buffered medium, and the profiles of dissolved As(III) and As were followed throughout growth. As shown in Fig. 5, in all cases, the concentrations of dissolved As(III) and As decreased drastically in the medium after 1 to 2 days of growth at 30°C. The crystal chemistry of the solids formed in all these experiments was strictly similar to that observed with strain CC1 (XRD and XANES, data not shown). Arsenic removal from the medium by coprecipitation with Fe(III) was therefore a property of A. ferrooxidans.
FIG. 5.
Arsenic removal by different A. ferrooxidans strains in Fe(II)-As(III) medium. Strains: type strain ATCC 23270 (triangles); ATCC 33020 (squares); ATCC 19859 (diamonds); and BRGM1 (circles). The concentrations of soluble As (open symbols) and As(III) (solid symbols) were determined.
DISCUSSION
A. ferrooxidans was detected in all the samples collected around the Carnoulès mine, not only where arsenic and iron were present, but also where natural arsenic attenuation was established (9, 26; this paper). One acidophilic autotrophic iron-oxidizing microorganism was isolated from the Reigous spring at station C, where soluble arsenic was disappearing. This bacterium, designed strain CC1, has been unambiguously identified as Acidithiobacillus ferrooxidans. Indeed, it was able to grow on Fe(II) or S0 as the sole energy source in the absence of an organic carbon source. Furthermore, it encoded and synthesized rusticyanin, a protein which is involved in iron oxidation and which has been identified so far only in A. ferrooxidans species. Finally, according to phylogenetic analyses based on the sequence of the 16S rDNA and of the 16S-23S rDNA intergenic region, this microorganism belonged to the A. ferrooxidans species.
Under laboratory conditions, dissolved As(III) and As disappeared from a defined liquid medium in the presence of A. ferrooxidans CC1; the same held true for different collection and private strains (ATCC 23270, ATCC 33020, ATCC 19859, and BRGM1). Arsenic removal therefore seems to be a general property of the A. ferrooxidans species. These results are in agreement with the hypothesis that arsenic precipitation in the Reigous spring is mediated, at least in part, by A. ferrooxidans (24, 26, 27). However, A. ferrooxidans did not precipitate arsenic as As(V), as proposed by these authors, but, surprisingly, as As(III). Indeed, the A. ferrooxidans strains studied in the present work did not oxidize As(III) to As(V) directly or indirectly, as previously reported (45).
It is noteworthy that removal of arsenic was only observed when A. ferrooxidans strain CC1 was grown on iron and not when it was grown on S0 alone. This observation is fully consistent with the fact that As(III) is scavenged by the newly formed Fe(III) solid phases in our biotic experiments. It is known that adsorption of As(III) on ferrihydrite can occur at relatively high arsenic concentrations (40) but only slightly at low pH (6, 40). However, ill-ordered As(III)-Fe(III) mixed hydroxide can be formed in acidic conditions (35).
In our hands, in abiotic conditions, with either Fe(III) or Fe(II), or even with a filtered supernatant of an A. ferrooxidans grown on Fe(II), more than 80% of As(III) remained in solution. Even if Fe(III) was massively incorporated with sulfate in crystalline jarosite, this phase was almost devoid of arsenic. It is possible that the presence of high K+ levels in our experimental media favored the crystallization of jarosite having a small surface area, which was not able to scavenge the large arsenite pool present in the system (115 mg liter−1). In the presence of the bacteria, As(III) was not adsorbed on the jarosite produced after iron oxidation, but 95% was precipitated with poorly ordered schwertmannite. Under these conditions, the rapid precipitation of As(III) could have lowered the influence of the As(III) oxidation by intermediate chemical species produced by spontaneous oxidation of Fe(II) to Fe(III) in the presence of oxygen (21). This rapid precipitation of As-bearing schwertmannite under biotic conditions might be related to kinetic effects arising from the rapid oxidation of Fe(II) via metabolic reactions that lead to high oversaturation with respect to jarosite, thus favoring a more disordered phase, schwertmannite, containing more arsenic and less sulfate than jarosite.
The mode of association of As(III) with schwertmannite is still unclear, and neither chemical nor spectroscopic data are yet available for either the sorption affinity of As(III) for this mineral or the molecular structure of As(III)-doped schwertmannite. At pHs lower than 9, dissolved As(III) is only present as arsenious acid, H3AsO3, which has no electric charge. This neutral species might not easily substitute to HSO4− or SO42− groups in the channels of the schwertmannite structure. On the contrary, by analogy with the sorption of As(III) on other iron oxides (19), one may infer that H3AsO3 might be linked by edges and double corners to FeO6 octahedra on the surface of the schwertmannite particles. Such behavior has already been described for the coprecipitation of As(V) species with this mineral, and, in this case, it alters the crystallization of schwertmannite and leads to the formation of poorly ordered mixed As-Fe oxyhydroxides when solutions exhibit high As/S ratios (8, 35, 46).
The present work shows that the biotic coprecipitation of As(III) with Fe(III) efficiently removes As(III) from heavily contaminated waters. Recent analyses of the Reigous sediments where the A. ferrooxidans strain CC1 was collected have shown the absence of jarosite in these natural precipitates (35). It might be related to the relatively low K+ concentration in the Reigous water compared to that in our experiments. More importantly, XRD and XANES analyses of upstream sediments from the Reigous creek revealed the abundance of a rare Fe(III)-As(III) mineral, tooeleite, occurring in nanocrystalline form in association with As-rich XRD-amorphous As(III)-Fe(III) or As(V)-Fe(III) hydrous precipitates. Surprisingly, tooeleite was not detected by XRD in any of our experiments with four distinct A. ferrooxidans strains, including the CC1 strain isolated from the Reigous creek. This difference in the nature of the Fe(III)-As(III) minerals formed under A. ferrooxidans activity in As(III)-rich systems is still unexplained and might be related to changes in either physicochemical or biological parameters. In any case, the process of As(III) mineralization by Fe(III) mediated by A. ferrooxidans may be relevant to the formation of schwermannite or tooeleite in acidic water sediments.
A. ferrooxidans cells grown on Fe(II) were reported to be surrounded by a polynuclear Fe(III) complex (23 and references therein). This complex was described as a “system of Fe(III) ions strongly interacting with each other through dipolar interactions and exchange coupling.” Furthermore, isolated extracellular polymeric substances (EPS) from Fe(II)-grown A. ferrooxidans cells have been shown to contain tightly bound Fe(III) (20). These EPS were supposed to constitute a “special reaction compartment with the required low pH value and high amounts of Fe(III)” for pyrite bioleaching. It is noteworthy that the authors specified that metals other than Fe(III) could be complexed in EPS. Therefore, one possibility is that As(III) is trapped with Fe(III) in the EPS. Microbial precipitation of metals at the cell surface has been already reported, for example in Ralstonia eutropha (30, 37) and Geobacter sulfurreducens (30, 31). It is therefore possible that, at the cell surface of A. ferrooxidans, the Fe(II) oxidation site serves as the As(III) nucleation site. Another but not exclusive possibility suggested by the work of Crundwell (14 and references therein) is that the pH in the exopolysaccharide layer may temporarily rise above that of the bulk solution due to proton consumption during iron oxidation. Arsenic is known to be soluble across a wide pH range, but the pH can influence the saturation indices of solutes such as iron (48). This pH increase can lead to As and Fe coprecipitation.
It is noteworthy that A. ferrooxidans grown on Fe(II) was shown to be more resistant to metal ions than cells grown on thiosulfate or S0 (42, 44). More strikingly, in the presence of heavy metals, the growth of A. ferrooxidans on S0 medium was observed only when Fe(II) was added (42). It would be interesting to investigate whether the nucleation sites created by Fe(II) oxidation at the cell surface allow precipitation not only of As(III), but also of metal ions and whether this model can be applied to other iron-oxidizing microorganisms.
Acknowledgments
We gratefully acknowledge Y. Quentin (Laboratoire de Chimie Bactérienne, Marseille, France) for his help in the phylogenetic analyses and G. Giordano's group (Laboratoire de Chimie Bactérienne, Marseille, France) for assistance in the arsenite oxidase activity determination. We thank C. C. Zhang's group (Laboratoire de Chimie Bactérienne, Marseille, France) for the shaker under white light. We acknowledge F. Chaspoul from P. Gallice's group (Laboratoire de Chimie Générale, Faculté de Pharmacie, Marseille, France) for arsenic concentration and speciation measurements. We are grateful to J. A. DeMoss (Laboratoire de Chimie Bactérienne, Marseille, France) for his interest in this work and for critical reading of the manuscript. We acknowledge one of the reviewers, whose comments allowed us to improve the manuscript.
K.D. acknowledges the support of a graduate scholarship from the Agence de l'Environnement et de la Maîtrise de l'Energie (A.D.E.M.E.) and the Bureau de Recherches Géologiques et Minières (B.R.G.M.). This study was financed by the Programme Environnement Vie et Société (I.N.S.U.-C.N.R.S.), the A.C.I.-Ecologie Quantitative (French Ministery of Research), and GEOMEX (C.N.R.S.).
REFERENCES
- 1.Anderson, G. L., J. Williams, and R. Hille. 1992. The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J. Biol. Chem. 267:23674-23682. [PubMed] [Google Scholar]
- 2.Barham, J. 1997. Schwertmannite: a unique mineral, contains replaceable ligand, transforms to jarosites, hematites, and/or basic iron sulfate. J. Mater. Res. 12:2751-2758. [Google Scholar]
- 3.Barrett, J., D. K. Ewart, M. N. Hughes, and R. K. Poole. 1993. Chemical and biological pathways in the bacterial oxidation of arsenopyrite. FEMS Microbiol. Rev. 11:57-62. [Google Scholar]
- 4.Bengrine, A. 1997. Ph. D. thesis. Université de la Méditerranée, Aix-Marseille II, France.
- 5.Bigham, J. M., L. Carlson, and E. Murad. 1994. Schwertmannite, a new iron oxyhydroxy-sulfate from Pyhäsalmi, Finland, and other localities. Mineralogical Magazine 58:641-648. [Google Scholar]
- 6.Bowell, R. J. 1994. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl. Geochem. 9:279-286. [Google Scholar]
- 7.Butcher, B. G., S. M. Deane, and D. E. Rawlings. 2000. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol. 66:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson, L., J. M. Bigham, U. Schwertmann, A. Kyek, and F. Wagner. 2002. Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ. Sci. Technol. 36:1712-1719. [DOI] [PubMed] [Google Scholar]
- 9.Casiot, C., G. Morin, F. Juillot, O. Bruneel, J.-C. Personné, M. Leblanc, K. Duquesne, V. Bonnefoy, and F. Elbaz-Poulichet. 2003. Bacterial immobilization and oxidation of arsenic in acid mine drainage (Carnoulès creek, France). Water Res. 37:2929-2936. [DOI] [PubMed] [Google Scholar]
- 10.Cervantes, C., G. Ji, J. L. Ramírez, and S. Silver. 1994. Resistance to arsenic compounds in microorganism. FEMS Microbiol. Rev. 15:355-367. [DOI] [PubMed] [Google Scholar]
- 11.Cherry, J. A., A. U. Shaikh, D. E. Tallman, and R. V. J. Nicholson. 1979. Arsenic species as an indicator of redox conditions in groundwater. J. Hydrol. 43:373-392. [Google Scholar]
- 12.Collinet M.-N., and D. Morin. 1990. Characterization of arsenopyrite oxidizing Thiobacillus. Tolerance to arsenite, arsenate, ferrous and ferric iron. Antonie van Leeuwenhoek 57:237-244. [DOI] [PubMed] [Google Scholar]
- 13.Coram, N. J., and D. E. Rawlings. 2002. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40°C. Appl. Environ. Microbiol. 68:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crundwell, F. K. 2001. How do bacteria interact with minerals?, p. 149-157. In V. S. T. Ciminelli and O. Garcia (ed.), Biohydrometallurgy: fundamentals, technology and sustainable development. Elsevier, Amsterdam, The Netherlands.
- 15.Eary, L. E. and J. A. Schramke. 1990. Rates of inorganic oxidation reactions involving dissolved oxygen, p. 379-396. In D. C. Melchior and R. L. Bassett (ed.), Chemical modeling of aqueous systems II. American Chemical Society, Washington, D.C.
- 16.Ehrlich, H. L. 1963. Bacterial action on orpiment. Econ. Geol. 58:991-994. [Google Scholar]
- 17.Ehrlich, H. L. 1964. Bacterial oxidation of arsenopyrite and enargite. Econ. Geol. 59:1306-1312. [Google Scholar]
- 18.Emett, M. T., and G. H. Khoe. 2001. Photochemical oxidation of arsenic by oxygen and iron in acidic solutions. Water Res. 35:649-656. [DOI] [PubMed] [Google Scholar]
- 19.Farquar, M., J. M. Charnock, F. Livens, and D. J. Vaughan. 2002. Mechanism of arsenic uptake from aqueous solution by interaction with goethite, lepidocrocite, mackinawite, pyrite: an X-ray adsorption spectroscopy study. Environ. Sci. Technol. 36:1757-1762. [DOI] [PubMed] [Google Scholar]
- 20.Gehrke, T., R. Hallmann, K. Kinzler, and W. Sand. 2001. The EPS of Acidithiobacillus ferrooxidans — a model for structure-function relationships of attached bacteria and their physiology. Water Sci. Technol. 43:159-167. [PubMed] [Google Scholar]
- 21.Hug, S. J., and O. Leupin. 2003. Iron-catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction. Environ. Sci. Technol. 37:2734-2742. [DOI] [PubMed]
- 22.Hutchins, S. R., M. S. Davidson, J. A. Brierley, and C. L. Brierley. 1986. Microorganisms in reclamation of metals. Annu. Rev. Microbiol. 40:311-336. [DOI] [PubMed] [Google Scholar]
- 23.Ingledew, W. J. 1986. Ferrous iron oxidation by Thiobacillus ferrooxidans. Biotechnol. Bioeng. Symp. 16:23-33. [Google Scholar]
- 24.Kilty, H., D. Moran, J. Roussy, J. M. Tobin, C. Drakides, and J. R. Degorce-Dumas. 2001. Bioprecipitation of As and Fe ions, p. 365-371. In V. S. T. Ciminelli and O. Garcia (ed.), Biohydrometallurgy: fundamentals, technology and sustainable development. Elsevier, Amsterdam, The Netherlands.
- 25.Kilty, H., J. Roussy, J. M. Tobin, and J. R. Degorce-Dumas. 2001. Acid mine water treatment: a laboratory scale study, p. 357-364. In V. S. T. Ciminelli and O. Garcia (ed.), Biohydrometallurgy: fundamentals, technology an sustainable development. Elsevier, Amsterdam, The Netherlands.
- 26.Leblanc, M., B. Achard, D. Ben Othman, J. M. Luck, J. Bertrand-Sarfati, and J.-C. Personné. 1996. Accumulation of arsenic from acidic mine waters by ferruginous bacterial accretions (stromatolites). Appl. Geochem. 11: 541-554. [Google Scholar]
- 27.Leonard, P., C. M. Estrada Rendon, G. Amara, J. Roussy, J. M. Tobin, J. R. Degorce-Dumas. 1999. Natural attenuation study of the impact of acid mine drainage (AMD) in the site of Carnoulès, p. 587-594. In R. Amils and A. Ballester (ed.), Biohydrometallurgy and the environment toward the mining of the 21st century. Elsevier, Amsterdam, The Netherlands.
- 28.Liu, Z., F. Borne, J. Ratouchniak, and V. Bonnefoy. 2001. Genetic transfer of IncP, IncQ and IncW plasmids to four Thiobacillus ferrooxidans strain by conjugation. Hydrometallurgy 59:339-345. [Google Scholar]
- 29.Liu, Z., N. Guiliani, C. Appia-Ayme, F. Borne, J. Ratouchniak, and V. Bonnefoy. 2000. Construction and characterization of a recA mutant of Thiobacillus ferrooxidans by marker exchange mutagenesis. J. Bacteriol. 182:2269-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd, J. R., and D. R. Lovley. 2001. Microbial detoxification of metals and radionuclides. Curr. Opin. Biotechnol. 12:248-253. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd, J. R., V. A. Sole, C. V. Van Praagh, and D. R. Lovley. 2000. Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl. Environ. Microbiol. 66:3743-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandl, M., P. Matulova, and H. Docekalova. 1992. Migration of AsIII during bacterial oxidation of arsenopyrite in chalcopyrite concentrates by Thiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 38:429-431. [Google Scholar]
- 33.Mandl, M., and M. Vykovsky. 1994. Kinetics of AsIII oxidation by iron(III) catalyzed by pyrite in the presence of Thiobacillus ferrooxidans. Biotechnol. Lett. 16:1199-1204. [Google Scholar]
- 34.Monroy Fernandez, M. G., C. Mustin, P. de Donato, O. Barres, P. Marion, and J. Berthelin. 1995. Occurrences at mineral-bacteria interface during oxidation of arsenopyrite by Thiobacillus ferrooxidans. Biotechnol. Bioeng. 46:13-21. [DOI] [PubMed] [Google Scholar]
- 35.Morin, G., F. Juillot, C. Casiot, O. Bruneel, J-C. Personné, F. Elbaz-Poulichet, M. Leblanc, P. Ildefonse, and G. Calas. 2003. Bacterial formation of tooeleite and mixed As(III)/(V)-Fe(III) gels in the Carnoulès acid mine drainage, France. A XANES, XRD and SEM study. Environ. Sci. Technol. 37:1705-1712. [DOI] [PubMed] [Google Scholar]
- 36.Muir, M. K., and T. Anderson. 1977. Determination of ferrous iron in copper-process metallurgical solutions by the o-phenanthroline colorimetric method. Metallurg. Trans. 8B:517. [Google Scholar]
- 37.Nies, D. H. 2000. Heavy metal-resistant bacteria as extremophiles: molecular physiology and biotechnical use of Ralstonia sp. CH34. Extremophiles 4:77-82. [DOI] [PubMed] [Google Scholar]
- 38.Norris, P. R. 1983. Iron and mineral oxidation with Leptospirillum-like bacteria, p. 83-86. In G. Rossi and A. E. Torma (ed.), Recent progress in biohydrometallurgy. Associazione Mineraria Sarda, Iglesias, Italy.
- 39.Nyashanu, R. M., A. J. Monhemius, and D. L. Buchanan. 1999. The effects of ore mineralogy on the speciation of arsenic in bacterial oxidation of refractory arsenical gold ores, p. 431-441. In R. Amils and A. Ballester (ed.), Biohydrometallurgy and the environment toward the mining of the 21st century. Elsevier, Amsterdam, The Netherlands.
- 40.Raven, K. R., A. Jain, and R. H. Loeppert. 1998. Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environ. Sci. Technol. 32:344-349. [Google Scholar]
- 41.Selenska-Pobell, S., A. Otto, and S. Kutschke. 1998. Identification and discrimination of thiobacilli with ARDREA, RAPD and rep-APD. J. Appl. Microbiol. 84:1085-1091. [Google Scholar]
- 42.Sugio, T., C. Domatsu, T. Tano, and K. Imai. 1984. Role of ferrous ions in synthetic cobaltous sulfide leaching of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 48:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuovinen, O. H., S. I. Niemela, and H. G. Gyllenberg. 1971. Tolerance of Thiobacillus ferrooxidans to some metals. Antonie van Leeuwenhoek 37:489-496. [DOI] [PubMed] [Google Scholar]
- 45.Wakao, N., H. Koyatsu, Y. Komai, H. Shimokawara, Y. Sakurai, and H. Shiota. 1988. Microbial oxidation of arsenite and occurrence of arsenite-oxidizing bacteria in acid mine water from a sulfur-pyrite mine. Geomicrobiol. J. 6:11-24. [Google Scholar]
- 46.Waychunas, G. A., N. Xu, C. C. Fuller, J. A. Davis, and J. M. Bigham. 1995. XAS study of AsO43− and SeO42− substituted schwertmannites. Physica B 208/209:481-483. [Google Scholar]
- 47.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams, M. 2001. Arsenic in mine waters: an international study. Environ. Geol. 40:267-278. [Google Scholar]
- 49.Yarzábal A., K. Duquesne, and V. Bonnefoy. Rusticyanin gene expression of Acidithiobacillus ferrooxidans ATCC 33020 in sulfur- and in ferrous iron-media,. Hydrometallurgy, in press.